Potentiation of Recombinant NP and M1-Induced Cellular Immune Responses and Protection by Physical Radiofrequency Adjuvant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. RF Device
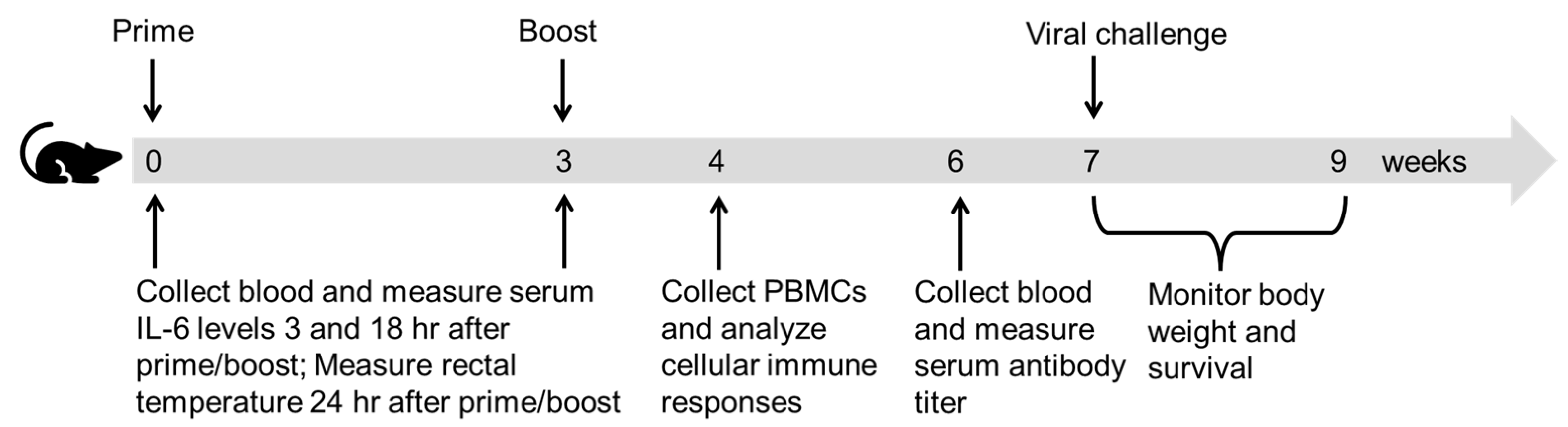
2.4. Immunization
2.5. Antibody Titer Measurement
2.6. Cellular Immune Response
2.7. Lethal Viral Challenge
2.8. Cytokine Levels
2.9. Statistics
3. Results
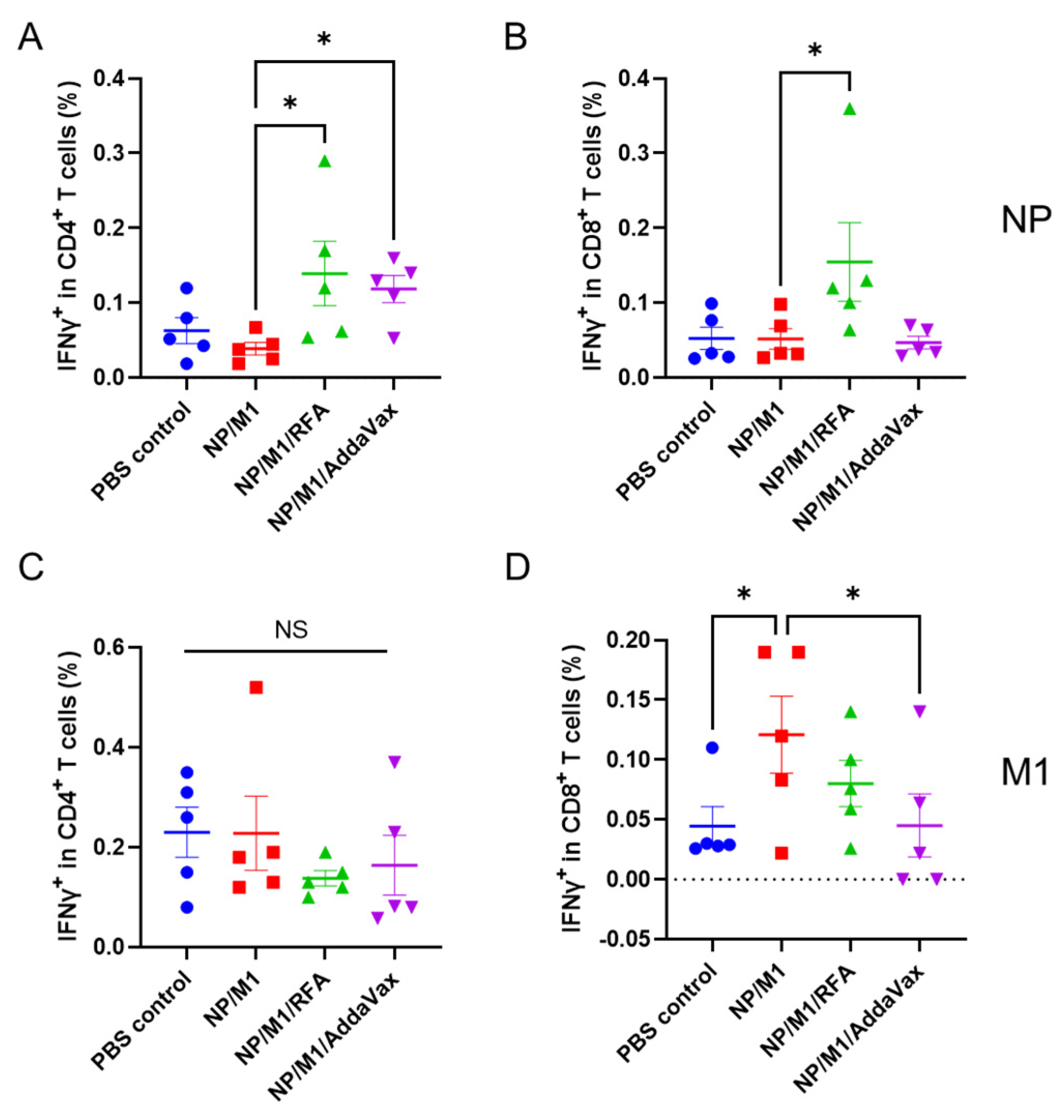
3.1. RFA Enhances NP-Induced Cellular Immune Responses
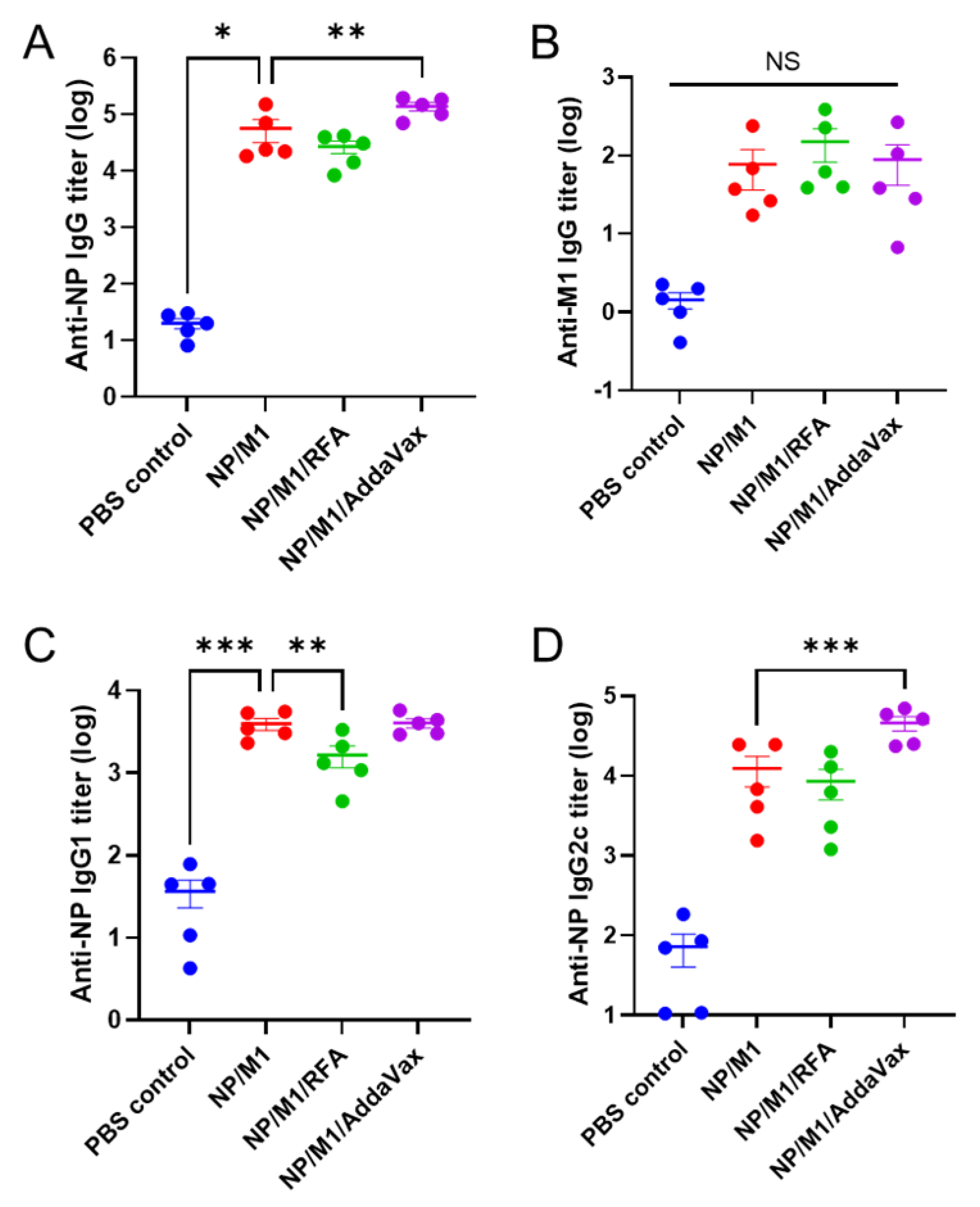
3.2. RFA Has a Minimal Effect on Humoral Immune Responses
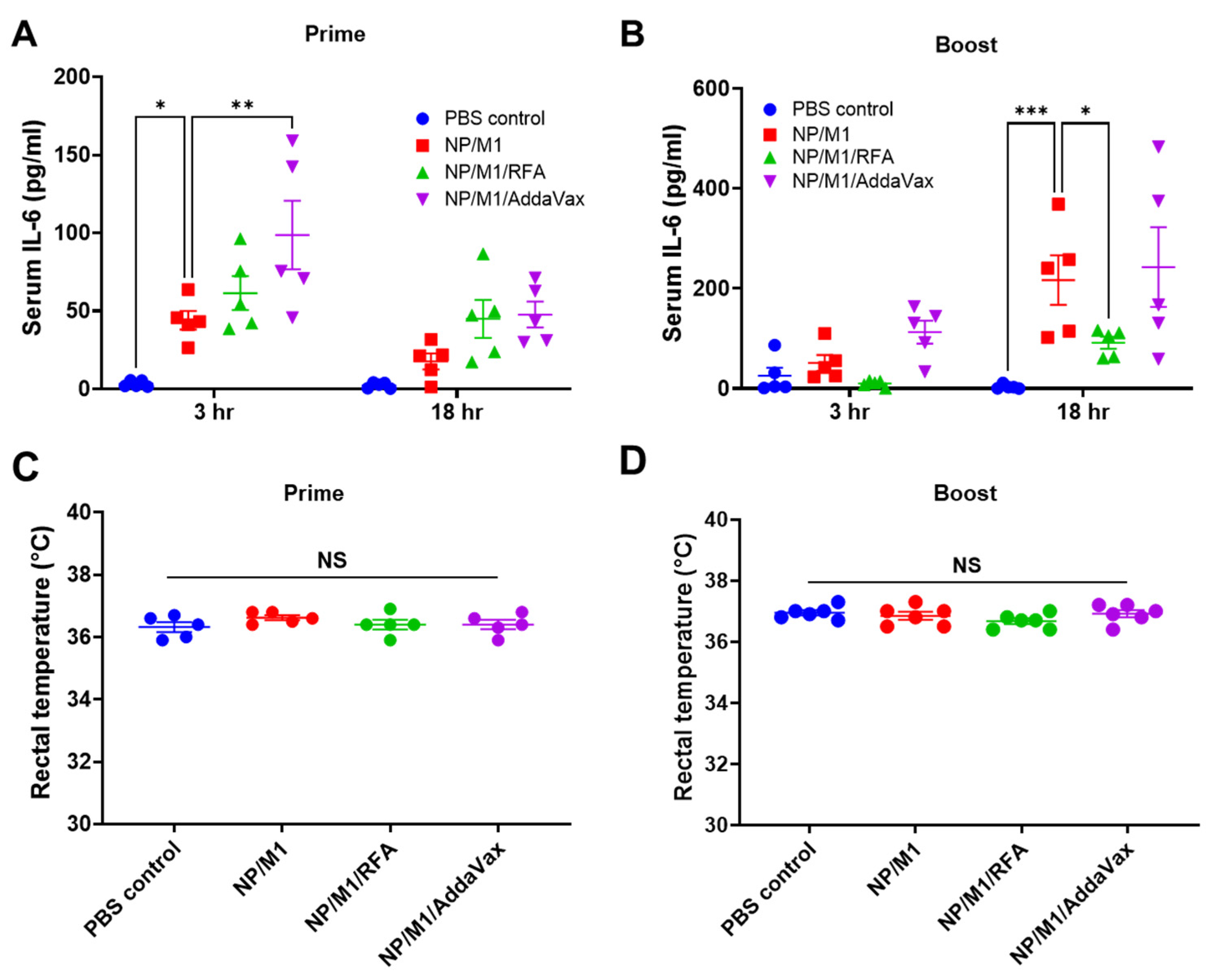
3.3. RFA Safely Boosts NP/M1 Immunization
3.4. RFA Increases NP/M1-Induced Protection against Body Weight Loss
3.5. RFA Increased NP/M1-Induced Protection against Lethality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, S.S.; Webby, R.J. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef] [Green Version]
- Yamayoshi, S.; Kawaoka, Y. Current and future influenza vaccines. Nat. Med. 2019, 25, 212–220. [Google Scholar] [CrossRef]
- Ostrowsky, J.; Arpey, M.; Moore, K.; Osterholm, M.; Friede, M.; Gordon, J.; Higgins, D.; Molto-Lopez, J.; Seals, J.; Bresee, J. Tracking progress in universal influenza vaccine development. Curr. Opin. Virol. 2020, 40, 28–36. [Google Scholar] [CrossRef]
- Uchida, T. Development of a cytotoxic T-lymphocyte-based, broadly protective influenza vaccine. Microbiol. Immunol. 2011, 55, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cargnelutti, D.E.; Sanchez, M.V.; Mattion, N.M.; Scodeller, E.A. Development of a universal CTL-based vaccine for influenza. Bioengineered 2013, 4, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.Y.; Ha, D.L.A.; Simmons, C.; de Jong, M.D.; Chau, N.V.V.; Schumacher, R.; Peng, Y.C.; McMichael, A.J.; Farrar, J.J.; Smith, G.L.; et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Investig. 2008, 118, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.L.; Kong, W.P.; Misplon, J.A.; Lo, C.Y.; Tumpey, T.M.; Xu, L.; Nabel, G.J. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 2005, 23, 5404–5410. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kobinger, G.P.; Lin, J.; Figueredo, J.; Calcedo, R.; Kobasa, D.; Wilson, J.M. Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein. Vaccine 2007, 25, 6845–6851. [Google Scholar] [CrossRef]
- Joe, P.T.; Christopoulou, I.; van Hoecke, L.; Schepens, B.; Ysenbaert, T.; Heirman, C.; Thielemans, K.; Saelens, X.; Aerts, J.L. Intranodal administration of mRNA encoding nucleoprotein provides cross-strain immunity against influenza in mice. J. Transl. Med. 2019, 17, 242. [Google Scholar] [CrossRef]
- McMahon, M.; Asthagiri Arunkumar, G.; Liu, W.C.; Stadlbauer, D.; Albrecht, R.A.; Pavot, V.; Aramouni, M.; Lambe, T.; Gilbert, S.C.; Krammer, F. Vaccination With Viral Vectors Expressing Chimeric Hemagglutinin, NP and M1 Antigens Protects Ferrets Against Influenza Virus Challenge. Front. Immunol. 2019, 10, 2005. [Google Scholar] [CrossRef]
- Asthagiri; Arunkumar, G.; McMahon, M.; Pavot, V.; Aramouni, M.; Ioannou, A.; Lambe, T.; Gilbert, S.; Krammer, F. Vaccination with viral vectors expressing NP, M1 and chimeric hemagglutinin induces broad protection against influenza virus challenge in mice. Vaccine 2019, 37, 5567–5577. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, A.; Quirion, M.R.; Lo, C.Y.; Misplon, J.A.; Grabowska, A.K.; Pierantoni, A.; Ammendola, V.; Price, G.E.; Soboleski, M.R.; Cortese, R.; et al. Vaccination to conserved influenza antigens in mice using a novel Simian adenovirus vector, PanAd3, derived from the bonobo Pan paniscus. PLoS ONE 2013, 8, e55435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambe, T.; Carey, J.B.; Li, Y.; Spencer, A.J.; van Laarhoven, A.; Mullarkey, C.E.; Vrdoljak, A.; Moore, A.C.; Gilbert, S.C. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci. Rep. 2013, 3, 1443. [Google Scholar] [CrossRef] [Green Version]
- Neefjes, J.; Jongsma, M.L.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; Marrack, P. Old and new adjuvants. Curr. Opin. Immunol. 2017, 47, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nguyen, M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune. Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Nanishi, E.; Dowling, D.J.; Levy, O. Toward precision adjuvants: Optimizing science and safety. Curr. Opin. Pediatr. 2020, 32, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhu, X.; Hossen, M.N.; Kakar, P.; Zhao, Y.; Chen, X. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat. Commun. 2018, 9, 3695. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Y.; Li, Y.; Zhao, Y.; Chen, X. Vaccine delivery alerts innate immune systems for more immunogenic vaccination. JCI Insight. 2021, 6, e144627. [Google Scholar] [CrossRef]
- Cottey, R.; Rowe, C.A.; Bender, B.S. Influenza virus. In Current Protocols in Immunology; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Herve, C.; Laupeze, B.; Del.Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.F.; Chiu, S.Y.; Huang, H.W.; Cheng, B.H.; Pan, H.M.; Huang, W.L.; Chang, H.H.; Liao, C.C.; Jiang, S.T.; Su, Y.C. A reporter mouse for non-invasive detection of toll-like receptor ligands induced acute phase responses. Sci. Rep. 2019, 9, 19065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, Z.; Zhu, X.; Cao, Y.; Chen, X. Improving immunogenicity and safety of flagellin as vaccine carrier by high-density display on virus-like particle surface. Biomaterials 2020, 249, 120030. [Google Scholar] [CrossRef]
- Chen, X.; Wu, M.X. Laser vaccine adjuvant for cutaneous immunization. Expert. Rev. Vaccines 2011, 10, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Carragher, D.M.; Kaminski, D.A.; Moquin, A.; Hartson, L.; Randall, T.D. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 2008, 181, 4168–4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee., L.Y.Y.; Izzard, L.; Hurt, A.C. A Review of DNA Vaccines Against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef] [Green Version]
- Quan, F.S.; Kim, M.C.; Lee, B.J.; Song, J.M.; Compans, R.W.; Kang, S.M. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology 2012, 430, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Z.; Xu, R.; Quan, F.S.; Kang, S.M.; Wang, L.; Compans, R.W. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS ONE 2010, 5, e13972. [Google Scholar] [CrossRef] [PubMed] [Green Version]

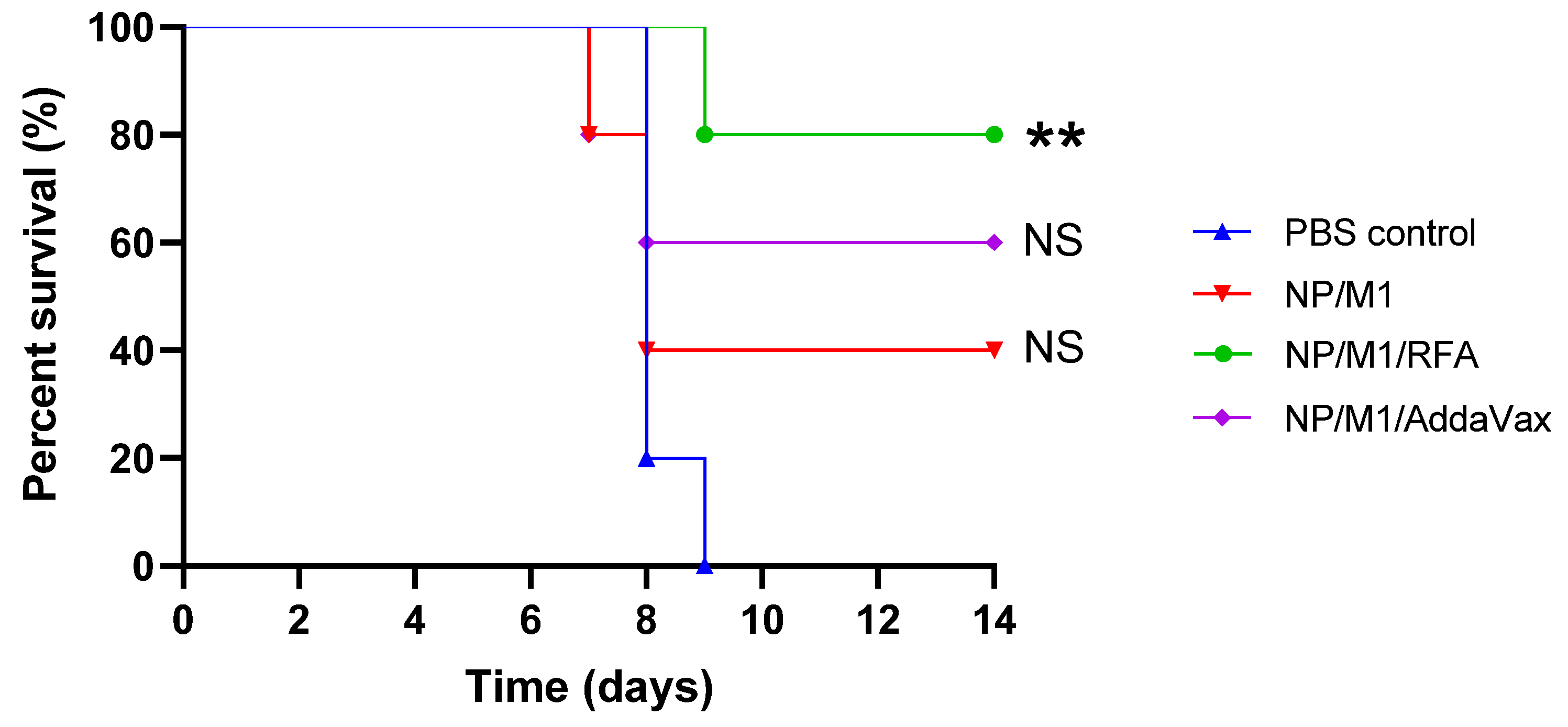
| NP/M1 | NP/M1/RFA | NP/M1/AddaVax | Reference Group | |
|---|---|---|---|---|
| Day 8 | NS | p < 0.05 | p < 0.01 | PBS control |
| Day 9 | - | p < 0.05 | p < 0.05 | NP/M1 |
| Day 10 | - | p < 0.05 | p < 0.05 | NP/M1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Z.; Zhao, Y.; Chen, X. Potentiation of Recombinant NP and M1-Induced Cellular Immune Responses and Protection by Physical Radiofrequency Adjuvant. Vaccines 2021, 9, 1382. https://doi.org/10.3390/vaccines9121382
Li Y, Li Z, Zhao Y, Chen X. Potentiation of Recombinant NP and M1-Induced Cellular Immune Responses and Protection by Physical Radiofrequency Adjuvant. Vaccines. 2021; 9(12):1382. https://doi.org/10.3390/vaccines9121382
Chicago/Turabian StyleLi, Yibo, Zhuofan Li, Yiwen Zhao, and Xinyuan Chen. 2021. "Potentiation of Recombinant NP and M1-Induced Cellular Immune Responses and Protection by Physical Radiofrequency Adjuvant" Vaccines 9, no. 12: 1382. https://doi.org/10.3390/vaccines9121382
APA StyleLi, Y., Li, Z., Zhao, Y., & Chen, X. (2021). Potentiation of Recombinant NP and M1-Induced Cellular Immune Responses and Protection by Physical Radiofrequency Adjuvant. Vaccines, 9(12), 1382. https://doi.org/10.3390/vaccines9121382








