PICK-ing Malaysia’s Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Environment
2.2. Screening Method Study Design, Population, and Methodology
- Proportion of population that is vaccinated, PPV;
- Proportion of disease outcomes that are vaccinated, PCV.
2.3. Retrospective Cohort Study Design and Population
3. Results
3.1. Screening Method: Vaccine Effectiveness
3.2. Cohort of Confirmed COVID-19 Cases: Vaccine Effectiveness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
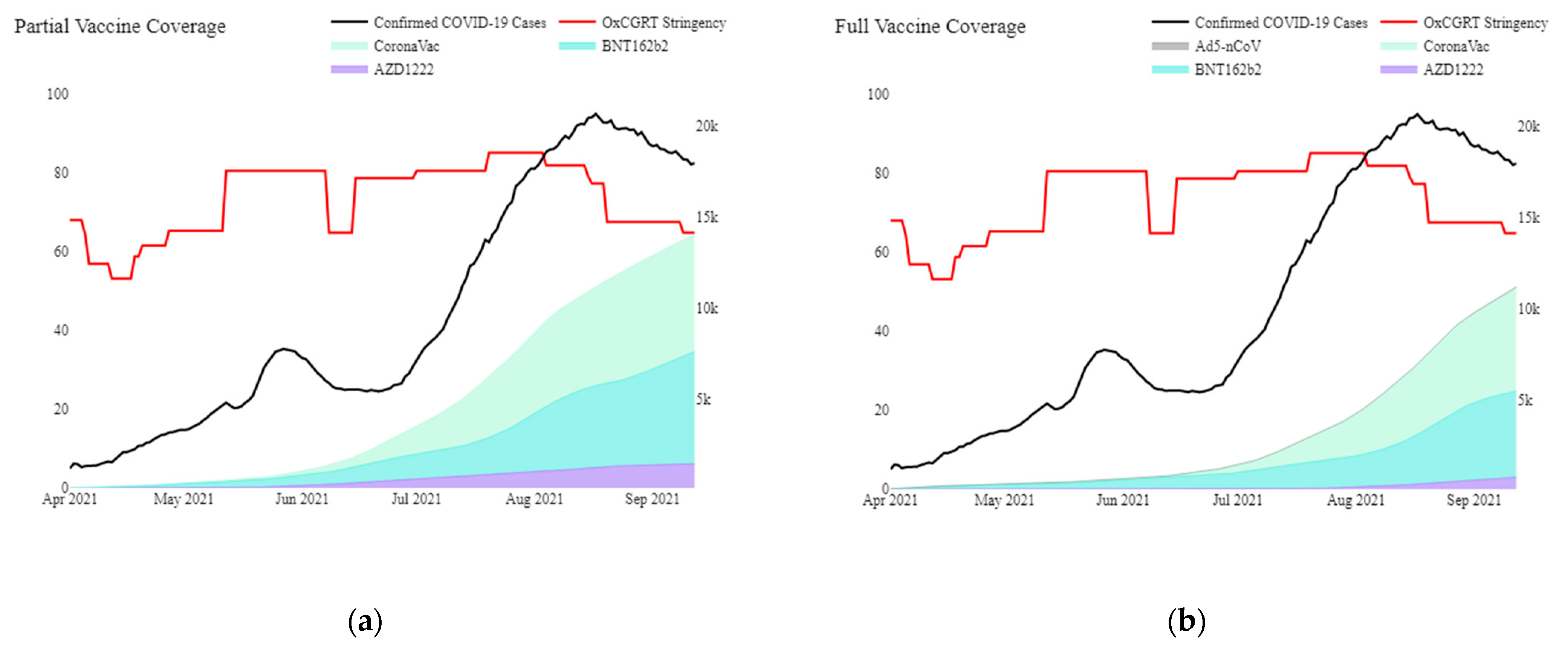
Appendix A. Additional Context on Malaysia (Setting) and COVID-19 Pandemic
Appendix B. Programme Imunisasi COVID-19 Kebangsaan (PICK)
- Phase 1 targeted ‘frontline’ workers, mostly in the healthcare, defence, and security sectors, involving approximately 500,000 individuals (1.5% of the population);
- Phase 2, which began in April 2021, prioritised senior (aged 60 years and above), disabled and high-risk individuals for severe diseases. Phase 2 involved approximately 9.4 million individuals (28.8% of the population);
- Phase 3 involved the remaining population aged 18 years and above and prioritises locations with high disease burdens to minimise the adverse effects of COVID-19.
Appendix C. Definition of Outcome Measures
- COVID-19 confirmed casesA COVID-19 confirmed case was defined as a person with laboratory confirmation* of infection with the COVID-19 irrespective of clinical signs or symptoms.*A person with a positive molecular test (RT-PCR or rapid molecular) or a person with positive RTK-Ag in pre-determined areas/locality with the prevalence of COVID-19 ≥ 10%
- Symptomatic COVID-19 confirmed casesA COVID-19 confirmed case with reported signs or symptoms such as fever, chill, rigours, myalgia, headache, sore throat, nausea or vomiting, diarrhoea, fatigue, nasal congestion, cough, shortness of breath, difficulty in breathing, sudden onset of anosmia, and/or ageusia.
- COVID-19 ICU admissionsA confirmed COVID-19 case that required intensive care and was admitted to ICU based on clinical judgement due to disease progression to severe diseases, such as category 4 (requiring oxygen therapy) or category 5 (requiring mechanical ventilation).
- COVID-19 deathsA COVID-19 death refers to any “death attributable to COVID-19” and took two settings as follows:
- Criteria for death during hospitalisation:
- COVID-19-positive case that has been confirmed through laboratory tests (RT-PCR)
- Death resulting from clinically compatible disease
- No clear alternative cause of death that is not related to COVID-19 infection (examples: death due to trauma, road traffic accident, suicide, etc.)
- No period of complete recovery from COVID-19 between illness and death
- Even with pre-existing disease (e.g., cancer), it is considered death due to COVID-19 if the reason for the severe course is due to COVID-19 infection
- Criteria for cases that were brought in dead (BID):
- If a postmortem is conducted, the classification of COVID-19 death shall be in accordance with the autopsy death report;
- If the autopsy is not performed, the death of COVID-19 must meet the following criteria:
- COVID-19-positive case that has been confirmed through laboratory test (RT-PCR) with CT value; and
- Has an epidemiological link with another COVID-19 case.
- Supportive radiological investigation, if any (examples: chest x-ray, postmortem computed tomography scan, etc.), which shows signs of COVID-19 infection, can also be used as a supportive modality for the attending specialist in charge. However, it is not a criterion that must be met for the confirmation of “Death Due to COVID-19”.
Appendix D. Data Sources
- The COVID-19 cases line listingData source: Malaysia national electronic COVID-19 cases register (e-COVID/COVID-19 line listing).Data on the COVID-19 confirmed cases were obtained from the national electronic COVID-19 cases register, which is the national COVID-19 surveillance system. This surveillance system detects cases from multiple sources such as the surveillance system, point of entry, targeted screening, international health regulation focal points, pre-admission of COVID-19 screening, passive case detection from healthcare facilities, and any screening for brought-in dead. Each patient diagnosed as a confirmed case will be assigned a unique case number. Data elements include patient demographics, location, date of positive test, presence of comorbidities, and symptoms at diagnosis.
- The intensive care unit (ICU) admissions registerData source: Malaysia national COVID-19 clinical admissions registerData on ICU admissions for COVID-19 patients were obtained from the National COVID-19 clinical admission register. This is an administrative register collecting information on allCOVID-19 ICU admissions containing elements such as patient demographic, location, symptoms, disease category at admission, and comorbidities.
- The COVID-19 deaths line listingData source: Malaysia national COVID-19 deaths line listingThe criteria for the classification of COVID-19 death have been developed and established by a consensus from Infectious Diseases Physicians, Forensic Pathologists, and the National Committee of COVID-19 Mortality Review. Every death underwent an audit process based on the WHO guidelines before it was classified as COVID-19 death. Data elements included patient demographics, location, date of death, and comorbidities.
- The COVID-19 cases line listingData source: Malaysia national COVID-19 vaccinations registerVaccination records for the Malaysian population were obtained from the National COVID-19 vaccination register, available via the Vaccine Management System of the Ministry of Health Malaysia (Malaysia Vaccine Administration System, MyVAS).Data elements included patient-level demographics and comorbidities, date of vaccination, site of vaccination, vaccine batch numbers, and vaccine type. Data fields pertaining to background characteristics were self-reported when patients registered for the COVID-19 vaccine via PICK, while data fields on vaccination were reported by healthcare providers at vaccination centres via MyVAS. Verification steps were built in. Firstly, selected workers at the vaccination centres with administrator rights verified the validity of data fields for respective patients via MyVAS, including the actual administration of vaccines and demographics. Secondly, patients were required to scan QR codes at various stations to validate the vaccine administration through Malaysia’s contact tracing application, MySejahtera. For non-MySejahtera users, workers at the vaccination centres perform the validation via MyVAS.
Appendix E. Methodology
Appendix E.1. Screening Method
- The proportion of the population that is vaccinated, or vaccination coverage in population, PPV.
- The proportion of outcomes (infections, or symptomatic infections) that are vaccinated, PCV, since 1 April 2021.
- The number of outcomes that are vaccinated, CV, since 1 April 2021.
- The number of outcomes that are unvaccinated, CnV, since 1 April 2021.
- The number of vaccinated individuals, V.
- The number of unvaccinated individuals, nV.
Appendix E.2. Retrospective Cohort: Logistic Regression
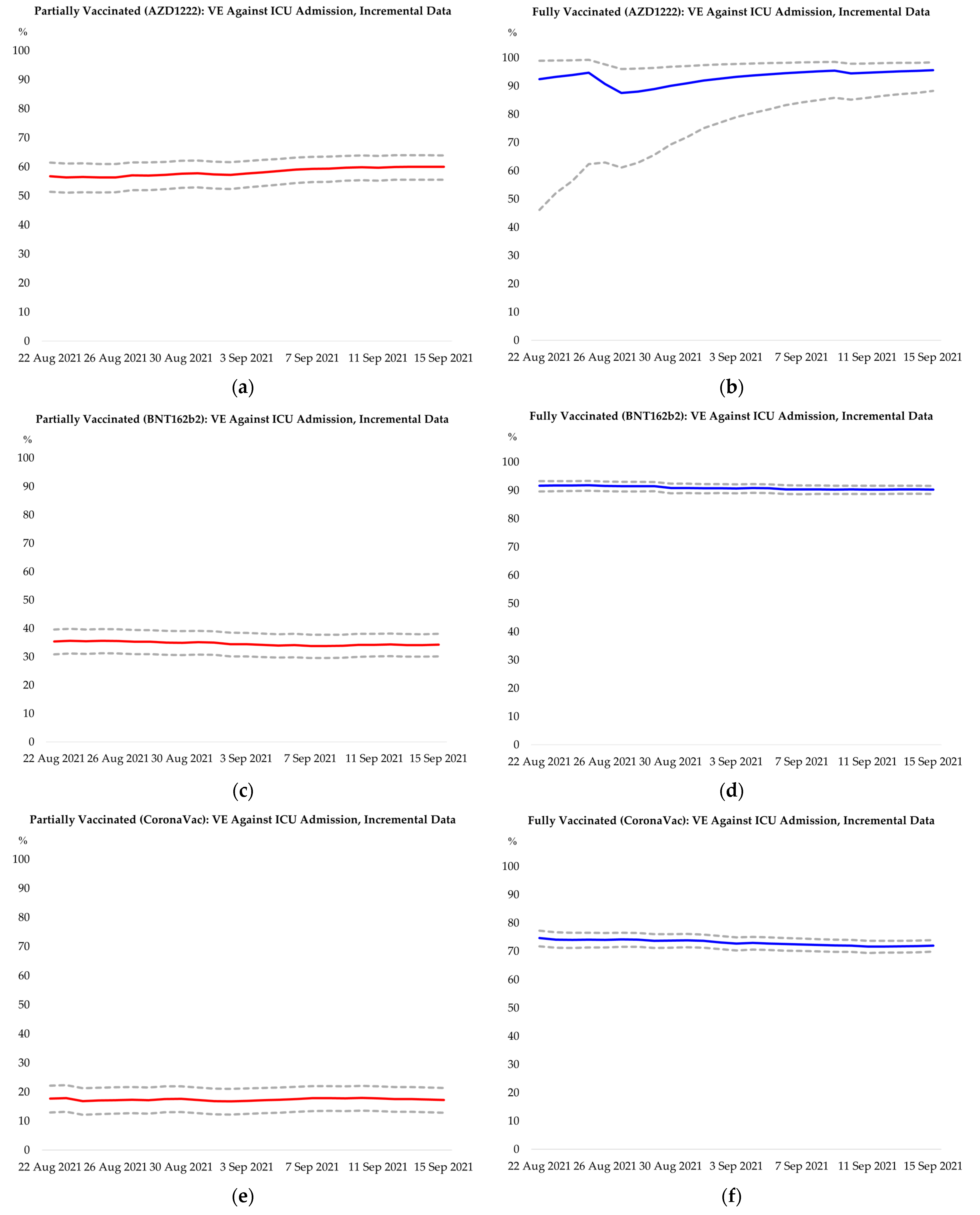
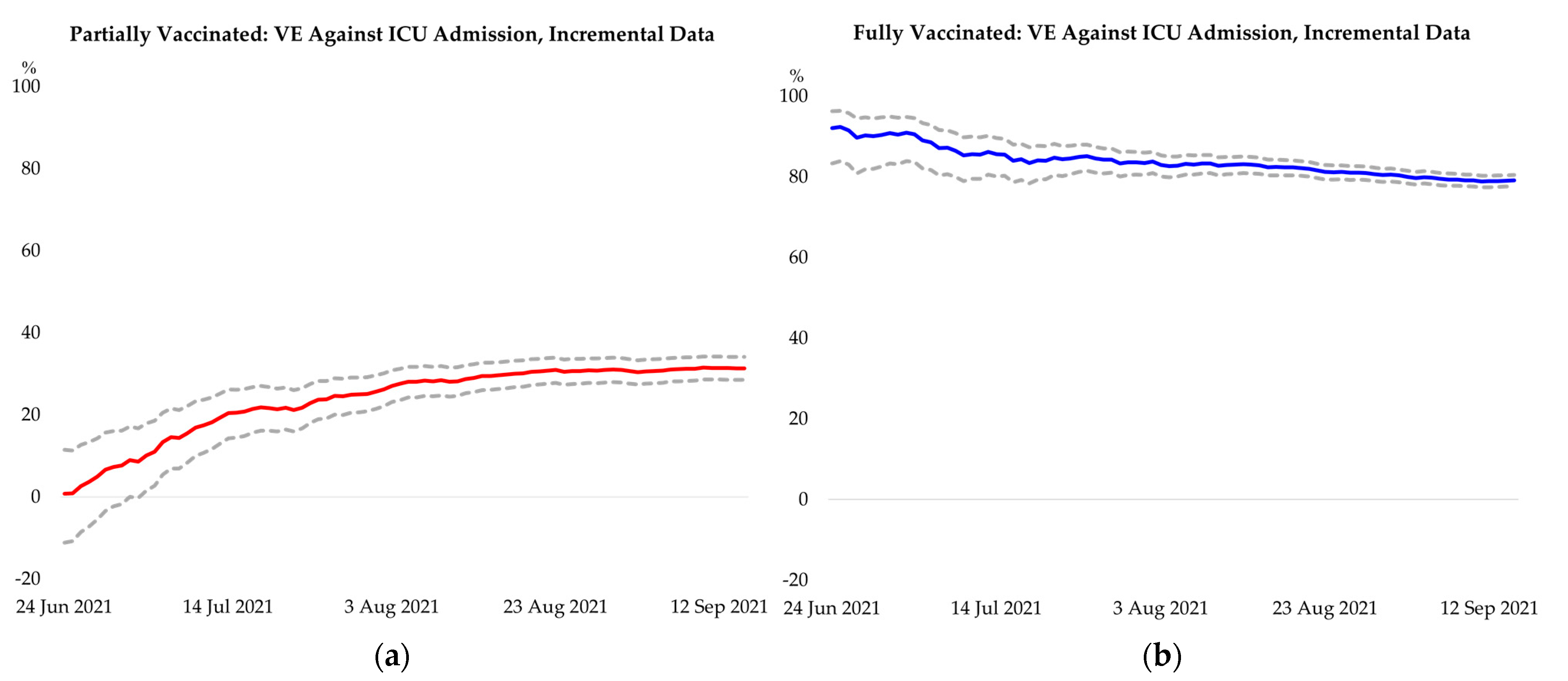
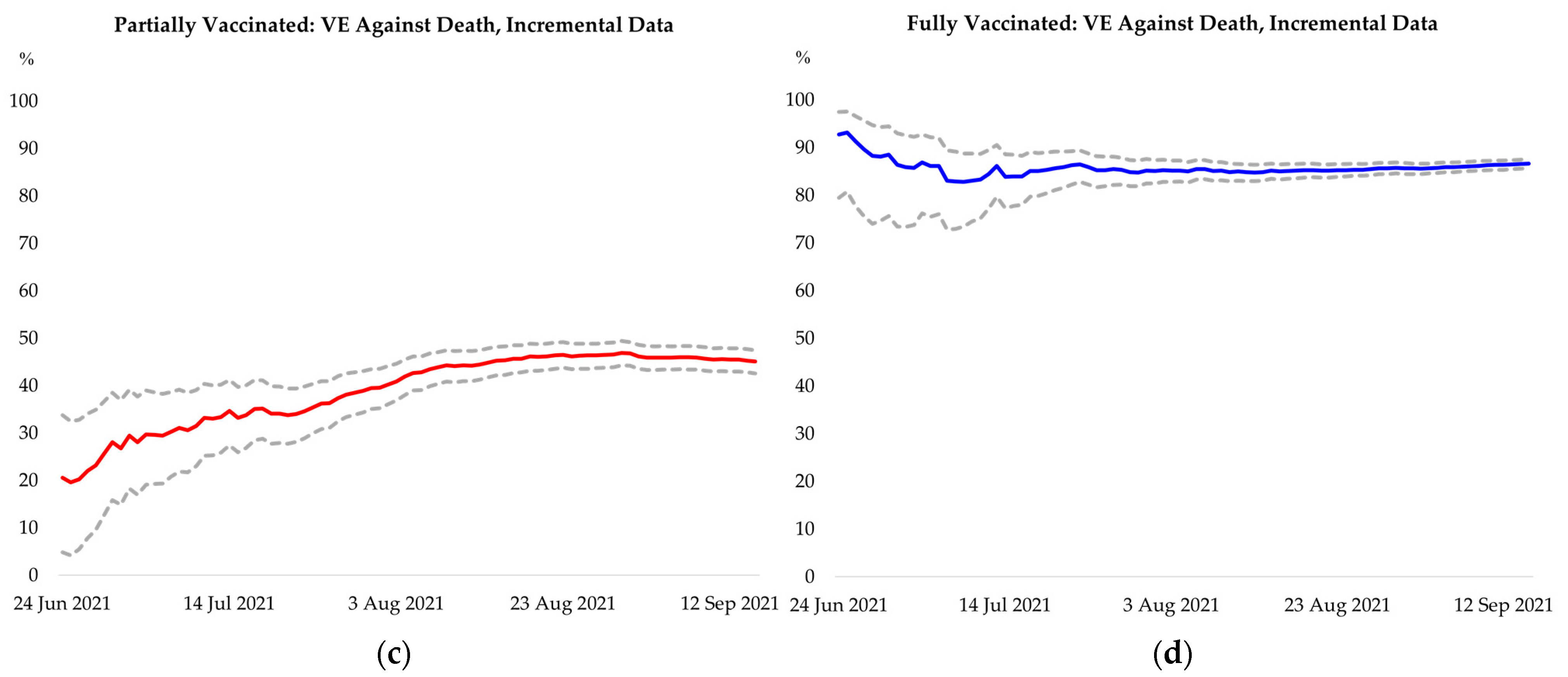
Appendix F. Additional Results
| Characteristic | Cohort | Unvaccinated | Partially Vaccinated: AZD1222 | Fully Vaccinated: AZD1222 | Partially Vaccinated: BNT162b2 | Fully Vaccinated: BNT162b2 | Partially Vaccinated: CoronaVac | Fully Vaccinated: CoronaVac | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Participants no. | 1,286,881 | 100.0 | 788,464 | 61.3 | 45,736 | 3.6 | 4687 | 0.4 | 92,677 | 7.2 | 83,923 | 6.5 | 131,115 | 10.2 | 140,279 | 10.9 | |
| AZD1222 | 50,423 | 3.9 | 45,736 | 3.6 | 4687 | 0.4 | |||||||||||
| BNT162b2 | 176,600 | 13.7 | 92,677 | 7.2 | 83,923 | 6.5 | |||||||||||
| CoronaVac | 271,394 | 21.1 | 131,115 | 10.2 | 140,279 | 10.9 | |||||||||||
| Sex | <0.001 | ||||||||||||||||
| Female | 551,330 | 42.8 | 317,937 | 40.3 | 22,819 | 49.9 | 2218 | 47.3 | 45,891 | 49.5 | 43,378 | 51.7 | 53,144 | 40.5 | 65,943 | 47.0 | |
| Male | 735,551 | 57.2 | 470,527 | 59.7 | 22,917 | 50.1 | 2469 | 52.7 | 46,786 | 50.5 | 40,545 | 48.3 | 77,971 | 59.5 | 74,336 | 53.0 | |
| Age group | <0.001 | ||||||||||||||||
| Below 20 | 49,217 | 3.8 | 35,447 | 4.5 | 1440 | 3.1 | 77 | 1.6 | 3807 | 4.1 | 793 | 0.9 | 4277 | 3.3 | 3376 | 2.4 | |
| 20 to 29 | 381,826 | 29.7 | 248,752 | 31.5 | 14,854 | 32.5 | 1474 | 31.4 | 28,048 | 30.3 | 17,449 | 20.8 | 40,189 | 30.7 | 31,060 | 22.1 | |
| 30 to 39 | 332,779 | 25.9 | 207,440 | 26.3 | 11,630 | 25.4 | 1038 | 22.1 | 23,331 | 25.2 | 24,790 | 29.5 | 34,655 | 26.4 | 29,895 | 21.3 | |
| 40 to 49 | 205,691 | 16.0 | 123,544 | 15.7 | 6903 | 15.1 | 537 | 11.5 | 15,291 | 16.5 | 12,933 | 15.4 | 23,219 | 17.7 | 23,264 | 16.6 | |
| 50 to 59 | 139,563 | 10.8 | 71,931 | 9.1 | 4324 | 9.5 | 385 | 8.2 | 12,544 | 13.5 | 11,843 | 14.1 | 13,659 | 10.4 | 24,877 | 17.7 | |
| 60 to 69 | 84,915 | 6.6 | 40,096 | 5.1 | 4662 | 10.2 | 887 | 18.9 | 5617 | 6.1 | 9178 | 10.9 | 6433 | 4.9 | 18,042 | 12.9 | |
| 70 to 79 | 33,563 | 2.6 | 15,687 | 2.0 | 1295 | 2.8 | 207 | 4.4 | 2333 | 2.5 | 5312 | 6.3 | 2033 | 1.6 | 6696 | 4.8 | |
| 80 and above | 11,891 | 0.9 | 6627 | 0.8 | 441 | 1.0 | 72 | 1.5 | 822 | 0.9 | 1482 | 1.8 | 634 | 0.5 | 1813 | 1.3 | |
| Missing | 47,436 | 3.7 | 38,940 | 4.9 | 187 | 0.4 | 10 | 0.2 | 884 | 1.0 | 143 | 0.2 | 6016 | 4.6 | 1256 | 0.9 | |
| Nationality | <0.001 | ||||||||||||||||
| Malaysian | 1,038,080 | 80.7 | 587,240 | 74.5 | 43,704 | 95.6 | 4571 | 97.5 | 85,103 | 91.8 | 82,861 | 98.7 | 101,110 | 77.1 | 133,491 | 95.2 | |
| Non-Malaysian | 248,801 | 19.3 | 201,224 | 25.5 | 2032 | 4.4 | 116 | 2.5 | 7574 | 8.2 | 1062 | 1.3 | 30,005 | 22.9 | 6788 | 4.8 | |
| Presentation of Symptoms | <0.001 | ||||||||||||||||
| Asymptomatic | 647,740 | 50.3 | 426,377 | 54.1 | 17,721 | 38.7 | 2105 | 44.9 | 35,524 | 38.3 | 34,761 | 41.4 | 61,877 | 47.2 | 69,375 | 49.5 | |
| Symptomatic | 639,141 | 49.7 | 362,087 | 45.9 | 28,015 | 61.3 | 2582 | 55.1 | 57,153 | 61.7 | 49,162 | 58.6 | 69,238 | 52.8 | 70,904 | 50.5 | |
| Presence of Comorbidities | <0.001 | ||||||||||||||||
| No comorbidities | 1,032,442 | 80.2 | 622,953 | 79.0 | 39,539 | 86.5 | 4336 | 92.5 | 67,281 | 72.6 | 65,287 | 77.8 | 109,385 | 83.4 | 123,661 | 88.2 | |
| Comorbid | 254,439 | 19.8 | 165,511 | 21.0 | 6197 | 13.5 | 351 | 7.5 | 25,396 | 27.4 | 18,636 | 22.2 | 21,730 | 16.6 | 16,618 | 11.8 | |
| Outcomes and Vaccine Effectiveness | Vaccine Effectiveness (95% CI): Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fully Adjusted | Unadjusted | Partially Unadjusted (No Trend and State Dummies) | Partially Adjusted (No State Dummies) | Partially Adjusted (No Comorbidities Dummy) | ||||||
| VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | |
| Admission to ICU | ||||||||||
| Partially vaccinated | 31.3 | 28.5, 34.1 | 27.2 | 24.5, 29.8 | 40.3 | 38.1, 42.5 | 28.4 | 25.4, 31.2 | 35.4 | 32.7, 37.9 |
| Fully vaccinated | 79.1 | 77.7, 80.4 | 74.1 | 72.5, 75.6 | 84.3 | 83.4, 85.3 | 79.0 | 77.6, 80.3 | 83.3 | 82.2, 84.4 |
| Confirmed death | ||||||||||
| Partially vaccinated | 45.1 | 42.6, 47.5 | 15.1 | 12.3, 17.8 | 23.5 | 20.7, 26.2 | 29.9 | 27.2, 32.5 | 45.9 | 43.9, 47.8 |
| Fully vaccinated | 86.7 | 85.7, 87.6 | 67.5 | 65.7, 69.1 | 82.9 | 81.9, 83.8 | 85.3 | 84.5, 86.2 | 88.7 | 88, 89.3 |
| Outcomes and Vaccine Effectiveness | Vaccine Effectiveness (95% CI): AZD1222 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fully Adjusted | Unadjusted | Partially Unadjusted (No Trend and State Dummies) | Partially Adjusted (No State Dummies) | Partially Adjusted (No Comorbidities Dummy) | ||||||
| VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | |
| Admission to ICU | ||||||||||
| Partially vaccinated | 60.0 | 55.6, 64.0 | 55.6 | 51.0, 59.9 | 63.7 | 59.8, 67.2 | 55.0 | 50.1, 59.4 | 64.7 | 60.9, 68.2 |
| Fully vaccinated | 95.6 | 88.3, 98.4 | 95.7 | 88.4, 98.4 | 96.8 | 91.5, 98.8 | 95.4 | 87.8, 98.3 | 96.9 | 91.6, 98.8 |
| Confirmed death | ||||||||||
| Partially vaccinated | 70.7 | 67.3, 73.7 | 34.7 | 29.3, 39.7 | 38.1 | 32.5, 43.3 | 44.8 | 39.6, 49.4 | 71.5 | 69.0, 73.8 |
| Fully vaccinated | 95.3 | 91.3, 97.4 | 88.2 | 79.2, 93.3 | 91.7 | 85.3, 95.4 | 93.3 | 88.0, 96.2 | 97.1 | 94.9, 98.4 |
| Outcomes and Vaccine Effectiveness | Vaccine Effectiveness (95% CI): BNT162b2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fully Adjusted | Unadjusted | Partially Unadjusted (No Trend and State Dummies) | Partially Adjusted (No State Dummies) | Partially Adjusted (No Comorbidities Dummy) | ||||||
| VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | |
| Admission to ICU | ||||||||||
| Partially vaccinated | 34.3 | 30.2, 38.1 | 25.2 | 20.8, 29.3 | 47.2 | 44.1, 50.2 | 36.4 | 32.5, 40.1 | 36.8 | 32.9, 40.4 |
| Fully vaccinated | 90.3 | 88.8, 91.6 | 88.2 | 86.4, 89.8 | 93.3 | 92.3, 94.2 | 91.2 | 89.9, 92.4 | 92.1 | 90.8, 93.1 |
| Confirmed death | ||||||||||
| Partially vaccinated | 48.1 | 44.5, 51.4 | 14.7 | 10.3, 18.9 | 39.8 | 36.4, 43.1 | 45.1 | 41.9, 48.1 | 46.2 | 43.2, 49.0 |
| Fully vaccinated | 92.7 | 91.7, 93.6 | 80.9 | 78.8, 82.8 | 91.6 | 90.6, 92.5 | 92.8 | 92.0, 93.6 | 93.5 | 92.8, 94.2 |
| Outcomes and Vaccine Effectiveness | Vaccine Effectiveness (95% CI): CoronaVac | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fully Adjusted | Unadjusted | Partially Unadjusted (No Trend and State Dummies) | Partially Adjusted (No State Dummies) | Partially Adjusted (No Comorbidities Dummy) | ||||||
| VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | |
| Admission to ICU | ||||||||||
| Partially vaccinated | 17.3 | 12.9, 21.4 | 18.3 | 14.3, 22.0 | 24.0 | 20.3, 27.6 | 10.4 | 5.8, 14.8 | 21.7 | 17.7, 25.6 |
| Fully vaccinated | 72.0 | 69.9, 73.9 | 64.8 | 62.4, 67.1 | 78.1 | 76.6, 79.5 | 70.3 | 68.1, 72.3 | 77.9 | 76.3, 79.5 |
| Confirmed death | ||||||||||
| Partially vaccinated | 29.8 | 25.7, 33.7 | 8.2 | 4.1, 12.0 | 0.0 | −4.9, 4.6 | 7.7 | 3.1, 12.1 | 31.5 | 28.3, 34.6 |
| Fully vaccinated | 82.4 | 81.0, 83.7 | 58.6 | 56.1, 60.9 | 75.9 | 74.3, 77.3 | 79.5 | 78.1, 80.8 | 85.6 | 84.6, 86.5 |
| Definition of Partial Vaccination Status | Vaccine Effectiveness | Cohort of Confirmed COVID-19 Cases | Vaccine Effectiveness (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. | Event No. | Rate of Event | Fully Adjusted | Unadjusted | No Trend and State Dummies | No State Dummies | No Comorbidities Dummy | |||||||
| (per 1000 Persons) | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | ||||
| 1 day post dose 1 | Unvaccinated | 749,524 | 14,497 | 19.3 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Partially vaccinated | 262,441 | 3715 | 14.2 | 31.3 | 28.5, 34.1 | 27.2 | 24.5, 29.8 | 40.3 | 38.1, 42.5 | 28.4 | 25.4, 31.2 | 35.4 | 32.7, 37.9 | |
| Fully vaccinated | 227,480 | 1156 | 5.1 | 79.1 | 77.7, 80.4 | 74.1 | 72.5, 75.6 | 84.3 | 83.4, 85.3 | 79.0 | 77.6, 80.3 | 83.3 | 82.2, 84.4 | |
| Partially vaccinated: AZD1222 | 45,549 | 395 | 8.7 | 60.0 | 55.6, 64.0 | 55.6 | 51.0, 59.9 | 63.7 | 59.8, 67.2 | 55.0 | 50.1, 59.4 | 64.7 | 60.9, 68.2 | |
| Fully vaccinated: AZD1222 | 4677 | 4 | 0.9 | 95.6 | 88.3, 98.4 | 95.7 | 88.4, 98.4 | 96.8 | 91.5, 98.8 | 95.4 | 87.8, 98.3 | 96.9 | 91.6, 98.8 | |
| Partially vaccinated: BNT162b2 | 91,793 | 1335 | 14.5 | 34.3 | 30.2, 38.1 | 25.2 | 20.8, 29.3 | 47.2 | 44.1, 50.2 | 36.4 | 32.5, 40.1 | 36.8 | 32.9, 40.4 | |
| Fully vaccinated: BNT162b2 | 83,780 | 194 | 2.3 | 90.3 | 88.8, 91.6 | 88.2 | 86.4, 89.8 | 93.3 | 92.3, 94.2 | 91.2 | 89.9, 92.4 | 92.1 | 90.8, 93.1 | |
| Partially vaccinated: CoronaVac | 125,099 | 1985 | 15.9 | 17.3 | 12.9, 21.4 | 18.3 | 14.3, 22.0 | 24.0 | 20.3, 27.6 | 10.4 | 5.8, 14.8 | 21.7 | 17.7, 25.6 | |
| Fully vaccinated: CoronaVac | 139,023 | 958 | 6.9 | 72.0 | 69.9, 73.9 | 64.8 | 62.4, 67.1 | 78.1 | 76.6, 79.5 | 70.3 | 68.1, 72.3 | 77.9 | 76.3, 79.5 | |
| 14 days post dose 1 | Unvaccinated | 749,524 | 14,497 | 19.3 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Partially vaccinated | 147,032 | 1786 | 12.1 | 46.4 | 43.5, 49.2 | 37.7 | 34.5, 40.7 | 53.0 | 50.6, 55.3 | 43.8 | 40.7, 46.7 | 50.2 | 47.5, 52.8 | |
| Fully vaccinated | 227,480 | 1156 | 5.1 | 79.8 | 78.4, 81.1 | 74.1 | 72.5, 75.6 | 84.4 | 83.4, 85.3 | 79.7 | 78.3, 81.0 | 83.8 | 82.7, 84.8 | |
| Partially vaccinated: AZD1222 | 35,995 | 248 | 6.9 | 70.0 | 65.9, 73.7 | 64.8 | 60.1, 69.0 | 72.7 | 69.0, 75.9 | 66.5 | 61.9, 70.5 | 74.2 | 70.7, 77.3 | |
| Fully vaccinated: AZD1222 | 4677 | 4 | 0.9 | 95.8 | 88.7, 98.4 | 95.7 | 88.4, 98.4 | 96.8 | 91.6, 98.8 | 95.6 | 88.3, 98.4 | 97.0 | 91.9, 98.9 | |
| Partially vaccinated: BNT162b2 | 41,916 | 457 | 10.9 | 55.8 | 51.3, 59.9 | 44.1 | 38.6, 49.1 | 64.6 | 61.1, 67.8 | 57.6 | 53.3, 61.5 | 57.7 | 53.5, 61.6 | |
| Fully vaccinated: BNT162b2 | 83,780 | 194 | 2.3 | 90.6 | 89.1, 91.9 | 88.2 | 86.4, 89.8 | 93.3 | 92.3, 94.2 | 91.5 | 90.1, 92.6 | 92.3 | 91.1, 93.3 | |
| Partially vaccinated: CoronaVac | 69,121 | 1081 | 15.6 | 26.4 | 21.4, 31.2 | 19.4 | 14.3, 24.3 | 32.7 | 28.3, 36.8 | 20.6 | 15.2, 25.7 | 30.3 | 25.6, 34.7 | |
| Fully vaccinated: CoronaVac | 139,023 | 958 | 6.9 | 72.8 | 70.7, 74.7 | 64.8 | 62.4, 67.1 | 78.1 | 76.6, 79.6 | 71.3 | 69.2, 73.3 | 78.6 | 77.0, 80.0 | |
| Definition of Partial Vaccination Status | Vaccine Effectiveness | Cohort of Confirmed COVID-19 Cases | Vaccine Effectiveness (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. | Event No. | Rate of Event | Fully Adjusted | Unadjusted | No Trend and State Dummies | No State Dummies | No Comorbidities Dummy | |||||||
| (per 1000 persons) | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | ||||
| 1 day post dose 1 | Unvaccinated | 749,524 | 15976 | 21.3 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Partially vaccinated | 262,441 | 4766 | 18.2 | 45.1 | 42.6, 47.5 | 15.1 | 12.3, 17.8 | 23.5 | 20.7, 26.2 | 29.9 | 27.2, 32.5 | 45.9 | 43.9, 47.8 | |
| Fully vaccinated | 227,480 | 1601 | 7 | 86.7 | 85.7, 87.6 | 67.5 | 65.7, 69.1 | 82.9 | 81.9, 83.8 | 85.3 | 84.5, 86.2 | 88.7 | 88.0, 89.3 | |
| Partially vaccinated: AZD1222 | 45,549 | 639 | 14 | 70.7 | 67.3, 73.7 | 34.7 | 29.3, 39.7 | 38.1 | 32.5, 43.3 | 44.8 | 39.6, 49.4 | 71.5 | 69.0, 73.8 | |
| Fully vaccinated: AZD1222 | 4677 | 12 | 2.6 | 95.3 | 91.3, 97.4 | 88.2 | 79.2, 93.3 | 91.7 | 85.3, 95.4 | 93.3 | 88.0, 96.2 | 97.1 | 94.9, 98.4 | |
| Partially vaccinated: BNT162b2 | 91,793 | 1674 | 18.2 | 48.1 | 44.5, 51.4 | 14.7 | 10.3, 18.9 | 39.8 | 36.4, 43.1 | 45.1 | 41.9, 48.1 | 46.2 | 43.2, 49.0 | |
| Fully vaccinated: BNT162b2 | 83,780 | 347 | 4.1 | 92.7 | 91.7, 93.6 | 80.9 | 78.8, 82.8 | 91.6 | 90.6, 92.5 | 92.8 | 92.0, 93.6 | 93.5 | 92.8, 94.2 | |
| Partially vaccinated: CoronaVac | 125,099 | 2453 | 19.6 | 29.8 | 25.7, 33.7 | 8.2 | 4.1, 12.0 | 0.0 | −4.9, 4.6 | 7.7 | 3.1, 12.1 | 31.5 | 28.3, 34.6 | |
| Fully vaccinated: CoronaVac | 139,023 | 1242 | 8.9 | 82.4 | 81.0, 83.7 | 58.6 | 56.1, 60.9 | 75.9 | 74.3, 77.3 | 79.5 | 78.1, 80.8 | 85.6 | 84.6, 86.5 | |
| 14 days post dose 1 | Unvaccinated | 749,524 | 15976 | 21.3 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Partially vaccinated | 147,032 | 2687 | 18.3 | 54.9 | 52.3, 57.4 | 14.5 | 10.9, 18.0 | 32.0 | 28.8, 35.1 | 39.6 | 36.7, 42.4 | 55.4 | 53.3, 57.4 | |
| Fully vaccinated | 227,480 | 1601 | 7 | 86.9 | 86.0, 87.8 | 67.5 | 65.7, 69.1 | 82.9 | 81.9, 83.8 | 85.8 | 84.9, 86.6 | 89.0 | 88.3, 89.6 | |
| Partially vaccinated: AZD1222 | 35,995 | 462 | 12.8 | 77.1 | 74.0, 79.9 | 40.3 | 34.5, 45.6 | 47.7 | 42.1, 52.8 | 54.6 | 49.7, 59.0 | 77.3 | 75.0, 79.5 | |
| Fully vaccinated: AZD1222 | 4677 | 12 | 2.6 | 95.4 | 91.4, 97.5 | 88.2 | 79.2, 93.3 | 91.7 | 85.3, 95.4 | 93.5 | 88.5, 96.4 | 97.2 | 95, 98.4 | |
| Partially vaccinated: BNT162b2 | 41,916 | 761 | 18.2 | 59.5 | 55.4, 63.2 | 15.1 | 8.6, 21.1 | 50.1 | 45.9, 53.9 | 55.7 | 51.9, 59.1 | 56.9 | 53.4, 60.2 | |
| Fully vaccinated: BNT162b2 | 83,780 | 347 | 4.1 | 92.8 | 91.9, 93.7 | 80.9 | 78.8, 82.8 | 91.6 | 90.6, 92.5 | 93.0 | 92.2, 93.7 | 93.6 | 92.9, 94.3 | |
| Partially vaccinated: CoronaVac | 69,121 | 1464 | 21.2 | 35.8 | 31.0, 40.3 | 0.6 | −4.9, 5.9 | 7.0 | 1.2, 12.4 | 16.6 | 11.3, 21.6 | 36.7 | 32.9, 40.3 | |
| Fully vaccinated: CoronaVac | 139,023 | 1242 | 8.9 | 82.6 | 81.2, 83.9 | 58.6 | 56.1, 60.9 | 75.9 | 74.3, 77.3 | 80.1 | 78.8, 81.4 | 85.9 | 84.9, 86.7 | |

References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar] [CrossRef]
- Falzone, L.; Gattuso, G.; Tsatsakis, A.; Spandidos, D.A.; Libra, M. Current and innovative methods for the diagnosis of COVID 19 infection (Review). Int. J. Mol. Med. 2021, 47, 100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Weekly Epidemiological Update–Edition 64. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---2-november-2021 (accessed on 2 November 2021).
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 26 September 2021).
- Baraniuk, C. What do we know about China’s covid-19 vaccines? BMJ 2021, 373, n912. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- World Health Organization. Considerations for Evaluation of COVID-19 Vaccines. Available online: https://www.who.int/publications/m/item/considerations-for-the-assessment-of-covid-19-vaccines-for-listing-by-who (accessed on 10 October 2021).
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 vaccines against the B. 1.617. 2 (delta) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D.; et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.; Cravioto, A.; Rees, H.; Higgins, J.P.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker. Sinovac: CoronaVac. Available online: https://covid19.trackvaccines.org/vaccines/7/ (accessed on 10 October 2021).
- World Health Organization. Evidence Assessment: Sinovac/CoronaVac COVID-19 Vaccine. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf (accessed on 10 October 2021).
- COVID-19 Immunisation Task Force (CITF). Open data on Malaysia’s National COVID-19 Immunisation Programme. Available online: https://github.com/CITF-Malaysia/citf-public (accessed on 10 October 2021).
- Department of Statistics Malaysia. Current Population Estimates, Malaysia. 2020. Available online: https://www.dosm.gov.my (accessed on 26 September 2021).
- World Health Organization. COVID-19 Situation Report for the Western Pacific Region #70: 8–14 September 2021. Available online: https://www.who.int/westernpacific/internal-publications-detail/covid-19-situation-report-for-the-western-pacific-region-70-8-september-2021---14-september-2021 (accessed on 4 October 2021).
- Prime Minister Office of Malaysia. Kenyataan Media Pejabat Perdana Menteri. Available online: https://www.pmo.gov.my/wp-content/uploads/2021/06/Kenyataan-Media-PMO-Pelaksanaan-Total-Lockdown.pdf (accessed on 4 October 2021).
- Jawatankuasa Khas Jaminan Akses Bekalan Vaksin COVID-19 (JKJAV) [The Special Committee for Ensuring Access to COVID-19 Vaccine Supply (JKJAV)]. Available online: https://www.vaksincovid.gov.my/ (accessed on 26 September 2021).
- Ministry of Health Malaysia. Open Data on the COVID-19 Epidemic in Malaysia. Available online: https://github.com/MoH-Malaysia/covid19-public/ (accessed on 10 October 2021).
- Orenstein, W.A.; Bernier, R.H.; Dondero, T.J.; Hinman, A.R.; Marks, J.S.; Bart, K.J.; Sirotkin, B. Field evaluation of vaccine efficacy. Bull. World Health Organ. 1985, 63, 1055. [Google Scholar]
- Mazagatos, C.; Monge, S.; Olmedo, C.; Vega, L.; Gallego, P.; Martín-Merino, E.; Sierra, M.J.; Limia, A.; Larrauri, A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Eurosurveillance 2021, 26, 2100452. [Google Scholar] [CrossRef]
- Araki, K.; Hara, M.; Sakanishi, Y.; Shimanoe, C.; Nishida, Y.; Matsuo, M.; Tanaka, K. Estimating rotavirus vaccine effectiveness in Japan using a screening method. Hum. Vaccines Immunother. 2016, 12, 1244–1249. [Google Scholar] [CrossRef]
- Cohen, A.L.; Taylor, T., Jr.; Farley, M.M.; Schaffner, W.; Lesher, L.J.; Gershman, K.A.; Bennett, N.M.; Reingold, A.; Thomas, A.; Baumbach, J.; et al. An assessment of the screening method to evaluate vaccine effectiveness: The case of 7-valent pneumococcal conjugate vaccine in the United States. PLoS ONE 2012, 7, e41785. [Google Scholar] [CrossRef]
- Remschmidt, C.; Rieck, T.; Bödeker, B.; Wichmann, O. Application of the screening method to monitor influenza vaccine effectiveness among the elderly in Germany. BMC Infect. Dis. 2015, 15, 1–11. [Google Scholar] [CrossRef][Green Version]
- Hak, E.; Verheij, T.J.; Grobbee, D.; Nichol, K.; Hoes, A. Confounding by indication in non-experimental evaluation of vaccine effectiveness: The example of prevention of influenza complications. J. Epidemiol. Community Health 2002, 56, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Government of Chile. Effectiveness of the SARS-CoV-2 Vaccination Program. Available online: https://www.gob.cl/en/news/sars-cov-2-vaccines-used-chile-remain-highly-effective-preventing-hospitalization-icu-admission-and-death/ (accessed on 7 October 2021).
- Ranzani, O.T.; Hitchings, M.D.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; de Moura Villela, E.F.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 30 September 2021).
- Hashim, J.H.; Adman, M.A.; Hashim, Z.; Radi, M.F.M.; Kwan, S.C. COVID-19 Epidemic in Malaysia: Epidemic Progression, Challenges, and Response. Front. Public Health 2021, 9, 560592. [Google Scholar] [CrossRef]
- Rampal, L.; Liew, B. Malaysia’s third COVID-19 wave-a paradigm shift required. Med. J. Malays. 2021, 76, 1–4. [Google Scholar]
- World Health Organization. COVID-19 in Malaysia Situation Report 45. Available online: https://www.who.int/malaysia/internal-publications-detail/covid-19-in-malaysia-situation-report-45 (accessed on 4 October 2021).
- Institute for Public Health (IPH); National Institutes of Health; Ministry of Health Malaysia. National Health and Morbidity Survey (NHMS) 2019: Vol. I: NCDs–Non-Communicable Diseases: Risk Factors and other Health Problems; 2020. Available online: https://iku.moh.gov.my/images/IKU/Document/REPORT/NHMS2019/Report_NHMS2019-NCD_v2.pdf (accessed on 4 October 2021).
- Centers for Disease Control and Prevention. Science Brief: Evidence Used to Update the List of Underlying Medical Conditions that Increase a Person’s Risk of Severe Illness from COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html (accessed on 4 October 2021).
- National Pharmaceutical Regulatory Agency (NPRA); Ministry of Health Malaysia. Frequently Asked Questions (FAQ) about COVID-19 Vaccine. Available online: https://www.npra.gov.my/ (accessed on 26 September 2021).
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 4 October 2021).
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef] [PubMed]

| PCV (%) | PPV (%) | VE (%) | 95% CI | |
|---|---|---|---|---|
| COVID-19 (SARS-CoV-2) Infection | ||||
| Partial Vaccination | 16.7 | 28.2 | 48.8 | 46.8, 50.7 |
| Full Vaccination | 16.6 | 62.0 | 87.8 | 85.8, 89.7 |
| Symptomatic COVID-19 | ||||
| Partial Vaccination | 20.7 | 28.2 | 33.5 | 31.6, 35.5 |
| Full Vaccination | 19.2 | 62.0 | 85.4 | 83.4, 87.3 |
| Characteristic | Cohort | Unvaccinated | Partially Vaccinated | Fully Vaccinated | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Participants no. | 1,286,881 | 100.0 | 788,464 | 61.3 | 269,528 | 20.9 | 228,889 | 17.8 | |
| AZD1222 | 50,423 | 3.9 | 45,736 | 3.6 | 4687 | 0.4 | |||
| BNT162b2 | 176,600 | 13.7 | 92,677 | 7.2 | 83,923 | 6.5 | |||
| CoronaVac | 271,394 | 21.1 | 131,115 | 10.2 | 140,279 | 10.9 | |||
| Sex | <0.001 | ||||||||
| Female | 551,330 | 42.8 | 317,937 | 40.3 | 121,854 | 45.2 | 111,539 | 48.7 | |
| Male | 735,551 | 57.2 | 470,527 | 59.7 | 147,674 | 54.8 | 117,350 | 51.3 | |
| Age group | <0.001 | ||||||||
| Below 20 | 49,217 | 3.8 | 35,447 | 4.5 | 9524 | 3.5 | 4246 | 1.9 | |
| 20 to 29 | 381,826 | 29.7 | 248,752 | 31.5 | 83,091 | 30.8 | 49,983 | 21.8 | |
| 30 to 39 | 332,779 | 25.9 | 207,440 | 26.3 | 69,616 | 25.8 | 55,723 | 24.3 | |
| 40 to 49 | 205,691 | 16.0 | 123,544 | 15.7 | 45,413 | 16.8 | 36,734 | 16.0 | |
| 50 to 59 | 139,563 | 10.8 | 71,931 | 9.1 | 30,527 | 11.3 | 37,105 | 16.2 | |
| 60 to 69 | 84,915 | 6.6 | 40,096 | 5.1 | 16,712 | 6.2 | 28,107 | 12.3 | |
| 70 to 79 | 33,563 | 2.6 | 15,687 | 2.0 | 5661 | 2.1 | 12,215 | 5.3 | |
| 80 and above | 11,891 | 0.9 | 6627 | 0.8 | 1897 | 0.7 | 3367 | 1.5 | |
| Missing | 47,436 | 3.7 | 38,940 | 4.9 | 7087 | 2.6 | 1409 | 0.6 | |
| Nationality | <0.001 | ||||||||
| Malaysian | 1,038,080 | 80.7 | 587,240 | 74.5 | 229,917 | 85.3 | 220,923 | 96.5 | |
| Non-Malaysian | 248,801 | 19.3 | 201,224 | 25.5 | 39,611 | 14.7 | 7966 | 3.5 | |
| Presentation of Symptoms | <0.001 | ||||||||
| Asymptomatic | 647,740 | 50.3 | 426,377 | 54.1 | 115,122 | 42.7 | 106,241 | 46.4 | |
| Symptomatic | 639,141 | 49.7 | 362,087 | 45.9 | 154,406 | 57.3 | 122,648 | 53.6 | |
| Presence of Comorbidities | <0.001 | ||||||||
| No comorbidities | 1032,442 | 80.2 | 622,953 | 79.0 | 216,205 | 80.2 | 193,284 | 84.4 | |
| Comorbid | 254,439 | 19.8 | 165,511 | 21.0 | 53,323 | 19.8 | 35,605 | 15.6 | |
| Outcomes and Vaccine Effectiveness | Cohort of Confirmed COVID-19 Cases | Vaccine Effectiveness (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. | Event No. | Rate of Event | All Types | AZD1222 | BNT162b2 | CoronaVac | |||||
| (per 1000 Persons) | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI | |||
| Admission to ICU | |||||||||||
| Unvaccinated | 749,524 | 14,497 | 19.3 | ref | ref | ref | ref | ref | ref | ref | ref |
| Partially vaccinated | 262,441 | 3715 | 14.2 | 31.3 | 28.5, 34.1 | 60.0 | 55.6, 64 | 34.3 | 30.2, 38.1 | 17.3 | 12.9, 21.4 |
| Fully vaccinated | 227,480 | 1156 | 5.1 | 79.1 | 77.7, 80.4 | 95.6 | 88.3, 98.4 | 90.3 | 88.8, 91.6 | 72.0 | 69.9, 73.9 |
| Confirmed death | |||||||||||
| Unvaccinated | 749,524 | 15,976 | 21.3 | ref | ref | ref | ref | ref | ref | ref | ref |
| Partially vaccinated | 262,441 | 4766 | 18.2 | 45.1 | 42.6, 47.5 | 70.7 | 67.3, 73.7 | 48.1 | 44.5, 51.4 | 29.8 | 25.7, 33.7 |
| Fully vaccinated | 227,480 | 1601 | 7.0 | 86.7 | 85.7, 87.6 | 95.3 | 91.3, 97.4 | 92.7 | 91.7, 93.6 | 82.4 | 81.0, 83.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suah, J.L.; Tok, P.S.K.; Ong, S.M.; Husin, M.; Tng, B.H.; Sivasampu, S.; Thevananthan, T.; Appannan, M.R.; Muhamad Zin, F.; Mohd Zin, S.; et al. PICK-ing Malaysia’s Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio. Vaccines 2021, 9, 1381. https://doi.org/10.3390/vaccines9121381
Suah JL, Tok PSK, Ong SM, Husin M, Tng BH, Sivasampu S, Thevananthan T, Appannan MR, Muhamad Zin F, Mohd Zin S, et al. PICK-ing Malaysia’s Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio. Vaccines. 2021; 9(12):1381. https://doi.org/10.3390/vaccines9121381
Chicago/Turabian StyleSuah, Jing Lian, Peter Seah Keng Tok, Su Miin Ong, Masliyana Husin, Boon Hwa Tng, Sheamini Sivasampu, Thevesh Thevananthan, Maheshwara Rao Appannan, Faizah Muhamad Zin, Shahanizan Mohd Zin, and et al. 2021. "PICK-ing Malaysia’s Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio" Vaccines 9, no. 12: 1381. https://doi.org/10.3390/vaccines9121381
APA StyleSuah, J. L., Tok, P. S. K., Ong, S. M., Husin, M., Tng, B. H., Sivasampu, S., Thevananthan, T., Appannan, M. R., Muhamad Zin, F., Mohd Zin, S., Yahaya, H., Rusli, N., Ujang, M. F., Mohd Ibrahim, H., Abdullah, N. H., & Peariasamy, K. M. (2021). PICK-ing Malaysia’s Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio. Vaccines, 9(12), 1381. https://doi.org/10.3390/vaccines9121381






