Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers
Abstract
:1. Introduction
2. Materials and Methods
- (A)
- Post-first dose HWCs (fHCWs), when infection was detected <12 days after the first vaccine dose;
- (B)
- Partially vaccinated HCWs (PHCWs), when the positivity was detected from day 12 after the first dose to day 7 after the second dose;
- (C)
- Totally vaccinated HCWs (THCWs), when SARS-CoV-2 positivity was detected ≥8 days after the second dose.
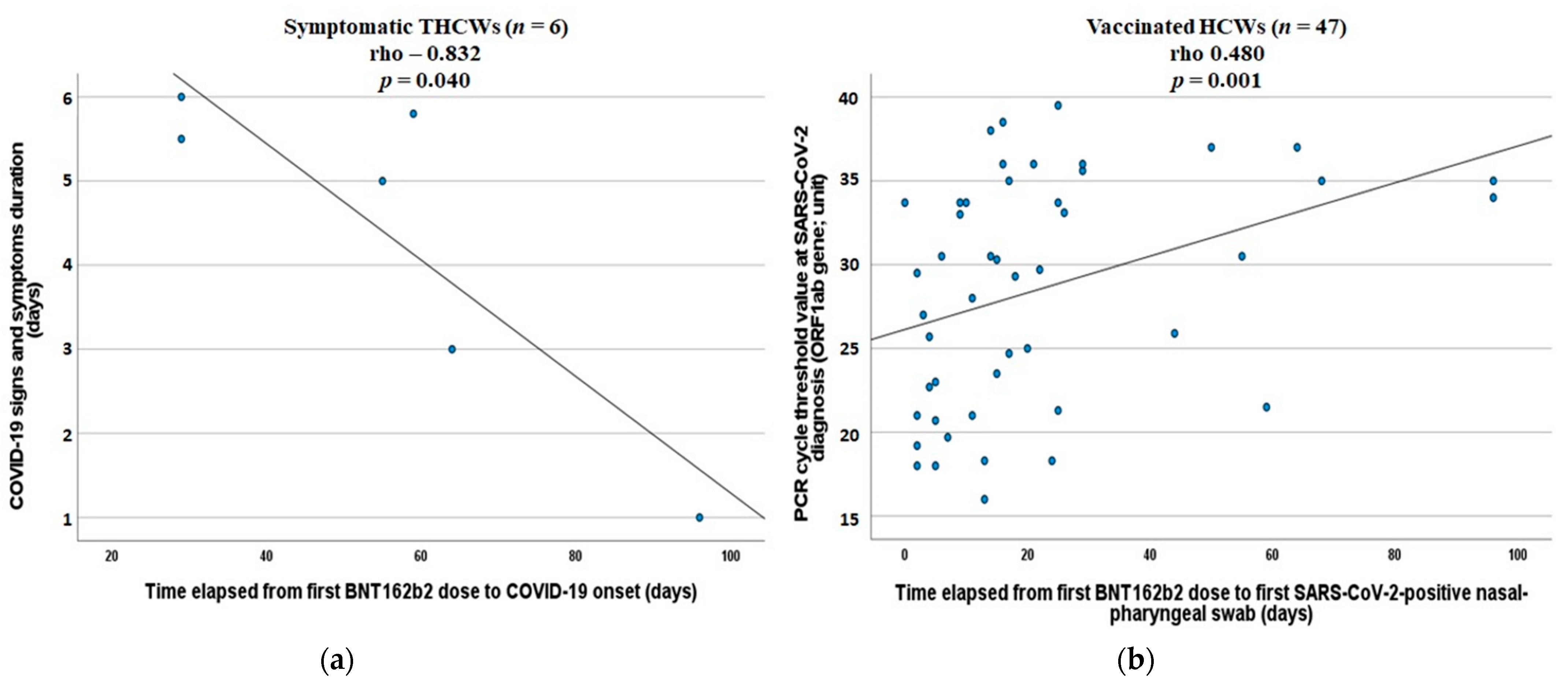
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Tov, A.B.; Cohen, D.; Muhsen, K. The Effectiveness of the First Dose of BNT162b2 Vaccine in Reducing SARS-CoV-2 Infection 13–24 Days after Immunization: Real-World Evidence. medRxiv 2021. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 MRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Keehner, J.; Horton, L.E.; Pfeffer, M.A.; Longhurst, C.A.; Schooley, R.T.; Currier, J.S.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine Side-Effects and SARS-CoV-2 Infection after Vaccination in Users of the COVID Symptom Study App in the UK: A Prospective Observational Study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Westhölter, D.; Taube, C. SARS-CoV-2 Outbreak in a Long-Term Care Facility after Vaccination with BNT162b2. Clin. Infect. Dis. 2021, 2021, ciab299. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early Rate Reductions of SARS-CoV-2 Infection and COVID-19 in BNT162b2 Vaccine Recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 Vaccines and Decreased Transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial Report of Decreased SARS-CoV-2 Viral Load after Inoculation with the BNT162b2 Vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Dall’Olmo, L.; Della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody Response to First and Second Dose of BNT162b2 in a Cohort of Characterized Healthcare Workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Kalimuddin, S.; Tham, C.Y.; Qui, M.; de Alwis, R.; Sim, J.X.; Lim, J.M.; Tan, H.-C.; Syenina, A.; Zhang, S.L.; Le Bert, N.; et al. Early T Cell and Binding Antibody Responses Are Associated with COVID-19 RNA Vaccine Efficacy Onset. Med 2021, 2, 682–688. [Google Scholar] [CrossRef]
- Tande, A.J.; Pollock, B.D.; Shah, N.D.; Farrugia, G.; Virk, A.; Swift, M.; Breeher, L.; Binnicker, M.; Berbari, E.F. Impact of the COVID-19 Vaccine on Asymptomatic Infection among Patients Undergoing Pre-Procedural COVID-19 Molecular Screening. Clin. Infect. Dis. 2021, 2021, ciab229. [Google Scholar] [CrossRef]
- Trunfio, M.; Venuti, F.; Alladio, F.; Longo, B.M.; Burdino, E.; Cerutti, F.; Ghisetti, V.; Bertucci, R.; Picco, C.; Bonora, S.; et al. Diagnostic SARS-CoV-2 Cycle Threshold Value Predicts Disease Severity, Survival, and Six-Month Sequelae in COVID-19 Symptomatic Patients. Viruses 2021, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Dipartimento della Protezione Civile, Consiglio dei Ministri. COVID-19 ITALIA. Available online: https://opendatadpc.maps.arcgis.com/apps/dashboards/b0c68bce2cce478eaac82fe38d4138b1 (accessed on 3 August 2021).
- Benenson, S.; Oster, Y.; Cohen, M.J.; Nir-Paz, R. BNT162b2 MRNA COVID-19 Vaccine Effectiveness among Health Care Workers. N. Engl. J. Med. 2021, 384, 1775–1777. [Google Scholar] [CrossRef]
- Tang, L.; Hijano, D.R.; Gaur, A.H.; Geiger, T.L.; Neufeld, E.J.; Hoffman, J.M.; Hayden, R.T. Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce. JAMA 2021, 325, 2500. [Google Scholar] [CrossRef] [PubMed]
- Angel, Y.; Spitzer, A.; Henig, O.; Saiag, E.; Sprecher, E.; Padova, H.; Ben-Ami, R. Association between Vaccination with BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections among Health Care Workers. JAMA 2021, 325, 2457. [Google Scholar] [CrossRef]
- Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Gethings, O.; Vihta, K.-D.; Jones, J.; House, T.; VanSteenHouse, H.; Bell, I.; et al. Impact of Vaccination on SARS-CoV-2 Cases in the Community: A Population-Based Study Using the UK’s COVID-19 Infection Survey. medRxiv 2021. [Google Scholar] [CrossRef]
- Speth, M.M.; Singer-Cornelius, T.; Oberle, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Mood, Anxiety and Olfactory Dysfunction in COVID-19: Evidence of Central Nervous System Involvement? Laryngoscope 2020, 130, 2520–2525. [Google Scholar] [CrossRef]
- Edén, A.; Kanberg, N.; Gostner, J.; Fuchs, D.; Hagberg, L.; Andersson, L.M.; Lindh, M.; Price, R.W.; Zetterberg, H.; Gisslén, M. CSF Biomarkers in Patients with COVID-19 and Neurologic Symptoms: A Case Series. Neurology 2021, 12, e294–e300. [Google Scholar] [CrossRef]
- Donadio, C.; Rainone, A.; Gouronnec, A.; Belmin, J.; Lafuente-Lafuente, C. Asymptomatic COVID-19 Cases among Older Patients despite BNT162b2 Vaccination: A Case Series in a Geriatric Rehabilitation Ward during an Outbreak. J. Infect. 2021, 83, 119–145. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- McEllistrem, M.C.; Clancy, C.J.; Buehrle, D.J.; Lucas, A.; Decker, B.K. Single Dose of a MRNA SARS-CoV-2 Vaccine Is Associated with Lower Nasopharyngeal Viral Load among Nursing Home Residents with Asymptomatic COVID-19. Clin. Infect. Dis. 2021, 73, e1365–e1367. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.B.; Pinsky, B.A.; Rath, M.E.M.; Wang, H.; Miller, J.A.; Skhiri, M.; Shepard, J.; Mathew, R.; Lee, G.; Bohman, B.; et al. Post-Vaccination SARS-CoV-2 Infections and Incidence of the B.1.427/B.1.429 Variant among Healthcare Personnel at a Northern California Academic Medical Center. medRxiv 2021. [Google Scholar] [CrossRef]
- Rao, S.N.; Manissero, D.; Steele, V.R.; Pareja, J. A Systematic Review of the Clinical Utility of Cycle Threshold Values in the Context of COVID-19. Infect. Dis. Ther. 2020, 9, 573–586. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, A.; Bergna, A.; Menzo, S.; Zehender, G.; Caucci, S.; Ghisetti, V.; Rizzo, F.; Maggi, F.; Cerutti, F.; Giurato, G.; et al. Circulating SARS-CoV-2 Variants in Italy, October 2020-March 2021. Virol. J. 2021, 18, 168. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Partially Vaccinated HCWs (n = 25) | Controls (n = 50) | p |
| Age, years | 54 (40–58) | 54 (40–58) | 1.00 |
| Male, n | 5 (20.0%) | 10 (20.0%) | 1.00 |
| Caucasian, n | 23 (92.0%) | 47 (94.0%) | 0.932 |
| Comorbidities/subject, n | 0 (0–1) | 0 (0–1) | 0.283 |
| Asymptomatic infections, n | 9 (36.0%) | 1 (2.0%) | <0.0001 |
| Signs and Symptoms, n * | |||
| Fever | 3 (18.7%) | 26 (53.1%) | 0.035 |
| Cough | 6 (37.5%) | 22 (44.9%) | 0.819 |
| Rhinorrhea | 5 (31.2%) | 6 (12.2%) | 0.169 |
| Pharyngitis | 3 (18.7%) | 8 (16.3%) | 0.873 |
| Dyspnea | 1 (6.2%) | 9 (18.4%) | 0.443 |
| O/G dysfunction | 3 (18.7%) | 31 (63.3%) | 0.005 |
| Headache | 7 (43.7%) | 21 (42.8%) | 0.819 |
| Arthromyalgia | 3 (18.7%) | 24 (49.0%) | 0.066 |
| Asthenia/Malaise | 3 (18.7%) | 25 (51.0%) | 0.048 |
| Nausea/Vomiting | 2 (12.5%) | 5 (10.2%) | 0.835 |
| Diarrhea | 2 (12.5%) | 7 (14.3%) | 0.812 |
| Signs/symptoms number, n * | 2 (1–2) | 3 (3–5) | 0.031 |
| Severe COVID-19, n | |||
| Hospitalization | 0 (0.0%) | 2 (4.0%) | 0.314 |
| Oxygen support | 0 (0.0%) | 2 (4.0%) | 0.314 |
| Sequelae | 3 (12.0%) | 11 (22.0%) | 0.298 |
| COVID-19 length, days * | 10 (7–17) | 12 (7–21) | 0.834 |
| Time since COVID-19 onset to swab collection, days * | 2 (1–3) | 3 (3–6) | 0.088 |
| PCR ORF1ab Cycle threshold | 30.3 (24.1–35.5) | 22.3 (19.6–30.6) | 0.023 |
| Parameter | Totally Vaccinated HCWs (n = 10) | Controls (n = 20) | p |
| Age, years | 49 (37–58) | 49 (37–58) | 1.00 |
| Male, n | 2 (20.0%) | 4 (20.0%) | 1.00 |
| Caucasian, n | 10 (100%) | 18 (90.0%) | 0.540 |
| Comorbidities/subject, n | 0 (0–1) | 0 (0–1) | 1.00 |
| Asymptomatic infections, n | 4 (40.0%) | 2 (10.0%) | 0.057 |
| Signs and Symptoms, n * | |||
| Fever | 1 (16.7%) | 14 (77.8%) | 0.028 |
| Cough | 3 (50.0%) | 6 (33.3%) | 0.808 |
| Rhinorrhea | 2 (33.3%) | 2 (11.1%) | 0.527 |
| Pharyngitis | 2 (33.3%) | 3 (16.7%) | 0.772 |
| Dyspnea | 0 (0.0%) | 3 (16.7%) | 0.546 |
| O/G dysfunction | 0 (0.0%) | 11 (61.1%) | 0.016 |
| Headache | 4 (66.7%) | 8 (44.4%) | 0.637 |
| Arthromyalgia | 0 (0.0%) | 12 (66.7%) | 0.014 |
| Asthenia/Malaise | 1 (16.7%) | 13 (72.2%) | 0.050 |
| Nausea/Vomiting | 0 (0.0%) | 3 (16.7%) | 0.546 |
| Diarrhea | 0 (0.0%) | 4 (22.2%) | 0.539 |
| Signs/symptoms number, n * | 2 (2–3) | 4 (3–6) | 0.014 |
| Severe COVID-19, n | |||
| Hospitalization | 0 (0.0%) | 1 (5.0%) | 0.846 |
| Oxygen support | 0 (0.0%) | 1 (5.0%) | 0.846 |
| Sequelae | 0 (0.0%) | 4 (20.0%) | 0.272 |
| COVID-19 length, days * | 5 (3–6) | 9 (7–14) | 0.028 |
| Time since COVID-19 onset to swab collection, days * | 3 (3–4) | 3 (1–5) | 0.98 |
| PCR ORF1ab Cycle threshold | 35.0 (31.3–35.9) | 22.5 (18.2–30.6) | 0.020 |
| Variable | Β (95% CI) | p |
|---|---|---|
| Vaccination status (reference: unvaccinated controls + post-first dose) | 3.81 (1.67–5.96) | 0.001 |
| Age | −0.006 (−0.089–0.76) | 0.883 |
| Sex (reference: female) | −1.64 (−4.64–1.37) | 0.282 |
| Number of comorbidities | 0.53 (−1.03–2.10) | 0.500 |
| Days from COVID-19 onset to swab collection | 0.48 (0.209–0.754) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunfio, M.; Verga, F.; Ghisetti, V.; Burdino, E.; Emanuele, T.; Bonora, S.; Di Perri, G.; Calcagno, A. Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers. Vaccines 2021, 9, 1377. https://doi.org/10.3390/vaccines9121377
Trunfio M, Verga F, Ghisetti V, Burdino E, Emanuele T, Bonora S, Di Perri G, Calcagno A. Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers. Vaccines. 2021; 9(12):1377. https://doi.org/10.3390/vaccines9121377
Chicago/Turabian StyleTrunfio, Mattia, Federica Verga, Valeria Ghisetti, Elisa Burdino, Teresa Emanuele, Stefano Bonora, Giovanni Di Perri, and Andrea Calcagno. 2021. "Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers" Vaccines 9, no. 12: 1377. https://doi.org/10.3390/vaccines9121377
APA StyleTrunfio, M., Verga, F., Ghisetti, V., Burdino, E., Emanuele, T., Bonora, S., Di Perri, G., & Calcagno, A. (2021). Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers. Vaccines, 9(12), 1377. https://doi.org/10.3390/vaccines9121377







