The Gut Microbiome in Psoriasis and Crohn’s Disease: Is Its Perturbation a Common Denominator for Their Pathogenesis?
Abstract
:1. Introduction
2. Clinical Features of Psoriasis and Associated Skin and Gut Microbiome Dysbiosis
3. Clinical Features of Crohn’s Disease and Associated Gut Microbiome Dysbiosis
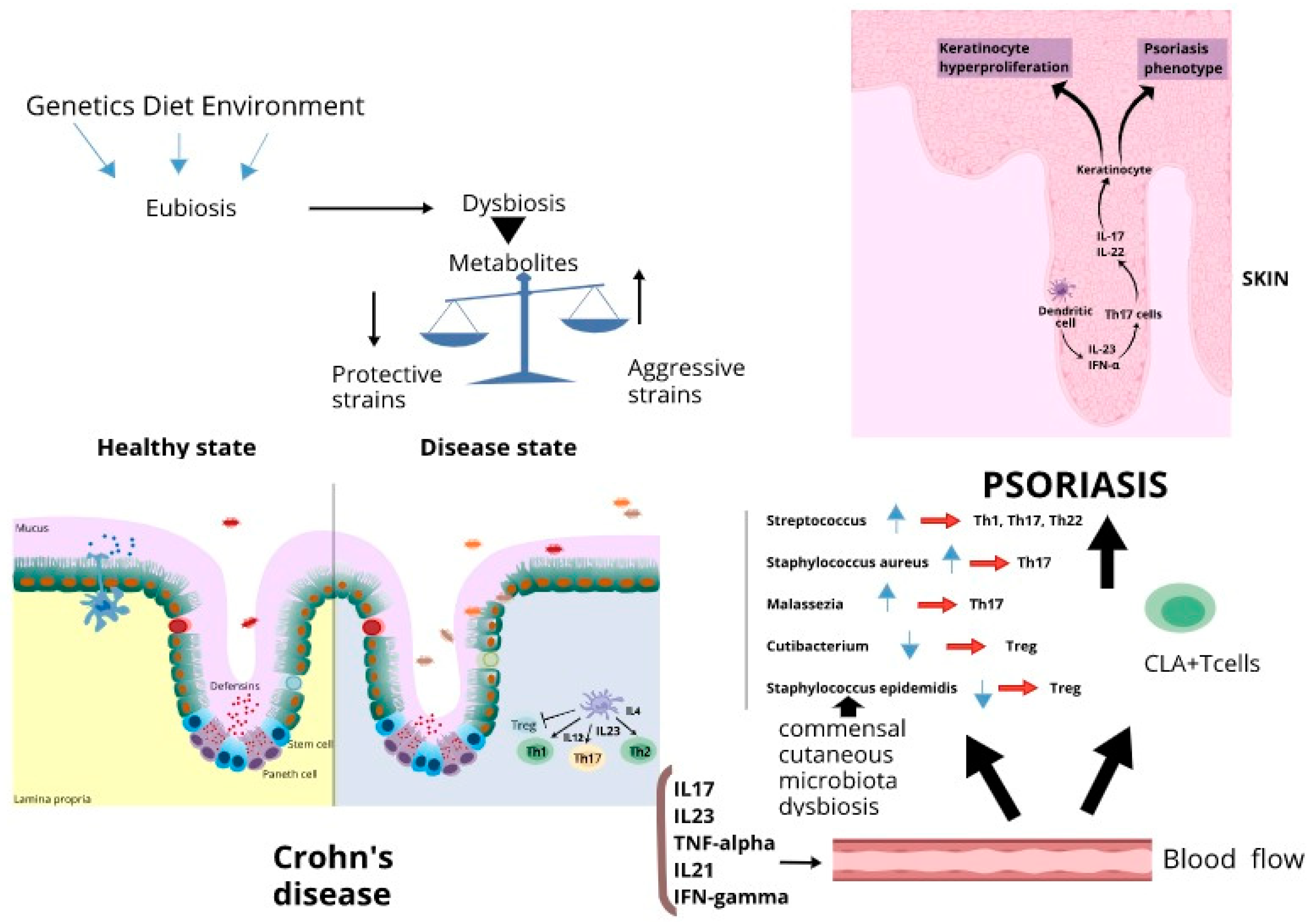
4. The Role of Gut Microbiota Perturbation in Psoriasis and Crohn’s Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makredes, M.; Robinson, D.; Bala, M.; Kimball, A.B. The burden of autoimmune disease: A comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis. J. Am. Acad. Dermatol. 2009, 61, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; Essers, I.; van Tubergen, A.; Boonen, A.; Bazelier, M.T.; De Bruin, M.L.; de Vries, F. The epidemiology of extra-articular manifestations in ankylosing spondylitis: A population-based matched cohort study. Ann. Rheum. Dis. 2015, 74, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Skroza, N.; Proietti, I.; Pampena, R.; La Viola, G.; Bernardini, N.; Nicolucci, F.; Tolino, E.; Uber, S.; Soccodato, V.; Potenza, C. Correlations between psoriasi sans inflammatory bowel diseases. Biomed. Res. Int. 2013, 2013, 983902. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E. Comorbidities in psoriasis. Clin. Dermatol. 2007, 25, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.I.; Bellary, S.V.; Francis, C. Increased occurrence of psoriasis in patients with Crohn’s disease and their relatives. Am. J. Gastroenterol. 1990, 85, 962–963. [Google Scholar]
- Nestle, F.O.; Kaplan, D.H.; Baker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Bassukas, I.D.; Gaitanis, G.; Katsanos, K.H.; Christodoulou, D.K.; Tsianos, E.; Vlachos, C. Psoriasis and inflammatory bowel disease: Links and risks. Psoriasis 2016, 6, 73–92. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–989. [Google Scholar] [CrossRef]
- Shah, S.C.; Khalili, H.; Gower-Rousseau, C.; Olen, O.; Benchimol, E.I.; Lynge, E.; Nielsen, K.R.; Brassard, P.; Vutcovici, M.; Bitton, A.; et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases—Pooled Analysis of Population-Based Studies from Western Countries. Gastroenterology 2018, 155, 1079–1089.e3. [Google Scholar] [CrossRef]
- Beeson, P.B. Age and sex associations of 40 autommnune diseases. Am. J. Med. 1994, 96, 457–462. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Hampe, J.; Heymann, K.; Krawczak, M.; Schreiber, S. Association of inflammatory bowel disease with indicators for childhood antigen and infection exposure. Int. J. Colorectal. Dis. 2003, 18, 413–417. [Google Scholar] [CrossRef]
- Bridger, S.; Lee, J.C.; Bjarnason, I.; Jones, J.E.; Macpherson, A.J. In siblings with similar genetic susceptibility for inflammatory bowel disease, smokers tend to develop Crohn’s disease and non-smokers develop ulcerative colitis. Gut 2002, 51, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, A.R.; Russell, R.K.; Wilson, M.L.; Gilmour, W.H.; Satsangi, J.; Wilson, D.C. Systematic Review: The Role of Breastfeeding in the Development of Pediatric Inflammatory Bowel Disease. J. Pediatr. 2009, 155, 421–426. [Google Scholar] [CrossRef]
- Hviid, A.; Svanström, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef]
- Mrowietz, U.; Steinz, K.; Gerdes, S. Psoriasis: To treat or to manage? Exp. Dermatol. 2014, 23, 705–709. [Google Scholar] [CrossRef]
- Chandra, A.; Ray, A.; Senapati, S.; Chatterjee, R. Genetic and epigenetic basis of psoriasis pathogenesis. Mol. Immunol. 2015, 64, 313–323. [Google Scholar] [CrossRef]
- Imielinski, M.; Baldassano, R.N.; Griffiths, A.; Russell, R.K.; Annese, V.; Dubinsky, M.; Kugathasan, S.; Bradfield, J.P.; Walters, T.D.; Sleiman, P.; et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 2009, 41, 1335–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Meha, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, L.C.; Stuart, P.E.; Tian, C.; Gudjonsson, J.E.; Das, S.; Zawistowski, M.; Ellinghaus, E.; Barker, J.N.; Chandran, V.; Dand, N.; et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat. Commun. 2017, 8, 15382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.; McGover, D.P.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Ellinghaus, D.; Ellinghaus, E.; Nair, R.P.; Stuart, P.E.; Esko, T.; Metspalu, A.; Debrus, S.; Raelson, J.V.; Tejasvi, T.; Belouchi, M.; et al. Combined analysis of genome wide association studies for Crohn disease and psoriasis identified seven hared susceptibility loci. Am. J Hum Genet. 2013, 90, 636–647. [Google Scholar] [CrossRef] [Green Version]
- Binus, A.M.; Han, J.; Qamar, A.A.; Mody, E.A.; Holt, E.W.; Qureshi, A.A. Associated comorbidities in psoriasis and inflammatory bowel disease. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 644–650. [Google Scholar] [CrossRef]
- Abuabara, K.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the U.K. Br. J. Dermatol. 2010, 163, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Onumah, N.; Kircik, L.H. Psoriasis and its comorbidities. J. Drugs Dermatol. 2012, 11, S5–S10. [Google Scholar]
- Gisondi, P.; Fostini, A.C.; Fossà, I.; Girolomoni, G.; Targher, G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 2018, 36, 21–28. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Vavricka, M.P.; Navarini, A.A.; French, L.E.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological order of appearance of extra intestinal manifestations relative to the time of IBD diagnosis in the Swiss inflammatory bowel disease cohort. Inflamm. Bowel Dis. 2015, 21, 1794–1800. [Google Scholar] [CrossRef]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Feagan, B.G.; Vermeire, S.; Panaccione, R.; Melmed, G.Y.; Blosch, C.; Newmark, R.; Zhang, N.; Chon, Y.; Lin, S.H.; et al. Mo2083 a randomized, placebo-controlled study to evaluate the safety, tolerability, and efficacy of AMG 827 in subjects with moderate to severe Crohn’s disease. Gastroenterology 2012, 143, e26. [Google Scholar] [CrossRef]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomized, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, K.; Leonardi, C.; Langley, R.G.; Warren, R.B.; Bachelez, H.; Romiti, R.; Ohtsuki, M.; Xu, W.; Acharya, N.; Solotkin, K.; et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: A presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J. Am. Acad. Dermatol. 2017, 76, 441–448.e2. [Google Scholar] [CrossRef] [Green Version]
- Wendling, D.; Joshi, A.; Reilly, P.; Jalundhwala, Y.J.; Mittal, M.; Bao, Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: An analysis of a large US claims database. Curr. Med. Res. Opin. 2014, 30, 2515–2521. [Google Scholar] [CrossRef]
- Tolu, S.; Rezvani, A.; Hindioglu, N.; Korkmaz, M.C. Etanercept-induced Crohn’s disease in ankylosing spondylitis: A case report and review of the literature. Rheumatol. Int. 2018, 38, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Nehring, P.; Przybylkowsk, A. Is psoriasis treatment a risk factor for inflammatory bowel disease? Pharmaceut. Med. 2020, 34, 257–262. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation and treatment of psoriasis. A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thysse, J.P.; Egeberg, A. Globl prevalence and bidirectional association between psoriasis and inflammatory bowel disease—A systematic review and meta-analysis. J. Crohns Colitis 2020, 14, 351–360. [Google Scholar] [CrossRef]
- Ljubenovic, M.; Lazarevic, V.; Golubovic, M.; Binic, I. Integrative Approach to Psoriasis Vulgaris. Holist. Nurs. Pract. 2018, 32, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Tonini, A.; Gualtieri, B.; Panduri, S.; Romanelli, M.; Chiricozzi, A. A new class of biologic agents facing the therapeutic paradigm in psoriasis: Anti-IL-23 agents. Expert Opin. Biol. Ther. 2018, 18, 135–148. [Google Scholar] [CrossRef]
- Micali, G.; Verzì, A.E.; Giuffrida, G.; Panebianco, E.; Musumeci, M.L.; Lacarrubba, F. Inverse Psoriasis: From Diagnosis to Current Treatment Options. Clin. Cosmet. Investig. Dermatol. 2019, 12, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, H.; Siegel, M.; Van Voorhees, A.S.; Merola, J.F. Treatment of Inverse/Intertriginous Psoriasis: Updated Guidelines from the Medical Board of the National Psoriasis Foundation. J. Drugs Dermatol. 2017, 16, 760–766. [Google Scholar] [PubMed]
- Singh, R.; Koppu, S.; Perche, P.O.; Feldman, S.R. The Cytokine Mediated Molecular Pathophysiology of Psoriasis and Its Clinical Implications. Int. J. Mol. Sci. 2021, 22, 12793. [Google Scholar] [CrossRef] [PubMed]
- Ladizinski, B.; Lee, K.C.; Wilmer, E.; Alavi, A.; Mistry, N.; Sibbald, R.G. A Review of the Clinical Variants and the Management of Psoriasis. Adv. Skin. Wound Care 2013, 26, 271–284. [Google Scholar] [CrossRef]
- Hoegler, K.M.; John, A.M.; Handler, M.Z.; Schwartz, R.A. Generalized pustular psoriasis: A review and update on treatment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1645–1651. [Google Scholar] [CrossRef]
- Wang, W.-M.; Jin, H.-Z. Biologics in the treatment of pustular psoriasis. Expert Opin. Drug Saf. 2020, 19, 969–980. [Google Scholar] [CrossRef]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; Li, K.; Methé, B.A.; Blaser, M.J. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Statnikov, A.; Alekseyenko, A.V.; Li, Z.; Henaff, M.; Perez-Perez, G.I.; Blaser, M.J.; Aliferis, C.F. Microbiomic Signatures of Psoriasis: Feasibility and Methodology Comparison. Sci. Rep. 2013, 3, 2620. [Google Scholar] [CrossRef]
- Takemoto, A.; Cho, O.; Morohoshi, Y.; Sugita, T.; Muto, M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J. Dermatol. 2015, 42, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmack, D.; Lee, K.; Afifim, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.-H.; Strober, B.E.; Pei, Z.; Blaser, M.J. Substantial Alterations of the Cutaneous Bacterial Biota in Psoriatic Lesions. PLoS ONE 2008, 3, e2719. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. S2), 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallen-Russell, C.; Wallen-Russell, S. Meta Analysis of Skin Microbiome: New Link between Skin Microbiota Diversity and Skin Health with Proposal to Use This as a Future Mechanism to Determine Whether Cosmetic Products Damage the Skin. Cosmetics 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Yerushalmi, M.; Elalouf, O.; Anderson, M.; Chandran, V. The skin microbiome in psoriatic disease: A systematic review and critical appraisal. J. Transl. Autoimmun. 2019, 2, 100009. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J.M.; Bruggermann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes 2014, 5, 201. [Google Scholar] [CrossRef]
- Lewis, D.J.; Chan, W.H.; Hinojosa, T.; Hsu, S.; Feldman, S.R. Mechanisms of microbial pathogenesis and the role of the skin microbiome in psoriasis: A review. Clin. Dermatol. 2019, 37, 160–166. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Shen, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 2018, 27, 144–149. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation I ape-/-mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhadou, F.; Minto, D.; Schnerbert, B.; Thio, H.B. Psoriasis and microbiota: A systematic review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Codoñer, F.M.; Ramírez-Bosca, A.; Climent, E.; Carrión-Gutierrez, M.; Guerrero, M.; Pérez-Orquín, J.M.; De La Parte, J.H.; Genovés, S.; Ramón, D.; Navarro-López, V.; et al. Gut microbial composition in patients with psoriasis. Sci. Rep. 2018, 8, 3812. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P.; Leclerc, M.; Hugot, J.P. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [Green Version]
- Eppinga, H.; Sperna Weiland, C.J.; Thio, H.B.; van der Woude, J.; Nijste, T.E.C.; Peppelenbosch, M.P.; Kostantinov, S.R. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease but not in hidradenitis suppurativa. J. Crohn Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. A Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Mary, J.-Y.; Lakatos, P.; D’Haens, G.; Moja, L.; D’Hoore, A.; Panes, J.; Reinisch, W.; Sandborn, W.J.; et al. Development of Red Flags Index for Early Referral of Adults with Symptoms and Signs Suggestive of Crohn’s Disease: An IOIBD Initiative. J. Crohn Colitis 2015, 9, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Roda, G.; Ng, S.C.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, T.W.; Barclay, M.L.; Gearry, R.B.; Frizelle, F.A. The Spectrum of Perianal Crohn’s Disease in a Population-Based Cohort. Dis. Colon Rectum 2012, 55, 773–777. [Google Scholar] [CrossRef]
- Ott, C.; Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef]
- Zallott, C.; Peyrin-Biroulet, L. Clinical risk factors for complicated disease: How reliable are they? Dig. Dis. 2012, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersading, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef] [Green Version]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current stat of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-troughput clone library analysis of the mucosa associated microbiota reveals dysbiosis and differences between inflamed and noninflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.-L.; Barnich, N.; Bringer, M.-A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.-F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Lapaquette, p.; Glasser, A.L.; Huett, A.; Xavier, R.J.; Darfeuille-Michaud, A. Crohn’s disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell. Microbiol. 2010, 12, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.; Roy, B.C.; Khan, S.A.; Septer, S.; Umar, S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Silva, S.; Sabino, J.; Valles-Colomer, M.; Falony, G.; Kathagen, G.; Caenepeel, C.; Cleynen, I.; Van der Merwe, S.; Vermeire, S.; Raes, J. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 2019, 4, 1826–1831. [Google Scholar] [CrossRef]
- Fujimoto, T.; Imaeda, H.; Takahashi, K.; Kasumi, E.; Bamba, S.; Fujiyama, Y.; Andoh, A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J. Gastroenterol. Hepatol. 2013, 28, 613–619. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Andoh, A.; Sugimoto, M. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Varela, e.; Manichanh, C.; Gallart, M.; Torrejon, A.; Borruel, N.; Casellas, F.; Guarner, F.; Antoli, M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 38, 151–161. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Loubinoux, J.; Bronowichi, J.P.; Pereira, I.A.C.; Mougenel, J.L.; Le Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zinchevich, V.V.; Beech, I.B. Screening of sulfate-reducing bacteria in colonoscopy samples from healthy and colitic human gut mucosa. FEMS Microbiol. Ecol. 2000, 34, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Coffey, J.C.; O’Connell, P.R. Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Dzutsev, A.; Goldszmid, R.S.; Viaud, S.; Zitvogel, L.; Trinchieri, G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur. J. Immunol. 2015, 45, 17–31. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.R.; Boland, C.R.; Patel, M.; Trash, B.; Menter, A. Cutaneous manifestations of gastrointestinal disease: Part I. J. Am. Acad. Dermatol. 2013, 68, 189. [Google Scholar] [CrossRef] [PubMed]
- Trash, B.; Patel, M.; Shah, K.R.; Boland, C.R.; Menter, A. Cutaneous manifestations of gastrointestinal disease: Part II. J. Am. Acad. Dermatol. 2013, 68, 211. [Google Scholar] [CrossRef]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic bacteria induce a “glow of health”. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef] [Green Version]
- Arck, p.; Handjiski, B.; Hagen, E.; Pincus, M.; Bruenahl, C.; Bienenstock, J.; Paus, R. Is there a “gut-brain-skin axis”? Exp. Dermatol. 2010, 19, 401–405. [Google Scholar] [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [Green Version]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teigen, L.M.; Geng, Z.; Sadowsky, M.J.; Vaughn, B.P.; Hamilton, M.J.; Khoruts, A. Dietary Factors in Sulfur Metabolism and Pathogenesis of Ulcerative Colitis. Nutrients 2019, 11, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.; Teft, W.A.; Morse, B.L.; Choi, Y.-H.; Woolsey, S.; DeGorter, M.K.; Hegele, R.A.; Tirona, R.G.; Kim, R.B. Trimethylamine-N-oxide: A Novel Biomarker for the Identification of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 3620–3630, Erratum in Dig. Dis. Sci. 2016, 61, 325. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Darlenski, R.; Hristakieva, E.; Aydin, U.; Gancheva, D.; Gancheva, T.; Zheleva, A.; Gadjeva, V.; Fluhr, J.W. Epidermal barrier and oxidative stress parameters improve during in 311 nm narrow band UVB phototherapy of plaque type psoriasis. J. Dermatol. Sci. 2018, 91, 28–34. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y., M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Vojvodic, A.; Peric-Hajzler, Z.; Matovic, D.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Sijan, G.; Dimitrijevic, S.; Stepic, N.; Wollina, U.; Badr, B.A.E.; et al. Gut Microbiota and the Alteration of Immune Balance in Skin Diseases: From Nutraceuticals to Fecal Transplantation. Open Access Maced. J. Med. Sci. 2019, 7, 3034–3038. [Google Scholar] [CrossRef] [Green Version]
- Ferran, M.; Romeu, E.R.; Rincon, C.; Sagristà, M.; Giménez Arnu, A.M.; Celada, A.; Pujol, R.; Hollo, P.; Jokaim, H.; Santamaria-Babì, L.F. Circulating CLA+ T lymphocytes as peripheral cell biomarkers in T-cell-mediated skin diseases. Exp. Dermatol. 2013, 22, 439–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus-Gil, C.; Ruiz-Romeu, E.; Ferran, M.; Chiriac, A.; Deza, G.; Holló, P.; Celada, A.; Pujol, R.M.; Santamaría-Babi, L.F. CLA+ T Cell Response to Microbes in Psoriasis. Front. Immunol. 2018, 9, 1488. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Fleming, C.D.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Conrad, C.C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid Predendritic Cells Initiate Psoriasis Through Interferon-Alpha Production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [Green Version]
- Maguire, M.; Maguire, G. The role of microbiota, and probiotics and prebiotics in skin health. Arch. Dermatol. Res. 2017, 309, 411–421. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masuoka, N.; Kano, M.; Iizuka, R. Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef. Microbes 2014, 5, 121–128. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut as a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability–A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integr. Biol. 2015, 7, 1339–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potgieter, M.; Bester, J.; Kell, D.B.; Pretorius, E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 2015, 39, 567–591. [Google Scholar] [CrossRef] [Green Version]
- Langan, E.A.; Griffiths, C.E.M.; Solbach, W.; Knobloch, J.K.; Zillikens, D.; Thaci, D. The Role of the Microbiome in Psoriasis: Moving from Disease Description to Treatment Selection? Br. J. Dermatol. 2018, 178, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
| Crohn’s Disease | Psoriasis | ||||||
|---|---|---|---|---|---|---|---|
| Increased * | Reference | Decreased § | Reference | Increased * | Reference | Decreased § | Reference |
| Escherichia coli | [83,84] | Bacteroides | [76,77,78,79,80,81,82] | Streptococcus pyogenes | [59] | Staphylococcus epidermidis | [54] |
| Enterococcus spp. | [87] | Clostridium clusters XIVa and IV | [76,77,78,79,80,81,82] | Staphylococcus aureus | [54] | Cutibacterium acnes | [54] |
| Fusobacterium spp. | [87] | Firmicutes prausnitzii | [87,88] | Malassezia spp. | [59] | Akkermansia muciniphila | [86,87] |
| Streptococcus spp. | [87] | Blautia faecis | [87,88] | Bacteroides | [65] | ||
| Veillonella spp. | [87] | Roseburia inulinivorans | [87,88] | Firmicutes praunitzii | [65,67] | ||
| Ruminococcus torques | [87,88] | ||||||
| Clostridium lavalense | [87,88] | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, M.A.; Caruso, A. The Gut Microbiome in Psoriasis and Crohn’s Disease: Is Its Perturbation a Common Denominator for Their Pathogenesis? Vaccines 2022, 10, 244. https://doi.org/10.3390/vaccines10020244
De Francesco MA, Caruso A. The Gut Microbiome in Psoriasis and Crohn’s Disease: Is Its Perturbation a Common Denominator for Their Pathogenesis? Vaccines. 2022; 10(2):244. https://doi.org/10.3390/vaccines10020244
Chicago/Turabian StyleDe Francesco, Maria Antonia, and Arnaldo Caruso. 2022. "The Gut Microbiome in Psoriasis and Crohn’s Disease: Is Its Perturbation a Common Denominator for Their Pathogenesis?" Vaccines 10, no. 2: 244. https://doi.org/10.3390/vaccines10020244
APA StyleDe Francesco, M. A., & Caruso, A. (2022). The Gut Microbiome in Psoriasis and Crohn’s Disease: Is Its Perturbation a Common Denominator for Their Pathogenesis? Vaccines, 10(2), 244. https://doi.org/10.3390/vaccines10020244







