Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Testing
2.3. Statistical Analysis
3. Results
3.1. LIAISON® SARS-CoV-2 TrimericS IgG Results
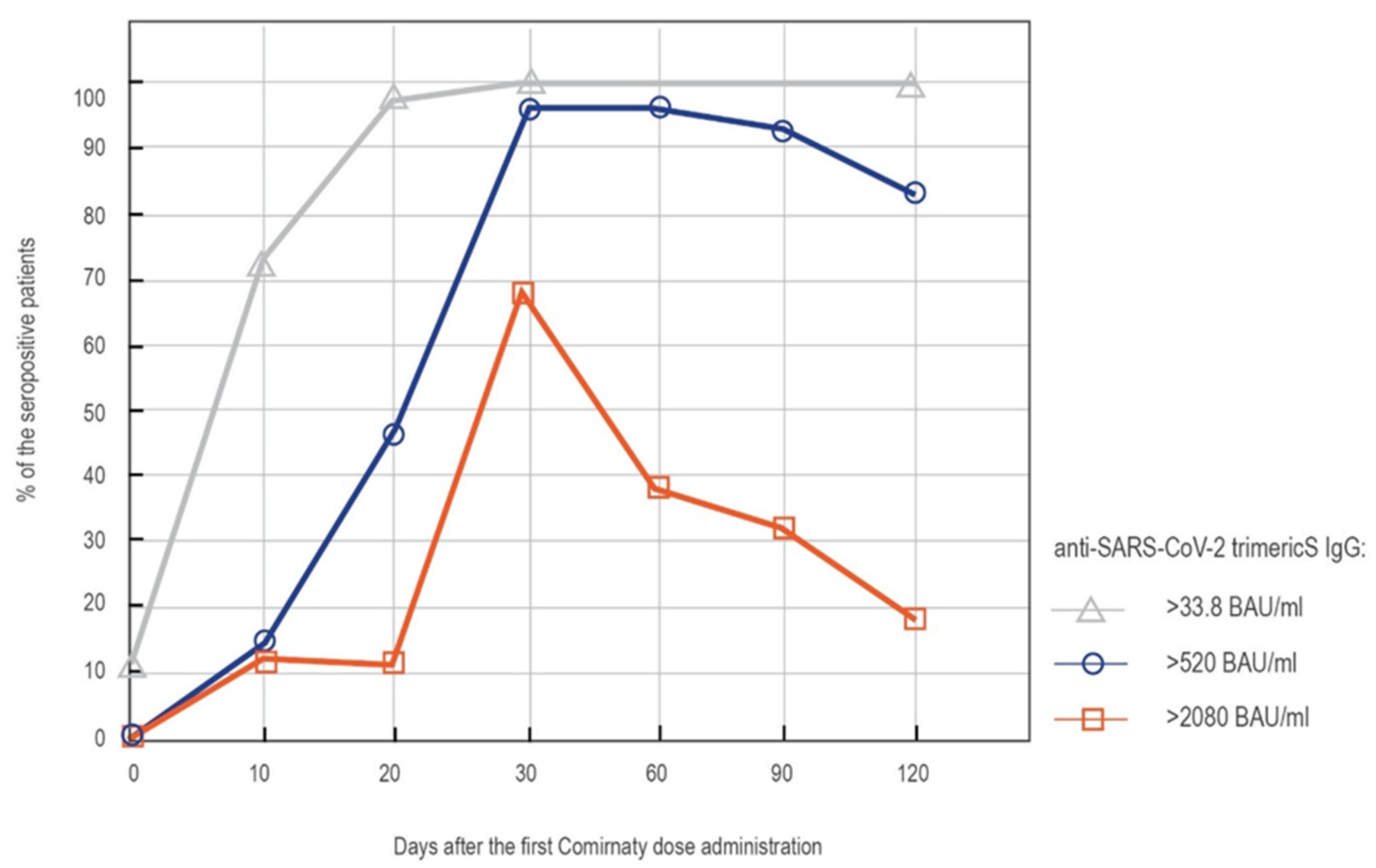
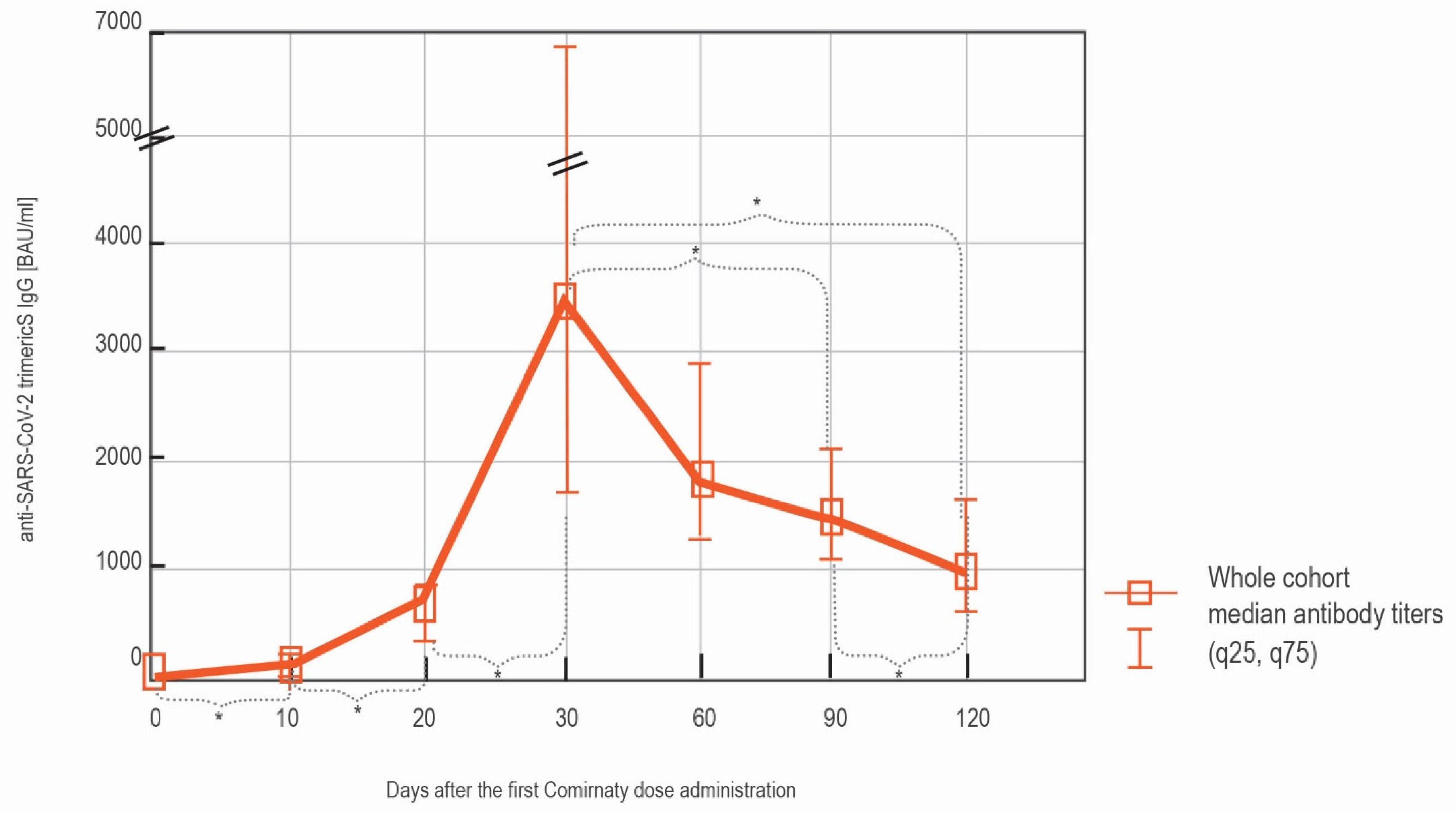
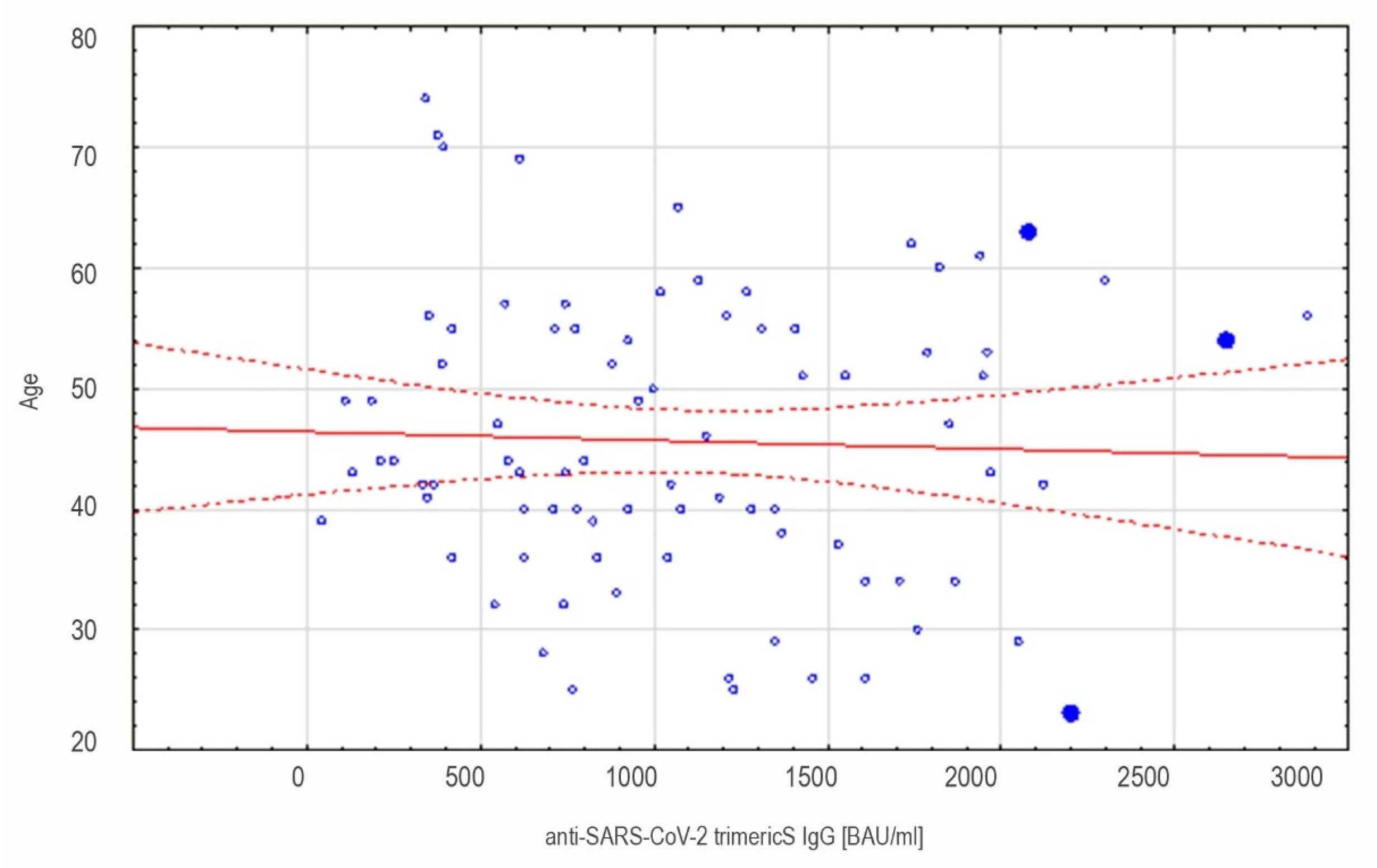
3.1.1. LIAISON® SARS-CoV-2 TrimericS IgG Results in the Whole Cohort
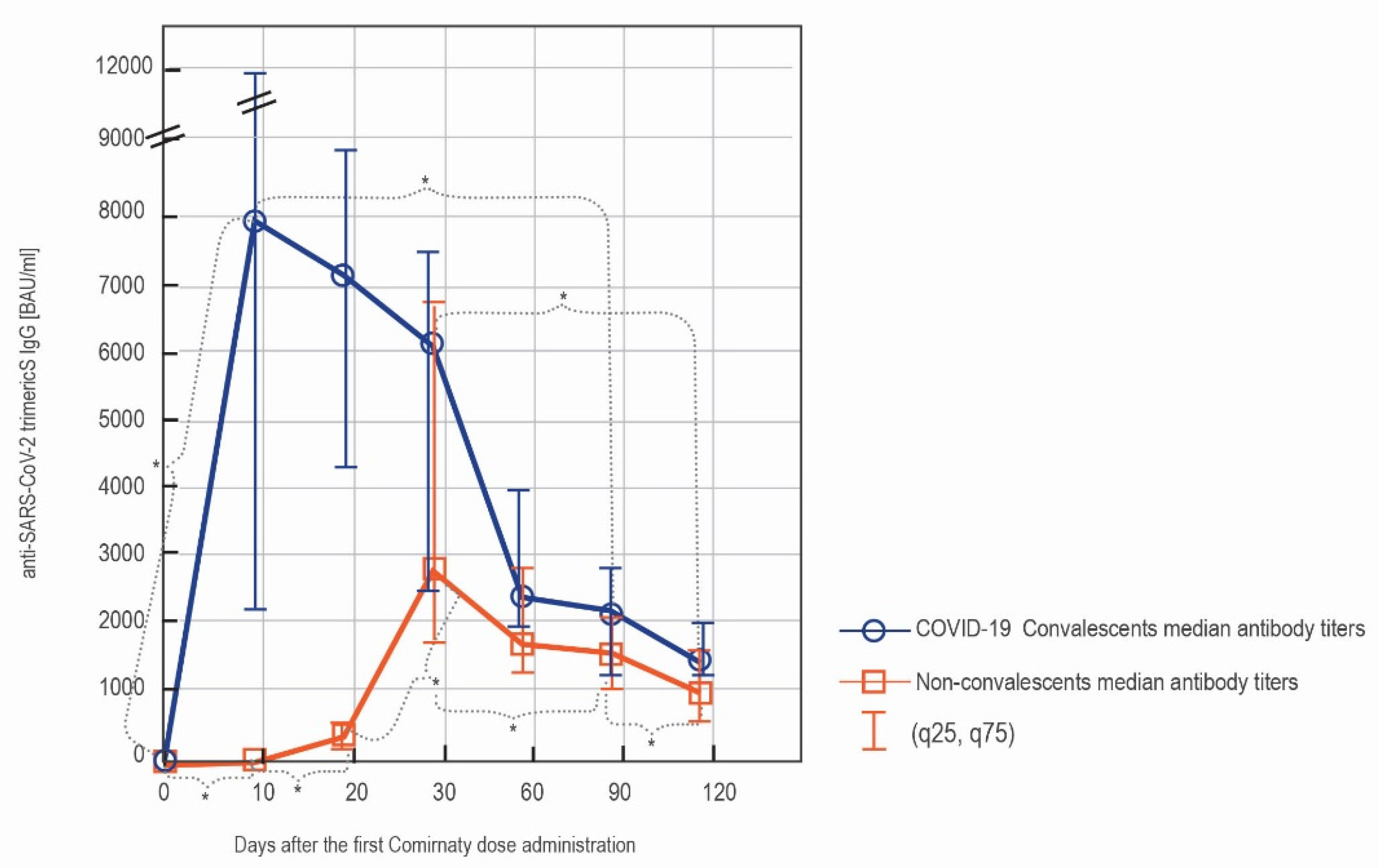
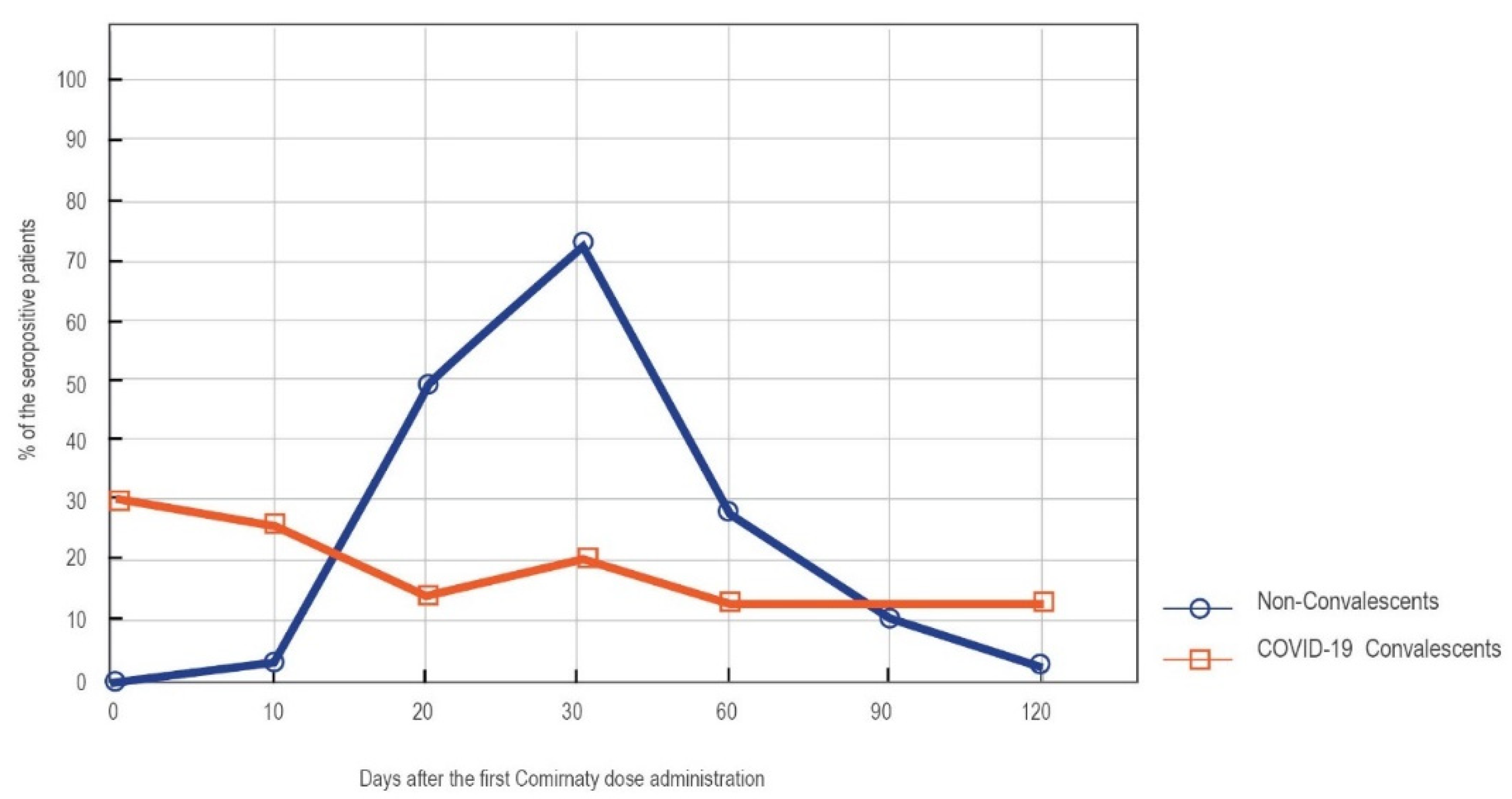
3.1.2. LIAISON® SARS-CoV-2 TrimericS IgG Results in in Convalescents and Non-Convalescents
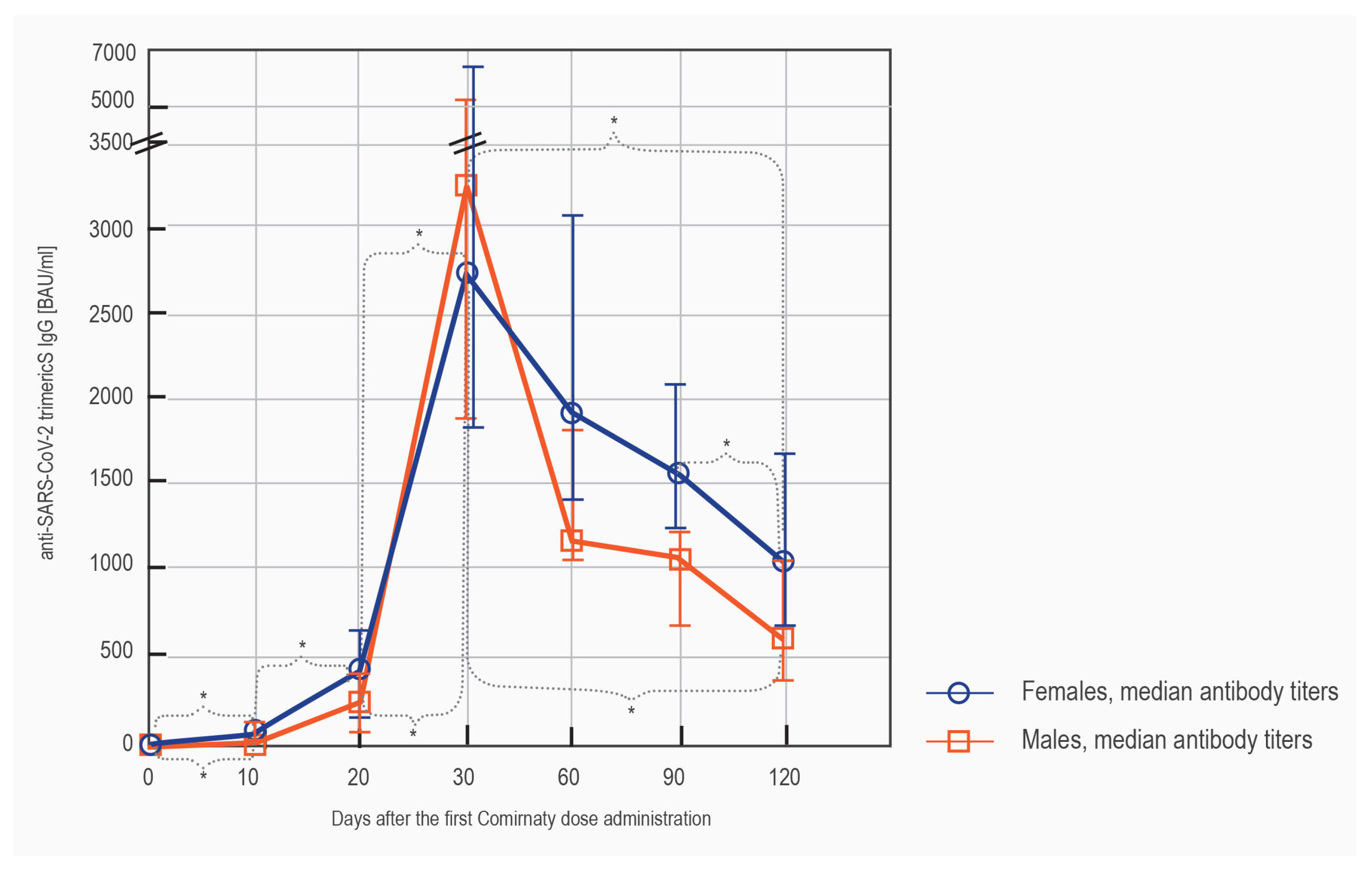
3.1.3. LIAISON® SARS-CoV-2 TrimericS IgG Results in Females vs. Males
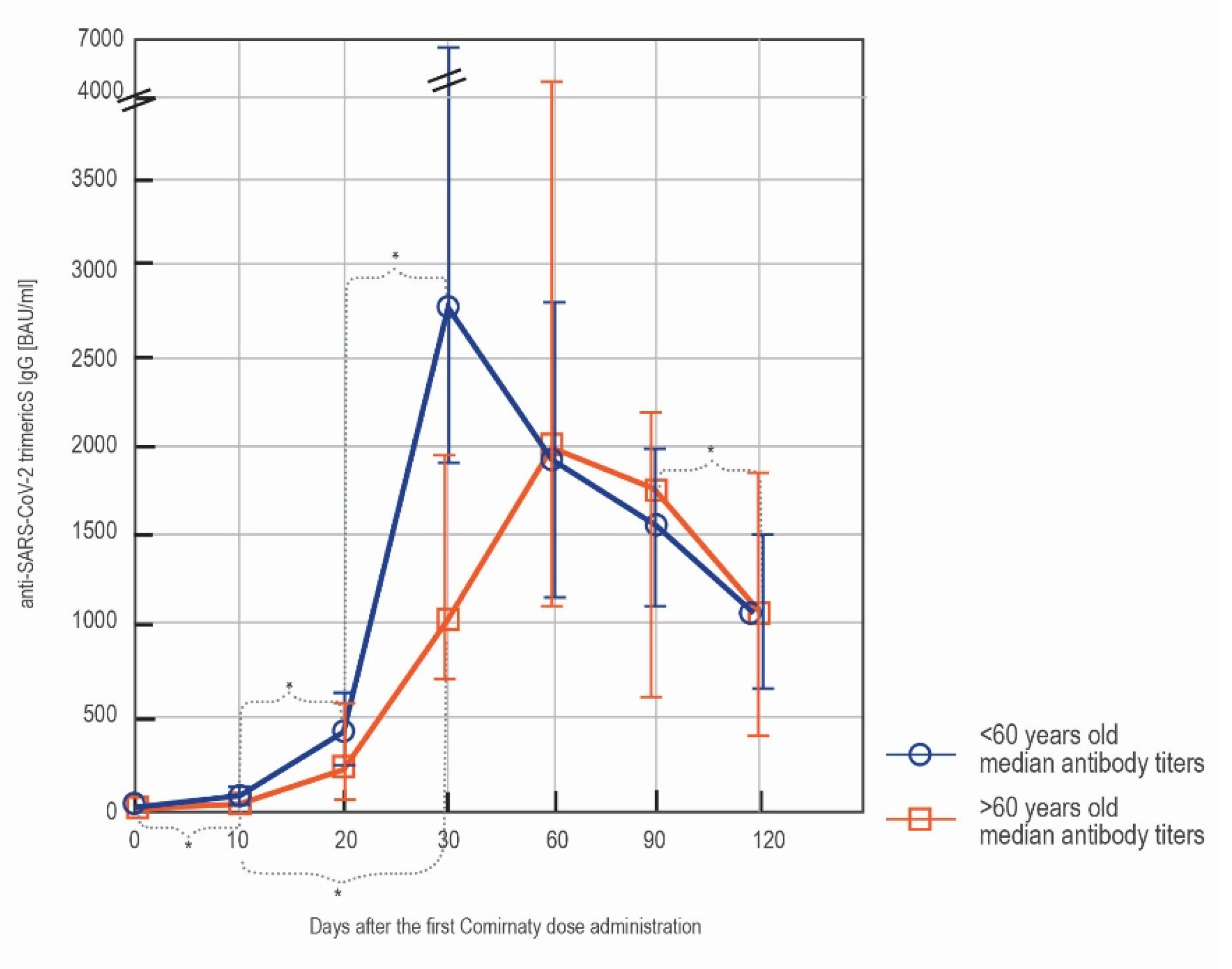
3.1.4. LIAISON® SARS-CoV-2 TrimericS IgG Results in Subjects below and over 60 y/o
3.2. Alinity SARS-CoV-2 S IgM Testing Results
3.3. SARS-CoV-2 N IgG Testing Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Treatments: Authorised. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-authorised (accessed on 2 September 2021).
- Kessels, R.; Luyten, J.; Tubeuf, S. Willingness to get vaccinated against Covid-19 and attitudes toward vaccination in general. Vaccine 2021, 39, 4716–4722. [Google Scholar] [CrossRef] [PubMed]
- Patelarou, E.; Galanis, P.; Mechili, E.A.; Argyriadi, A.; Argyriadis, A.; Asimakopoulou, E.; Brokaj, S.; Bucaj, J.; Carmona-Torres, J.M.; Cobo-Cuenca, A.I.; et al. Factors influencing nursing students’ intention to accept COVID-19 vaccination: A pooled analysis of seven European countries. Nurse Educ. Today 2021, 104, 105010. [Google Scholar] [CrossRef] [PubMed]
- Rastawicki, W.; Rokosz-Chudziak, N. Characteristics and assessment of the usefulness of serological tests in the diagnostic of infections caused by coronavirus SARS-CoV-2 on the basis of available manufacturer’s data and literature review. Prz. Epidemiol. 2020, 74, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Martin, E. A comparison of 7 commercial anti-SARS-CoV-2 antibody immunoassays. Arch. Med. Sci. 2020, 16. [Google Scholar] [CrossRef]
- Adams, E.R.; Ainsworth, M.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Bell, J.I.; Berry, T.; et al. Antibody testing for COVID-19: A report from the National COVID Scientific Advisory Panel. Wellcome Open Res. 2020, 5, 139. [Google Scholar] [CrossRef]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Kozlowska, D.; Panek, A.; Martin, E. The real life performance of 7 automated anti-SARS-CoV-2 IgG and IgM/IgA immunoassays. Pract. Lab. Med. 2021, 25, e00212. [Google Scholar] [CrossRef]
- Kohmer, N.; Westhaus, S.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J. Clin. Virol. 2020, 129, 104480. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [Green Version]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- First WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin (Human). NIBSC Code: 20/136. Instructions for Use. Version 2.0. Available online: https://www.nibsc.org/documents/ifu/20-136.pdf (accessed on 17 December 2020).
- Lippi, G.; Henry, B.; Plebani, M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics 2021, 11, 941. [Google Scholar] [CrossRef]
- Bonanni, P.; Cantón, R.; Gill, D.; Halfon, P.; Liebert, U.; Crespo, K.; Martín, J.; Trombetta, C. The Role of Serology Testing to Strengthen Vaccination Initiatives and Policies for COVID-19 in Europe. COVID 2021, 1, 20–38. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV -2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257. [Google Scholar] [CrossRef] [PubMed]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.P.; Ryan, D.T.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; D’Aquila, R.T.; Mustanski, B.; et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine 2021, 38, 101018. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Cornelius, A.R.; Gray-Gaillard, S.L.; Allen, J.R.; Karmacharya, T.; Wilson, J.P.; Hyman, S.W.; Tuen, M.; Koralov, S.B.; Mulligan, M.J.; et al. Robust immune responses after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2 experienced individuals. medRxiv 2021. [Google Scholar] [CrossRef]
- Salvagno, G.; Henry, B.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef]
- Focosi, D.; Baj, A.; Maggi, F. Is a single COVID-19 vaccine dose enough in convalescents? Hum. Vaccines Immunother. 2021, 17, 2959–2961. [Google Scholar] [CrossRef]
- Levi, R.; Azzolini, E.; Pozzi, C.; Ubaldi, L.; Lagioia, M.; Mantovani, A.; Rescigno, M. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J. Clin. Investig. 2021, 131, e149154. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Antoine-Moussiaux, T.; Della Vecchia, A.; Horeanga, A.; Papleux, E.; Vekemans, M.; Beukinga, I.; Blairon, L. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: A third dose is expected. J. Infect. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sasso, B.L.; Giglio, R.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.; Ciaccio, A.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.; Tober-Lau, P.; Hillus, D.; Helbig, E.T.; Lippert, L.J.; Thibeault, C.; Koch, W.; Landgraf, I.; Michel, J.; Bergfeld, L.; et al. Delayed Antibody and T-Cell Response to BNT162b2 Vaccination in the Elderly, Germany. Emerg. Infect. Dis. 2021, 27, 2174–2178. [Google Scholar] [CrossRef] [PubMed]
- Mahallawi, W.H.; Ibrahim, N.A.; Aljohani, A.S.; Shaikh, E.A.; Nafe, R.H.; Khan, A.M.; Mumena, W.A. Assessment of SARS-CoV-2 Anti-Spike IgG Antibody in Women and Children in Madinah, Saudi Arabia: A Single-Center Study. Int. J. Environ. Res. Public Health 2021, 18, 9971. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S.; Migliore, G.; Vimercati, L.; Martinelli, A.; Lobifaro, A.; Diella, G.; Stefanizzi, P. on behalf of the Control Room Working Group. BNT162b2 mRNA COVID-19 Vaccine Effectiveness in the Prevention of SARS-CoV-2 Infection and Symptomatic Disease in Five-Month Follow-Up: A Retrospective Cohort Study. Vaccines 2021, 9, 1143. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
| Naïve Vaccinees vs. COVID-19 Convalescents | |||||||
|---|---|---|---|---|---|---|---|
| Days after the First Comirnaty Dose Administration | Prior SARS-CoV-2 Contact | Number of Subjects | % of the Subjects Reaching a Given Antibody Concentration | Median Antibody Concentration | |||
| >33.8 BAU/mL | >520 BAU/mL | >2080 BAU/mL | BAU/mL | p | |||
| 0′ | Naïve vaccinees | 54 | 0 | 0 | 0 | 4.81 | p = 0.000 |
| COVID-19 convalescents | 13 | 61.5 | 0 | 0 | 41.5 * | ||
| 10′ | Naïve vaccinees | 85 | 68.2 | 1.2 | 0 | 67 | p = 0.000 |
| COVID-19 convalescents | 15 | 100 | 93.3 | 86.7 | 7945 * | ||
| 20′ | Naïve vaccinees | 85 | 96.5 | 38.8 | 0 | 408 | p = 0.000 |
| COVID-19 convalescents | 15 | 100 | 93.3 | 80.0 | 7180 * | ||
| 30′ | Naïve vaccinees | 85 | 100 | 96.5 | 65.9 | 2865 | p = 0.175 |
| COVID-19 convalescents | 15 | 100 | 93.3 | 80.0 | 6165 | ||
| 60′ | Naïve vaccinees | 85 | 100 | 95.3 | 34.1 | 1776 | p = 0.047 |
| COVID-19 convalescents | 15 | 100 | 100 | 66.7 | 2325 * | ||
| 90′ | Naïve vaccinees | 85 | 100 | 91.8 | 27.1 | 1522 | p = 0.022 |
| COVID-19 convalescents | 15 | 100 | 100 | 66.7 | 2165 * | ||
| 120′ | Naïve vaccinees | 85 | 100 | 81.2 | 14.1 | 1010 | p = 0.024 |
| COVID-19 convalescents | 15 | 100 | 93.3 | 46.7 | 1485 * | ||
| Females vs. Males n = 85 (72 vs. 13) | ||||||
|---|---|---|---|---|---|---|
| Days after the First Comirnaty Dose Administration | Sex | % of the Subjects Reaching a Given Antibody Concentration | Median Antibody Concentration | |||
| >33.8 BAU/mL | >520 BAU/mL | >2080 BAU/mL | BAU/mL | p | ||
| 10′ | Females | 73.6 | 1.4 | 0 | 76.4 | 0.082 |
| Males | 38.5 | 0 | 0 | 15.2 | ||
| 20′ | Females | 97.2 | 41.7 | 0 | 421 | 0.089 |
| Males | 92.3 | 23.1 | 0 | 253 | ||
| 30′ | Females | 100 | 95.8 | 66.7 | 2760 | 0.692 |
| Males | 100 | 100 | 61.5 | 3260 | ||
| 60′ | Females | 100 | 98.6 | 37.5 | 1932 | 0.017 |
| Males | 100 | 76.9 | 15.4 | 1193 * | ||
| 90′ | Females | 100 | 94.4 | 29.2 | 1570 | 0.004 |
| Males | 100 | 76.9 | 15.4 | 1089 * | ||
| 120′ | Females | 100 | 84.7 | 16.7 | 1070 | 0.024 |
| Males | 100 | 61.5 | 0 | 615 * | ||
| <60 y/o vs. >60 y/o n = 85 (76 vs. 9) | ||||||
|---|---|---|---|---|---|---|
| Days after the First Comirnaty Dose Administration | Age Group | % of the Subjects Reaching a Given Antibody Concentration | Median Antibody Concentration | |||
| >33.8 BAU/mL | >520 BAU/mL | >2080 BAU/mL | BAU/mL | p | ||
| 10′ | <60 y/o | 69.7 | 1.3 | 0 | 76.4 | 0.241 |
| >60 y/o | 55.6 | 0 | 0 | 33.8 | ||
| 20′ | <60 y/o | 97.4 | 38.2 | 0 | 416 | 0.241 |
| >60 y/o | 88.9 | 44.4 | 0 | 222 | ||
| 30′ | <60 y/o | 100 | 98.7 | 71.1 | 3360 | 0.009 |
| >60 y/o | 100 | 77.8 | 22.2 | 1050 * | ||
| 60′ | <60 y/o | 100 | 94.7 | 32.9 | 1771 | 0.478 |
| >60 y/o | 100 | 100 | 44.4 | 2000 | ||
| 90′ | <60 y/o | 100 | 92.1 | 26.3 | 1520 | 0.868 |
| >60 y/o | 100 | 88.9 | 33.3 | 1755 | ||
| 120′ | <60 y/o | 100 | 82.9 | 14.5 | 1000 | 0.885 |
| >60 y/o | 100 | 66.7 | 11.1 | 1070 | ||
| SARS-CoV-2 Anti-N IgG [Ratio] | |||||||
|---|---|---|---|---|---|---|---|
| Days after the First Comirnaty Dose Administration | 0′ | 10′ | 20′ | 30′ | 60′ | 90′ | 120′ |
| CASES: | |||||||
| Convalescent (confirmed 4 months prior to day 0′) | 2.44 | 2.67 | 2.69 | 2.29 | 1.74 | 1.32 | 0.58 |
| Convalescent (confirmed 4 months prior to day 0′) | 2.17 | 1.55 | 1.43 | 1.22 | 0.89 | 0.58 | 0.46 |
| Convalescent (confirmed 3 months prior to day 0′) | 2.64 | 1.98 | 1.98 | 1.82 | 1.6 | 1.04 | 0.54 |
| Convalescent (confirmed 2 months prior to day 0′) | 1.65 | 1.49 | 1.41 | 1.42 | 0.97 | 0.9 | 0.71 |
| Convalescent (confirmed 2 months prior to day 0′) | - | 5.01 | 4.62 | 4.28 | 3.11 | 2.11 | 1.48 |
| Convalescent (baseline seropositive) | 6.09 | 5.85 | 5.59 | 5.68 | 5.77 | 5.88 | 5.65 |
| Convalescent (baseline seropositive) | 2.11 | 1.15 | 0.95 | 0.9 | 0.49 | 0.39 | 0.25 |
| Convalescent (baseline seropositive) | 1.24 | 1.68 | 2.0 | 1.9 | 1.62 | 1.89 | 1.11 |
| Non-convalescent | - | 0,06 | 0,05 | 0,08 | 4.21 | 1.59 | 1.6 |
| Non-convalescent | 0.03 | 0.03 | 0.03 | 0.04 | 3.01 | 1.65 | 0.27 |
| Non-convalescent | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 1.59 |
| Non-convalescent | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 1.94 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swadźba, J.; Anyszek, T.; Panek, A.; Martin, E. Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines 2021, 9, 1367. https://doi.org/10.3390/vaccines9111367
Swadźba J, Anyszek T, Panek A, Martin E. Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines. 2021; 9(11):1367. https://doi.org/10.3390/vaccines9111367
Chicago/Turabian StyleSwadźba, Jakub, Tomasz Anyszek, Andrzej Panek, and Emilia Martin. 2021. "Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination" Vaccines 9, no. 11: 1367. https://doi.org/10.3390/vaccines9111367
APA StyleSwadźba, J., Anyszek, T., Panek, A., & Martin, E. (2021). Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines, 9(11), 1367. https://doi.org/10.3390/vaccines9111367






