Management of Invasive Infections due to a Rare Arthroconidial Yeast, Saprochaete capitata, in Two Patients with Acute Hematological Malignancies
Abstract
1. Introduction
2. Microbiological Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong-James, D.; Bicanic, T.; Brown, G.D.; Hoving, J.C.; Meintjes, G.; Nielsen, K.; Working Group from the EMBO Workshop on AIDS-Related Mycoses. AIDS-related mycoses: Current progress in the field and future priorities. Trends Microbiol. 2017, 25, 428–430. [Google Scholar] [CrossRef]
- Miceli, M.H.; Diaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Pande, A.; Non, L.R.; Santos, C.A. Pseudozyma and other non-Candida opportunistic yeast bloodstream infections in a large stem cell transplant center. Transpl. Infect. Dis. 2017, 19, e12664. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.W.; Hagen, F. Attack, defend and persist: How the fungal pathogen Candida auris was able to emerge globally in healthcare environments. Mycopathologia 2019, 184, 353–365. [Google Scholar] [CrossRef]
- Potenza, L.; Chitasombat, M.N.; Klimko, N.; Bettelli, F.; Dragonetti, G.; Del Principe, M.I.; Nucci, M.; Busca, A.; Fracchiolla, N.; Sciumè, M.; et al. Rhodotorula infection in haematological patient: Risk factors and outcome. Mycoses 2018, 62, 223–229. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Rudramurthy, S.; Kale, P.; Hariprasath, P.; Dhaliwal, M.; Singhi, S.; Rao, K.L.N. Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centre. Clin. Microbiol. Infect. 2014, 20, O83–O89. [Google Scholar] [CrossRef]
- Chagas-Neto, T.C.; Chaves, G.M.; Melo, A.S.; Colombo, A.L. Bloodstream infections due to Trichosporon spp.: Species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing and antifungal susceptibility testing. J. Clin. Microbiol. 2009, 47, 1074–1081. [Google Scholar] [CrossRef]
- Durán Graeff, L.; Seidei, D.; Vehreschild, M.J.G.T.; Hamprecht, A.; Kindo, A.; Racil, Z.; Demeter, J.; De Hoog, S.; Aurbach, U.; Ziegler, M.; et al. Invasive infections due to Saprochaete and Geotrichum species report of 23 cases from the FungiScope registry. Mycoses 2017, 60, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gulcan, A.; Gulcan, E.; Keles, M.; Aktas, E. Oral yeast colonization in peritoneal dialysis and hemodialysis patients and renal transplant recipients. Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 47–52. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Chiller, T.M. Fungal infections and new biologic therapies. Curr. Rheumatol. Rep. 2016, 18, 29. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The global burden of fungal diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef]
- Colombo, A.L.; de Almeida Jùnior, J.N.; Slavin, M.A.; Chen, S.C.; Sorrell, T.C. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect. Dis. 2017, 17, e344–e356. [Google Scholar] [CrossRef]
- Li, H.; Guo, M.; Wang, C.; Li, Y.; Fernandez, A.M.; Ferraro, T.N.; Yang, R.; Chen, Y. Epidemiological study of Trichosporon asahii infections over the past 23 years. Epidemiol Infect. 2020, 148, e169. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhoni, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Woosley, L.N.; Jones, R.N.; Castanheira, M. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: Application of new CLSI clinical breakpoint and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J. Clin. Microbiol. 2013, 51, 2571–2581. [Google Scholar] [PubMed]
- Diekema, D.J.; Petroelje, B.; Messer, S.A.; Hollis, R.J.; Pfaller, M.A. Activities of available and investigational antifungal agents against Rhodotorula species. J. Clin. Microbiol. 2005, 43, 476–478. [Google Scholar] [CrossRef][Green Version]
- Schuermans, C.; van Bergen, M.; Coorevits, L.; Verhaegen, J.; Lagrou, K.; Surmont, I.; Jeurissen, A. Breaktrough Saprochaete capitata infections in patients receiving echinocandins: Case report and review of the literature. Med. Mycol. 2011, 49, 414–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cornely, O.A.; Hoenigl, M.; Lass-Florl, C.; Chen, S.C.-A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R.; Mycoses Study Group Education and research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection-position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [PubMed]
- Trabelsi, H.; Neji, S.; Gargouri, L.; Sellami, H.; Guidara, R.; Cheikhrouhou, F.; Bellaaj, H.; Makni, F.; Elloumi, M.; Ayadiet, A. Geotrichum capitatum septicemia: Case report and review of the literature. Mycopathologia 2015, 179, 465–469. [Google Scholar] [CrossRef]
- Ulu-Kilic, A.; Atalay, M.A.; Metan, G.; Cevahir, F.; Koc, N.; Eser, B.; Çetin, M.; Kaynar, L.; Alp, E. Saprochaetae capitata as an emerging fungus among patients with haematological malignencies. Mycoses 2015, 58, 491–497. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Smith, M.T. The ribosomal gene phylogeny and species delimitation in Geotrichum and its teleomorphs. Stud. Mycol. 2004, 50, 489–515. [Google Scholar]
- Chang, W.W.; Buerger, L. Disseminated geotrichosis: Case report. Arch. Intern. Med. 1964, 113, 356–360. [Google Scholar] [CrossRef]
- Lo Cascio, G.; Vincenzi, M.; Soldani, F.; De Carolis, E.; Maccacaro, L.; Sorrentino, A.; Nadali, G.; Cesaro, S.; Sommavilla, M.; Niero, V.; et al. Outbreak of Saprochaete clavata Sepsis in Hematology Patients: Combined Use of MALDI-TOF and Sequencing Strategy to Identify and Correlate the Episodes. Front. Microbiol. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Vaux, S.; Criscuolo, A.; Desnos-Ollivier, M.; Diancourt, L.; Tarnaud, C.; Vandenbogaert, M.; Brisse, S.; Coignard, B.; Dromer, F. Multicenter outbreak of infections by Saprochaete clavata, an unrecognized opportunistic fungal pathogen. MBio 2014, 5, e02309-14. [Google Scholar] [CrossRef] [PubMed]
- Buchta, V.; Bolehovská, R.; Hovorková, E.; Cornely, O.A.; Seidel, D.; Žák, P. Saprochaete clavata Invasive Infections—A New Threat to Hematological-Oncological Patients. Front. Microbiol. 2019, 10, 2196. [Google Scholar] [CrossRef]
- Mazzocato, S.; Marchionni, E.; Fothergill, A.W.; Sutton, D.A.; Staffolani, S.; Gesuita, R.; Skrami, E.; Fiorentini, A.; Manso, E.; Barchiesi, F. Epidemiology and outcome of systemic infections due to Saprochaete capitata: Case report and review of the literature. Infection 2015, 43, 211–215. [Google Scholar] [CrossRef]
- Erman, B.; Fırtına, S.; Aksoy, B.A.; Aydogdu, S.; Genç, G.E.; Doğan, Ö.; Bozkurt, C.; Fışgın, T.; Çipe, F.E. Invasive Saprochaete capitata Infection in a Patient with Autosomal Recessive CARD9 Deficiency and a Review of the Literature. J. Clin. Immunol. 2020, 40, 466–474. [Google Scholar] [CrossRef]
- Pamidimukkala, U.; Kancharla, A.; Sudhaharan, S.; Gundeti, S.; Mandarapu, S.; Nagalla, V.K.; Raju, S.B.; Karanam, S.D. Isolation of the Rare Opportunistic Yeast Saprochaete capitata from Clinical Samples-Experience from a Tertiary Care Hospital in Southern India and a Brief Review of the Literature. J. Clin. Diagn. Res. 2017, 11, Dc36–Dc42. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, J.C.; Lopez-Soria, L.; Olazabal, I.; Amutio, E.; Arrieta-Aguirre, I.; Velasco-Benito, V.; Ponton, J.; Moragues, M.D. Invasive infections caused by Saprochaete capitata in patients with haematological malignancies: Report of five cases and review of the antifungal therapy. Rev. Iberoam. Micol. 2013, 30, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Desnos-Ollivier, M.; Blanc, C.; Garcia-Hermoso, D.; Hoinard, D.; Alanio, A.; Dromer, F. Misidentification of Saprochaete clavata as Magnusiomyces capitatus in clinical isolates: Utility of internal transcribed spacer sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry and importance of reliable databases. J. Clin. Microbiol. 2014, 52, 2196–2198. [Google Scholar]
- Kaplan, E.; Al-Hatmi, A.M.S.; Ilkit, M.; Gerrits van den Ende, A.H.G.; Hagen, F.; Meis, J.F.; de Hoog, G.S. Molecular Diagnostics of Arthroconidial Yeasts, Frequent Pulmonary Opportunists. J. Clin. Microbiol. 2018, 56, e01427-17. [Google Scholar] [CrossRef]
- Kolecka, A.; Khayhan, K.; Groenewald, M.; Theelen, B.; Arabatzis, M.; Velegraki, A.; Kostrzewa, M.; Mares, M.; Taj-Aldeen, S.J.; Boekhout, T. Identification of medically relevant species of arthroconidial yeasts by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 2491–2500. [Google Scholar] [CrossRef]
- Gadea, I.; Cuenca-Estrella, M.; Prieto, E.; Diaz-Guerra, T.M.; Garcia-Cia, J.I.; Mellado, E.; Tomas, J.F.; Rodriguez-Tuleda, J.F. Genotyping and antifungal susceptibility profile of Dipodascus capitatus isolates causing disseminated infection in seven hematological patients of a tertiary hospital. J. Clin. Microbiol. 2004, 42, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Christakis, G.; Perlorentzou, S.; Aslanidou, M.; Megalakaki, A.; Velegraki, A. Fatal blastoschizomyces capitatus sepsis in a neutropenic patient with acute myeloid leukemia: First documented case from Greece. Mycoses 2005, 48, 216–220. [Google Scholar] [CrossRef]
- Girmenia, C.; Pagano, L.; Martino, B.; D’Antonio, D.; Fanci, R.; Specchia, G.; Melillo, L.; Buelli, M.; Pizzarelli, G.; Venditti, M.; et al. GIMEMA Infection Program. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: A retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 2005, 43, 1818–1828. [Google Scholar] [CrossRef]
- Del Principe, M.I.; Sarmati, L.; Cefalo, M.; Fontana, C.; De Santis, G.; Buccisano, F.; Maurillo, L.; De Bellis, E.; Postorino, M.; Sconocchia, G.; et al. A cluster of Geotrichum clavatum (Saprochaete clavata) infection in haematological patients: A first Italian report and review of literature. Mycoses 2016, 59, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Esposto, M.C.; Prigitano, A.; Lo Cascio, G.; Ossi, C.; Grancini, A.; Cavanna, C.; Lallitto, F.; Tejada, M.; Bandettini, R.; Mularoni, A.; et al. Yeast-like filamentous fungi: Molecular identification and in vitro susceptibility study. Med. Mycol. 2018, 57, 909–913. [Google Scholar] [CrossRef]
- Leoni, M.; Riccardi, N.; Rotulo, G.A.; Godano, E.; Faraci, M.; Bandettini, R.; Esposto, M.C.; Castagnola, E. Magnusiomyces clavatus infection in a child after allogeneic hematotopoietic stem cell transplantation: Diagnostic and therapeutic implications. Med. Mycol. Case Rep. 2018, 23, 65–67. [Google Scholar] [CrossRef]
- Salgüero Fernández, I.; Nájera Botello, L.; Orden Martinez, B.; Roustan Gullón, G. Disseminated fungemia by Saprochaete clavata. Enferm. Infecc. Microbiol. Clin. 2018, 37, 283–284. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Bennett, J.E.; Pappas, P.G.; Maertens, J.; Lortholary, O.; et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [PubMed]
- Martino, P.; Venditti, M.; Micozzi, A.; Morace, G.; Polonelli, L.; Mantovani, M.P.; Petti, M.C.; Burgio, V.L.; Santini, C.; Serra, P.; et al. Blastoschizomyces capitatus: An emerging cause of invasive fungal disease in leukemia patients. Rev. Infect. Dis. 1990, 12, 570–582. [Google Scholar] [CrossRef]
- Martino, R.; Salavert, M.; Parody, R.; Tomas, J.F.; de la Camara, R.; Vazquez, L.; Jarque, I.; Prieto, E.; Sastre, J.L.; Gadea, I.; et al. Blastoschizomyces capitatus infection in patients with leukemia: Report of 26 cases. Clin. Infect. Dis. 2004, 38, 335–341. [Google Scholar] [CrossRef]
- Birrenbach, T.; Bertschy, S.; Aebersold, F.; Mueller, N.J.; Achermann, Y.; Muehlethaler, K.; Zimmerli, S. Emergence of Blastoschizomyces capitatus yeast infections, Central Europe. Emerg. Infect. Dis. 2012, 18, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Saghrouni, F.; Abdeljelil, J.B.; Youssef, Y.B.; Abdeljelil, N.B.; Gheith, S.; Fathallah, A.; Said, M.B. Geotrichum capitatum septicemia in patients with acute myeloid leukemia. Report of three cases. Med. Mycol. Case Rep. 2012, 1, 88–90. [Google Scholar] [CrossRef]
- Subramanya Supram, H.; Gokhale, S.; Chakrabarti, A.; Rudramurthy, S.M.; Gupta, S.; Honnavar, P. Emergence of Magnusiomyces capitatus infections in western Nepal. Med. Mycol. 2016, 54, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Aguirre, I.; Menendez-Manjon, P.; Cuétara, M.S.; de Larrinoa, I.F.; Garcia-Ruiz, J.C.; Moragues, M.D. Sequencing of FKS hot spot 1 from Saprochaete capitate to search a relationship to reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2018, 62, e01246-17. [Google Scholar] [CrossRef] [PubMed]
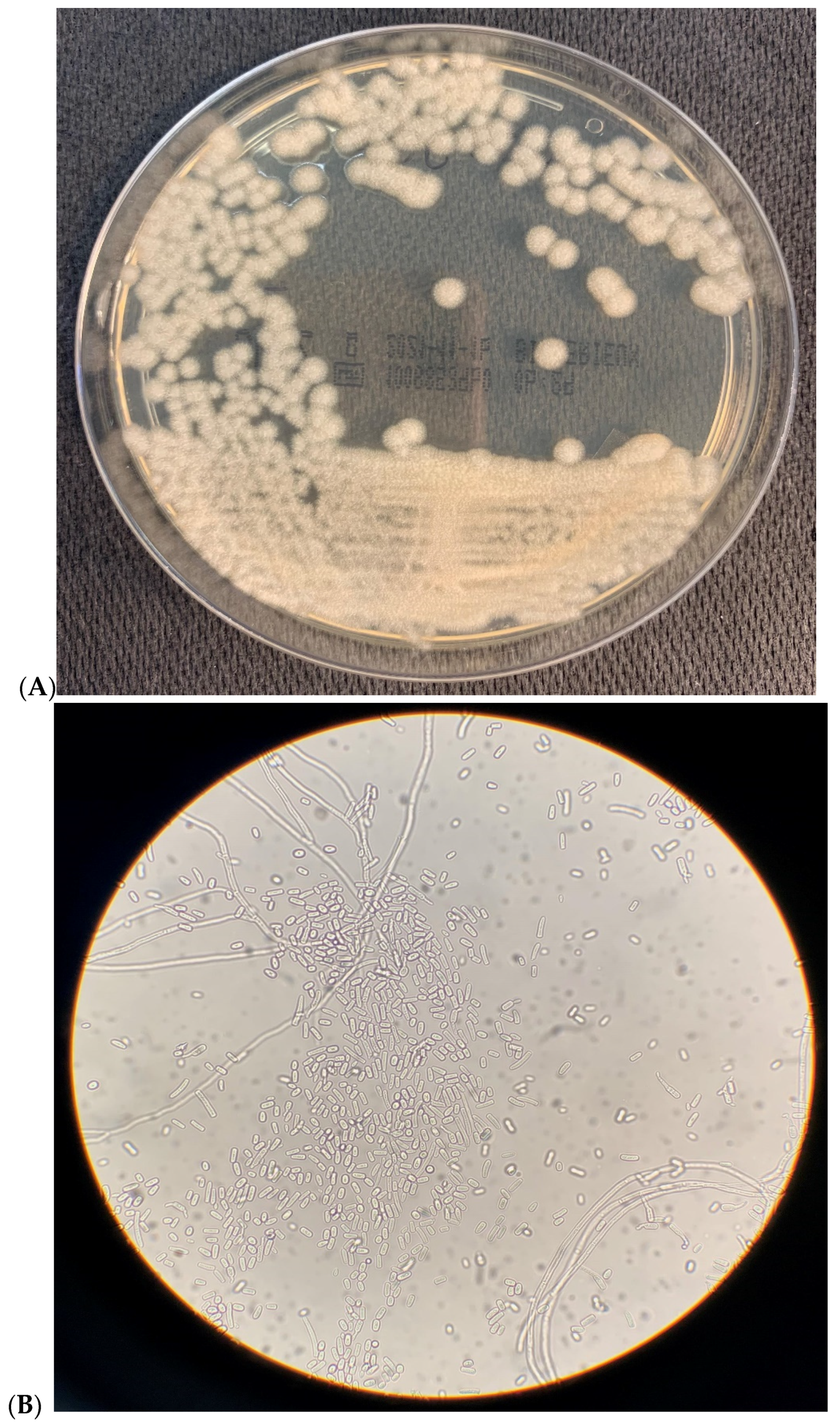
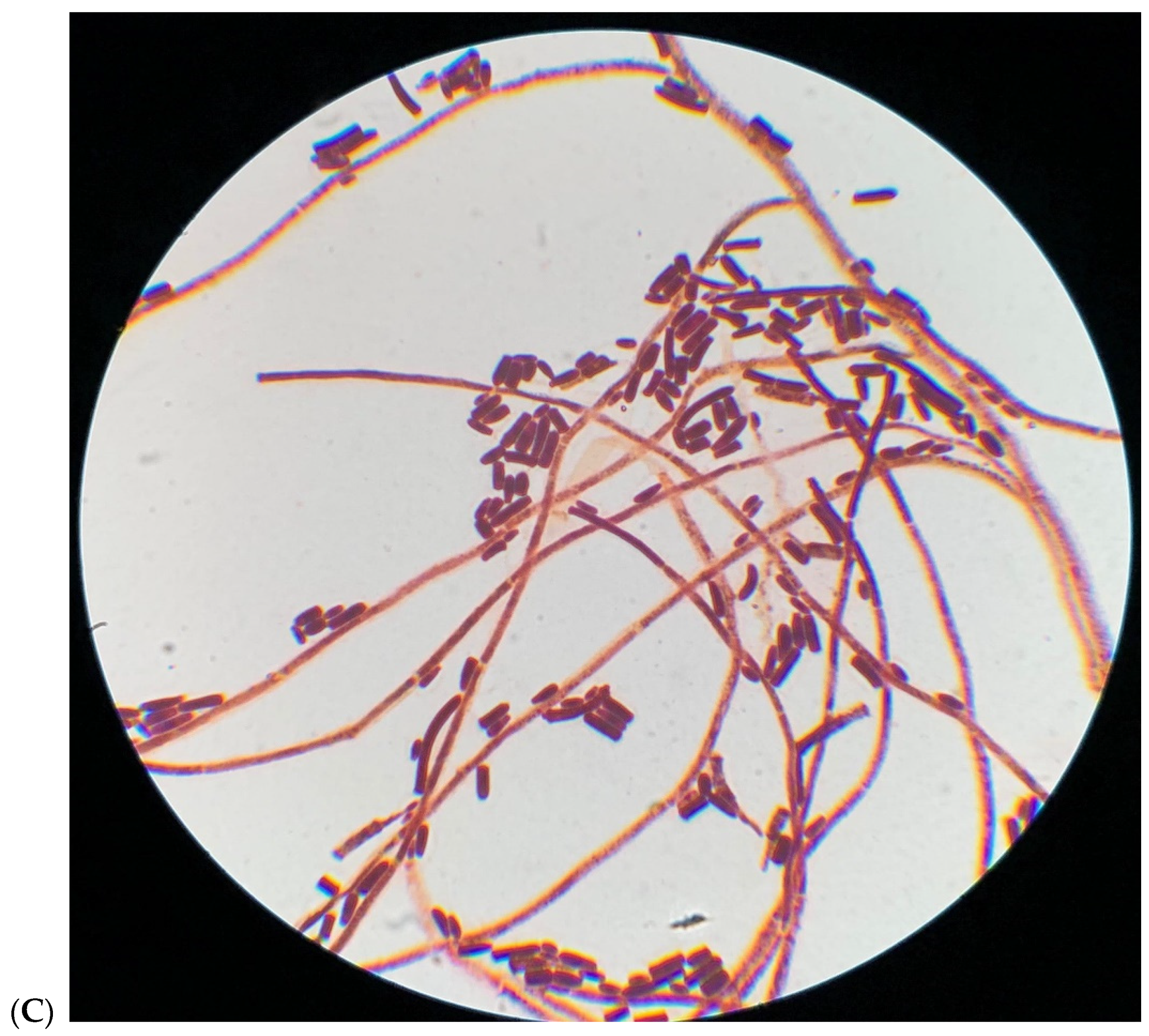
| Drugs (μg/mL) | Patient 1 | Patient 2 |
|---|---|---|
| 5-Fluorocytosine | 16 | 16 |
| Amphotericin B | 1 | 1 |
| Anidulafungin | 2 | 2 |
| Caspofungin | 8 | 8 |
| Fluconazole | 8 | 8 |
| Itraconazole | 0.25 | 0.5 |
| Micafungin | 2 | 8 |
| Posaconazole | 0.5 | 1 |
| Voriconazole | 0.12 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurrieri, F.; Corbellini, S.; Piccinelli, G.; Turra, A.; Morello, E.; Malagola, M.; Russo, D.; Caruso, A.; De Francesco, M.A. Management of Invasive Infections due to a Rare Arthroconidial Yeast, Saprochaete capitata, in Two Patients with Acute Hematological Malignancies. Vaccines 2021, 9, 1289. https://doi.org/10.3390/vaccines9111289
Gurrieri F, Corbellini S, Piccinelli G, Turra A, Morello E, Malagola M, Russo D, Caruso A, De Francesco MA. Management of Invasive Infections due to a Rare Arthroconidial Yeast, Saprochaete capitata, in Two Patients with Acute Hematological Malignancies. Vaccines. 2021; 9(11):1289. https://doi.org/10.3390/vaccines9111289
Chicago/Turabian StyleGurrieri, Francesca, Silvia Corbellini, Giorgio Piccinelli, Alessandro Turra, Enrico Morello, Michele Malagola, Domenico Russo, Arnaldo Caruso, and Maria Antonia De Francesco. 2021. "Management of Invasive Infections due to a Rare Arthroconidial Yeast, Saprochaete capitata, in Two Patients with Acute Hematological Malignancies" Vaccines 9, no. 11: 1289. https://doi.org/10.3390/vaccines9111289
APA StyleGurrieri, F., Corbellini, S., Piccinelli, G., Turra, A., Morello, E., Malagola, M., Russo, D., Caruso, A., & De Francesco, M. A. (2021). Management of Invasive Infections due to a Rare Arthroconidial Yeast, Saprochaete capitata, in Two Patients with Acute Hematological Malignancies. Vaccines, 9(11), 1289. https://doi.org/10.3390/vaccines9111289








