Potential Applications of Microparticulate-Based Bacterial Outer Membrane Vesicles (OMVs) Vaccine Platform for Sexually Transmitted Diseases (STDs): Gonorrhea, Chlamydia, and Syphilis
Abstract
1. Introduction
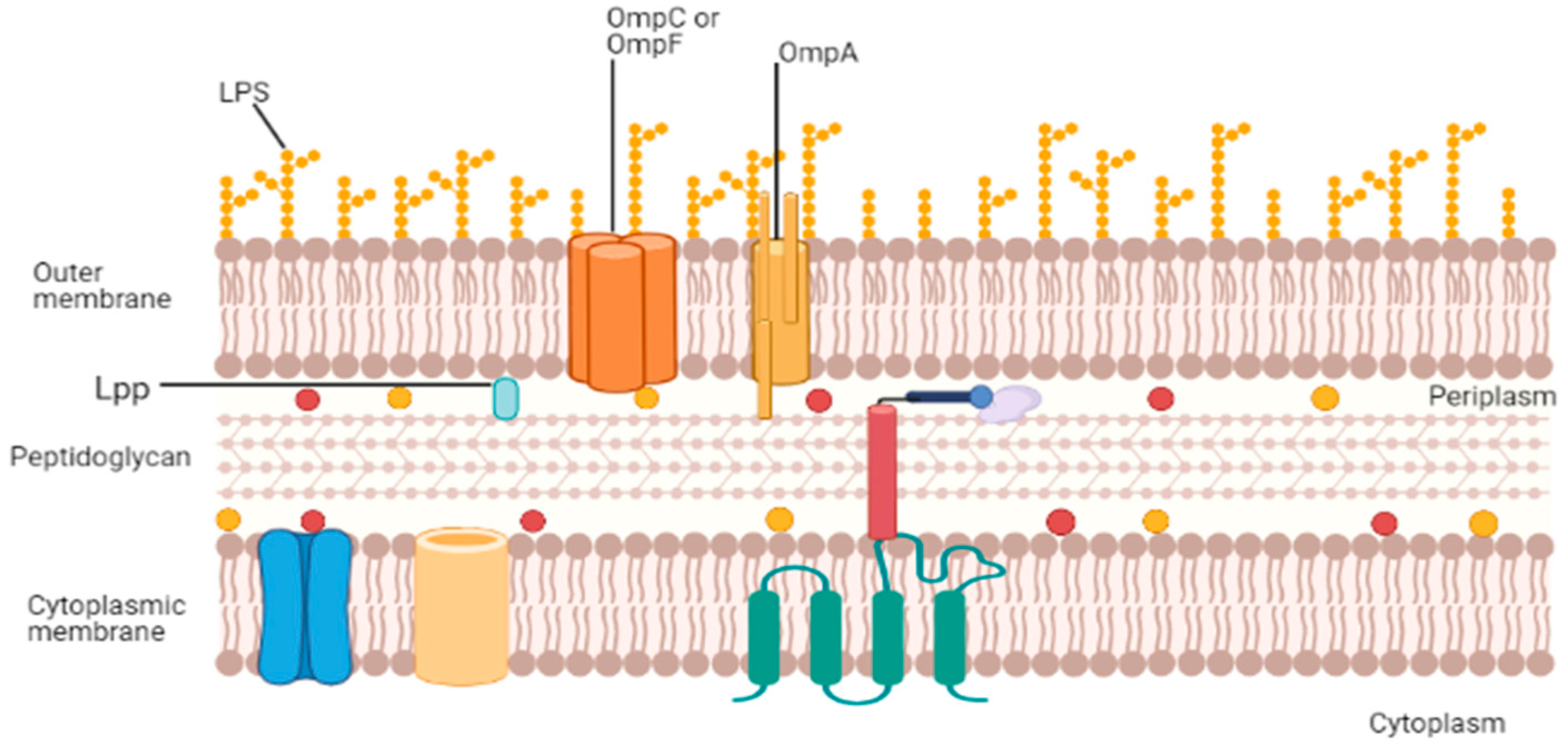
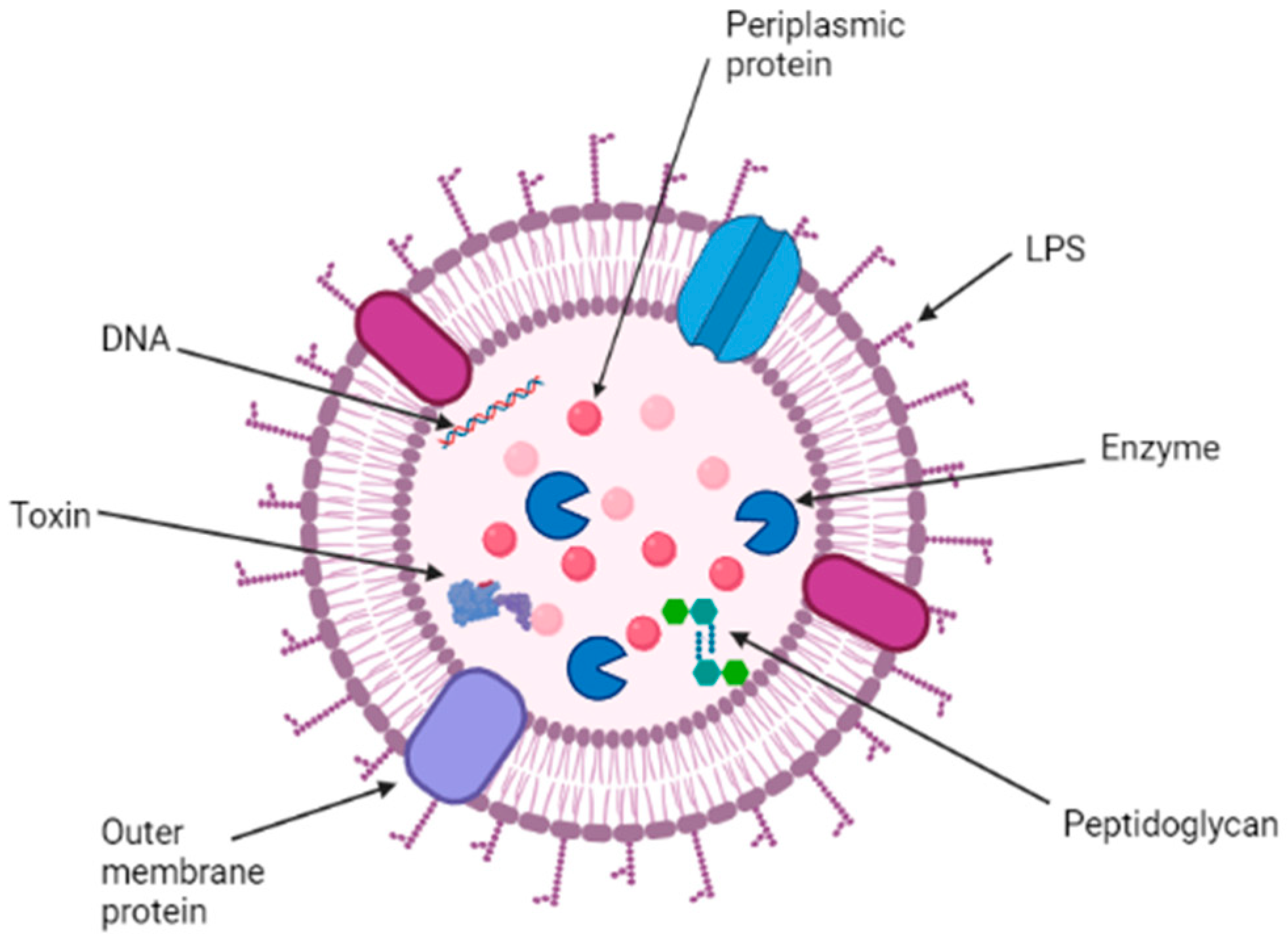
2. Structure, Function, and Composition of OMVs
3. Isolation of OMVs
4. OMVs as a Vaccine Candidate
5. Updates on the Pre-Clinical and Clinical Study on OMV-Based Vaccine Development
6. Particulate Delivery System of Outer Membrane Vesicles (OMVs)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC-STD Diseases & Related Conditions. Available online: https://www.cdc.gov/std/general/default.htm (accessed on 11 June 2021).
- Kirkcaldy, R.D.; Weston, E.; Segurado, A.C.; Hughes, G. Epidemiology of gonorrhoea: A global perspective. Sex. Health 2019, 16, 401–411. Available online: http://www.publish.csiro.au/?paper=SH19061 (accessed on 12 June 2021). [CrossRef]
- Table 3. Chlamydia—Reported Cases and Rates of Reported Cases by State/Territory and Region in Alphabetical Order, United States. 2015–2019. Available online: https://www.cdc.gov/std/statistics/2019/tables/3.htm (accessed on 11 June 2021).
- Schautteet, K.; De Clercq, E.; Vanrompay, D. Chlamydia trachomatis Vaccine Research through the Years. Infect. Dis. Obstet. Gynecol. 2011, 2011, 1–9. Available online: http://www.hindawi.com/journals/idog/2011/963513/ (accessed on 12 June 2021). [CrossRef]
- Chlamydia Infections Chlamydia Chlamydia Symptoms MedlinePlus. Available online: https://medlineplus.gov/chlamydiainfections.html (accessed on 11 June 2021).
- National Overview-Sexually Transmitted Disease Surveillance. 2019. Available online: https://www.cdc.gov/std/statistics/2019/overview.htm (accessed on 10 September 2021).
- STD Facts-Syphilis (Detailed). Available online: https://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm (accessed on 11 June 2021).
- McIntosh, E.D.G. Development of vaccines against the sexually transmitted infections gonorrhoea, syphilis, Chlamydia, herpes simplex virus, human immunodeficiency virus and Zika virus. Ther. Adv. VaccinesImmunother. 2020, 8, 2515135520923887. [Google Scholar] [CrossRef] [PubMed]
- Cyr, S.S.; Barbee, L.; Workowski, K.A.; Bachmann, L.H.; Pham, C.; Schlanger, K.; Torrone, E.; Weinstock, H.; Kersh, E.N.; Thorpe, P. Update to CDC’s Treatment Guidelines for Gonococcal Infection. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1911–1916. Available online: http://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm?s_cid=mm6950a6_w (accessed on 2 July 2021). [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. Available online: http://www.nature.com/articles/nrmicro3525 (accessed on 10 June 2021). [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. Available online: http://www.nature.com/articles/s41579-018-0112-2 (accessed on 11 June 2021). [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- McBroom, A.J.; Johnson, A.P.; Vemulapalli, S.; Kuehn, M.J. Outer Membrane Vesicle Production by Escherichia coli Is Independent of Membrane Instability. J. Bacteriol. 2006, 188, 5385–5392. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Sullivan, C.J.; Kuehn, M.J. Envelope Control of Outer Membrane Vesicle Production in Gram-Negative Bacteria. Biochemistry 2013, 52, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Maredia, R.; Devineni, N.; Lentz, P.; Dallo, S.F.; Yu, J.; Guentzel, N.; Chambers, J.; Arulanandam, B.; Haskins, W.E. Vesiculation from Pseudomonas aeruginosa under SOS. Sci. World J. 2012, 2012, 1–18. Available online: http://www.hindawi.com/journals/tswj/2012/402919/ (accessed on 14 June 2021). [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. Available online: http://journal.frontiersin.org/article/10.3389/fmicb.2017.01053/full (accessed on 14 June 2021). [CrossRef] [PubMed]
- Schooling, S.R.; Beveridge, T.J. Membrane Vesicles: An Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Schooling, S.R.; Hubley, A.; Beveridge, T.J. Interactions of DNA with Biofilm-Derived Membrane Vesicles. J. Bacteriol. 2009, 191, 4097–4102. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsumoto, T.; Tamura-Nakano, M.; Ozono, M.; Hashiguchi, S.; Suda, Y. Characterization of outer membrane vesicles of Acetobacter pasteurianus NBRC3283. J. Biosci. Bioeng. 2018, 125, 425–431. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1389172317303444 (accessed on 11 June 2021). [CrossRef] [PubMed]
- Solanki, K.S.; Pal, D.; Kaur, G.; Kumar, P.; Sahoo, M.; Chaudhuri, P. Isolation and Characterization of OMPs and OMVs of Brucella abortus S19 and Brucella abortus S19 per. J. Pure Appl. Microbiol. 2016, 10, 2121–2126. [Google Scholar]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochim. Biophys Acta-Mol. Cell Res. 2014, 1843, 1612–1619. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0167488913004382 (accessed on 11 June 2021). [CrossRef]
- Bauman, S.J.; Kuehn, M.J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006, 8, 2400–2408. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1286457906001808 (accessed on 11 June 2021). [CrossRef]
- Alves, N.J.; Turner, K.B.; DiVito, K.A.; Daniele, M.A.; Walper, S.A. Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a His-tag mutant. Res. Microbiol. 2017, 168, 139–146. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0923250816301176 (accessed on 12 June 2021). [CrossRef]
- Gnopo, Y.M.D.; Watkins, H.C.; Stevenson, T.C.; Delisa, M.P.; Putnam, D. Designer outer membrane vesicles as immunomodulatory systems-Reprogramming bacteria for vaccine delivery. Adv. Drug Deliv. Rev. 2017, 114, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–2018. Available online: http://www.nature.com/articles/nri3622 (accessed on 11 June 2021). [CrossRef] [PubMed]
- Nøkleby, H.; Aavitsland, P.; O’Hallahan, J.; Feiring, B.; Tilman, S.; Oster, P. Safety review: Two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine 2007, 25, 3080–3084. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0264410X0700045X (accessed on 11 June 2021). [CrossRef] [PubMed]
- van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. Available online: http://www.nature.com/articles/nri2868 (accessed on 12 June 2021). [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- Wu, X.; Lei, L.; Gong, S.; Chen, D.; Flores, R.; Zhong, G. The chlamydial periplasmic stress response serine protease cHtrA is secreted into host cell cytosol. BMC Microbiol. 2011, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. Available online: http://www.nature.com/articles/nrmicro.2016.30 (accessed on 11 June 2021). [CrossRef] [PubMed]
- Blanco, D.R.; Champion, C.I.; Dooley, A.; Cox, D.L.; Whitelegge, J.P.; Faull, K.; Lovett, M.A. A Monoclonal Antibody That Conveys In Vitro Killing and Partial Protection in Experimental Syphilis Binds a Phosphorylcholine Surface Epitope of Treponema pallidum. Infect. Immun. 2005, 73, 3083. [Google Scholar] [CrossRef]
- Parker, M.L.; Houston, S.; Wetherell, C.; Cameron, C.E.; Boulanger, M.J. The Structure of Treponema pallidum Tp0624 Reveals a Modular Assembly of Divergently Functionalized and Previously Uncharacterized Domains. Stevenson B, editor. PLoS ONE 2016, 11, e0166274. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Pennington, M.W.; Czerwinski, A.; Carter, D.; Zheng, B.; Nowak, N.A.; DeOliveira, R.B.; Shaughnessy, J.; Reed, G.W.; Ram, S.; et al. Preclinical Efficacy of a Lipooligosaccharide Peptide Mimic Candidate Gonococcal Vaccine. MBio 2019, 10, e02552-19. [Google Scholar] [CrossRef]
- Bartolini, E.; Ianni, E.; Frigimelica, E.; Petracca, R.; Galli, G.; Berlanda Scorza, F.; Norais, N.; Laere, D.; Giguti, F.; Pierleoni, A.; et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles 2013, 2, 20181. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A. Development of a Nanoemulsion-Based Vaccine for Chlamydia Infection. Available online: https://grantome.com/grant/NIH/R43-AI134168-01A1 (accessed on 22 October 2021).
- Blanco, D.R.; Champion, C.I.; Lovett, M.A. Use of the skin protection assay in experimental syphilis to assess protective immunity against a specific Treponema pallidum surface epitope. FEMS Microbiol. Lett. 2005, 249, 171–175. [Google Scholar] [CrossRef][Green Version]
- Petousis-Harris, H. Impact of meningococcal group B OMV vaccines, beyond their brief. Hum. Vaccin. Immunother. 2018, 14, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.; Del Campo, J.; Cuello, M.; González, E.; Nuñez, N.; Cabrera, O.; Llanes, R.; Acevedo, R.; Zayas, C.; Balboa, J.; et al. Mucosal approaches in Neisseria Vaccinology. Vaccimonitor 2009, 18, 53–55. [Google Scholar]
- Sexually Transmitted Infection (STI) Surveillance (Dashboard) ESR. Available online: https://www.esr.cri.nz/our-services/consultancy/public-health/sti/ (accessed on 22 October 2021).
- Paynter, J.; Goodyear-Smith, F.; Morgan, J.; Saxton, P.; Black, S.; Petousis-Harris, H. Effectiveness of a Group B Outer Membrane Vesicle Meningococcal Vaccine in Preventing Hospitalization from Gonorrhea in New Zealand: A Retrospective Cohort Study. Vaccines 2019, 7, 5. [Google Scholar] [CrossRef]
- Davenport, V.; Groves, E.; Horton, R.E.; Hobbs, C.G.; Guthrie, T.; Findlow, J.; Borrow, R.; Næss, L.M.; Oster, P.; Heyderman, R.S.; et al. Mucosal Immunity in Healthy Adults after Parenteral Vaccination with Outer-Membrane Vesicles from Neisseria meningitidis Serogroup B. J. Infect. Dis. 2008, 198, 731–740. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, K.-S.; Lee, S.-R.; Kim, E.; Kim, M.-S.; Lee, E.-Y.; Gho, Y.S.; Kim, J.-W.; Bishop, R.E.; Chang, K.-T. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim. Biophys Acta-Biomembr. 2009, 1788, 2150–2159. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0005273609002582 (accessed on 12 June 2021). [CrossRef] [PubMed]
- Ahmed, A.A.Q.; Qi, F.; Zheng, R.; Xiao, L.; Abdalla, A.M.; Mao, L.; Bakadia, B.M.; Liu, L.; Atta, O.M.; Li, X.; et al. The impact of ExHp-CD (outer membrane vesicles) released from Helicobacter pylori SS1 on macrophage RAW 264.7 cells and their immunogenic potential. Life Sci. 2021, 279, 119644. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0024320521006305 (accessed on 12 June 2021). [CrossRef]
- Harrell, J.E.; Kurtz, J.R.; Bauer, D.L.; Prior, J.T.; Gellings, P.S.; Morici, L.A.; McLachlan, J.B. An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection. Pathogens 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.B.; Prudencio, C.R.; De Gaspari, E. Experimental studies using OMV in a new platform of SARS-CoV-2 vaccines. Hum. Vaccines Immunother. 2021, 17, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Thomasin, C.; Corradin, G.; Men, Y.; Merkle, H.P.; Gander, B. Tetanus toxoid and synthetic malaria antigen containing poly(lactide)/poly(lactide-co-glycolide) microspheres: Importance of polymer degradation and antigen release for immune response. J. Control. Release 1996, 41, 131–145. Available online: https://linkinghub.elsevier.com/retrieve/pii/0168365996013636 (accessed on 12 June 2021). [CrossRef]
- Mallapragada, S.K.; Narasimhan, B. Immunomodulatory biomaterials. Int. J. Pharm. 2008, 364, 265–271. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18662761 (accessed on 12 June 2021). [CrossRef]
- Slütter, B.; Soema, P.C.; Ding, Z.; Verheul, R.; Hennink, W.; Jiskoot, W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J. Control. Release 2010, 143, 207–214. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0168365910000167 (accessed on 12 June 2021). [CrossRef] [PubMed]
- O’Hagan, D.T. Microparticles and polymers for the mucosal delivery of vaccines. Adv. Drug Deliv. Rev. 1998, 34, 305–320. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10837683 (accessed on 12 June 2021). [CrossRef]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21679733 (accessed on 12 June 2021). [CrossRef]
- Mönkäre, J.; Pontier, M.; van Kampen, E.E.; Du, G.; Leone, M.; Romeijn, S.; Nejadnik, M.R.; O’Mahony, C.; Slütter, B.; Jiskoot, W.; et al. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur. J. Pharm. Biopharm. 2018, 129, 111–121. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29803720 (accessed on 12 June 2021). [CrossRef]
- Bhowmik, T.; D’Souza, B.; Uddin, M.N.; D’Souza, M.J. Oral delivery of microparticles containing plasmid DNA encoding hepatitis-B surface antigen. J. Drug Target. 2012, 20, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Bielinska, A.U.; Kukowska-Latallo, J.F.; Baker, J.R. The interaction of plasmid DNA with polyamidoamine dendrimers: Mechanism of complex formation and analysis of alterations induced in nuclease sensitivity and transcriptional activity of the complexed DNA. Biochim. et Biophys. Acta (BBA)-Gene Struct. Expr. 1997, 1353, 180–190. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9294012 (accessed on 12 June 2021). [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24295808 (accessed on 12 June 2021). [CrossRef]
- Janes, K.; Calvo, P.; Alonso, M. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11251247 (accessed on 12 June 2021). [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22100638 (accessed on 12 June 2021). [CrossRef]
- Ali, O.A.; Lewin, S.A.; Dranoff, G.; Mooney, D.J. Vaccines Combined with Immune Checkpoint Antibodies Promote Cytotoxic T-cell Activity and Tumor Eradication. Cancer Immunol. Res. 2016, 4, 95–100. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26669718 (accessed on 12 June 2021). [CrossRef] [PubMed]
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28110170 (accessed on 12 June 2021). [CrossRef] [PubMed]
- O’Hagan, D.T.; Rahman, D.; McGee, J.P.; Jeffery, H.; Davies, M.; Williams, P.; Davis, S.S.; Challacombe, S. Biodegradable microparticles as controlled release antigen delivery systems. Immunology 1991, 73, 239–242. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2071168 (accessed on 12 June 2021). [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25656384 (accessed on 12 June 2021). [CrossRef] [PubMed]
- Uddin, M.N.; Kouzi, S.A.; Hussain, M.D. Strategies for Developing Oral Vaccines for Human Papillomavirus (HPV) Induced Cancer using Nanoparticle mediated Delivery System. J. Pharm. Pharm. Sci. 2015, 18, 220–234. Available online: https://journals.library.ualberta.ca/jpps/index.php/JPPS/article/view/22948 (accessed on 12 June 2021). [CrossRef] [PubMed]
- Balmert, S.; Little, S.R. Biomimetic Delivery with Micro- and Nanoparticles. Adv. Mater. 2012, 24, 3757–3778. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22528985 (accessed on 12 June 2021). [CrossRef]
- Gong, Y.-K.; Winnik, F.M. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale 2012, 4, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Wai, S.N.; Lindmark, B.; Söderblom, T.; Takade, A.; Westermark, M.; Oscarsson, J.; Jass, J.; Richter-Dahlfors, A.; Mizunoe, Y.; Uhlin, B.E. Vesicle-Mediated Export and Assembly of Pore-Forming Oligomers of the Enterobacterial ClyA Cytotoxin. Cell 2003, 115, 25–35. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14532000 (accessed on 12 June 2021). [CrossRef]
- Wu, G.; Ji, H.; Guo, X.; Li, Y.; Ren, T.; Dong, H.; Liu, J.; Liu, Y.; Shi, X.; He, B. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102148. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31887427 (accessed on 13 June 2021). [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chbib, C.; Shah, S.M.; Gala, R.P.; Uddin, M.N. Potential Applications of Microparticulate-Based Bacterial Outer Membrane Vesicles (OMVs) Vaccine Platform for Sexually Transmitted Diseases (STDs): Gonorrhea, Chlamydia, and Syphilis. Vaccines 2021, 9, 1245. https://doi.org/10.3390/vaccines9111245
Chbib C, Shah SM, Gala RP, Uddin MN. Potential Applications of Microparticulate-Based Bacterial Outer Membrane Vesicles (OMVs) Vaccine Platform for Sexually Transmitted Diseases (STDs): Gonorrhea, Chlamydia, and Syphilis. Vaccines. 2021; 9(11):1245. https://doi.org/10.3390/vaccines9111245
Chicago/Turabian StyleChbib, Christiane, Sarthak M. Shah, Rikhav P. Gala, and Mohammad N. Uddin. 2021. "Potential Applications of Microparticulate-Based Bacterial Outer Membrane Vesicles (OMVs) Vaccine Platform for Sexually Transmitted Diseases (STDs): Gonorrhea, Chlamydia, and Syphilis" Vaccines 9, no. 11: 1245. https://doi.org/10.3390/vaccines9111245
APA StyleChbib, C., Shah, S. M., Gala, R. P., & Uddin, M. N. (2021). Potential Applications of Microparticulate-Based Bacterial Outer Membrane Vesicles (OMVs) Vaccine Platform for Sexually Transmitted Diseases (STDs): Gonorrhea, Chlamydia, and Syphilis. Vaccines, 9(11), 1245. https://doi.org/10.3390/vaccines9111245






