Impact of COVID-19 Disruptions on Global BCG Coverage and Paediatric TB Mortality: A Modelling Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. BCG Disruption Data
2.2. BCG Disruption Magnitude
2.3. BCG Disruption Duration
2.4. Populations Affected by Disruption
2.5. Model Disruption Scenarios
2.6. Model
3. Results
3.1. BCG Disruption
3.2. Impact on Paediatric TB Mortality
4. Discussion
4.1. BCG in the Fight against Paediatric TB
4.2. Why Has BCG Been Impacted?
4.3. Other Threats to BCG Coverage
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation; Gavi; UNICEF. At Least 80 Million Children at Risk of Disease as COVID-19 Disrupts Vaccination Efforts, Warn Gavi, WHO and UNICEF. 2020. Available online: https://www.gavi.org/news/media-room/least-80-million-children-risk-disease-covid-19-disrupts-vaccination-efforts (accessed on 19 June 2020).
- Chiappini, E.; Parigi, S.; Galli, L.; Licari, A.; Brambilla, I.; Tosca, M.A.; Ciprandi, G.; Marseglia, G. Impact that the COVID-19 pandemic on routine childhood vaccinations and challenges ahead: A narrative review. Acta Paediatr. 2021, 110, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. UN, Department of Economic and Social Affairs, Population, Division, UNICEF. Geneva Palais Briefing Note on the Impact of COVID-19 Mitigation Measures on Vaccine Supply and Logistics. 2020. Available online: https://www.unicef.org/press-releases/geneva-palais-briefing-note-impact-covid-19-mitigation-measures-vaccine-supply-and (accessed on 19 June 2020).
- Abubakar, I.; Pimpin, L.; Ariti, C.; Beynon, R.; Mangtani, P.; Sterne, J.; Fine, P.E.M.; Smith, P.G.; Lipman, M.; Elliman, D.; et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette–Guérin vaccination against tuberculosis. Heal. Technol. Assess. 2013, 17, 1–372. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.; Lipman, M.; Whiting, P.; et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.; Sanderson, C. Timing of children’s vaccinations in 45 low-income and middle-income countries: An analysis of survey data. Lancet 2009, 373, 1543–1549. [Google Scholar] [CrossRef]
- Roy, P.; Vekemans, J.; Clark, A.; Sanderson, C.; Harris, R.C.; White, R.G. Potential effect of age of BCG vaccination on global paediatric tuberculosis mortality: A modelling study. Lancet Glob. Health 2019, 7, e1655–e1663. [Google Scholar] [CrossRef] [Green Version]
- Delamou, A.; El Ayadi, A.M.; Sidibe, S.; Delvaux, T.; Camara, B.S.; Sandouno, S.D.; Beavogui, A.H.; Rutherford, G.W.; Okumura, J.; Zhang, W.-H.; et al. Effect of Ebola virus disease on maternal and child health services in Guinea: A retrospective observational cohort study. Lancet Glob. Health 2017, 5, e448–e457. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.C.; Dodd, P.J.; White, R.G. The potential impact of BCG vaccine supply shortages on global paediatric tuberculosis mortality. BMC Med. 2016, 14, 138. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.C.; Chen, Y.; Côte, P.; Ardillon, A.; Nievera, M.C.; Ong-Lim, A.; Aiyamperumal, S.; Chong, C.P.; Kandasamy, K.V.; Mahenthiran, K.; et al. Impact of COVID-19 on routine immunisation in South-East Asia and Western Pacific: Disruptions and solutions. Lancet Reg. Health West. Pac. 2021, 10, 100140. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Pulse Survey on Continuity of Essential Health Services during the COVID-19 Pandemic: Interim Report. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1 (accessed on 19 June 2020).
- Chandir, S.; Siddiqi, D.A.; Setayesh, H.; Khan, A.J. Impact of COVID-19 lockdown on routine immunisation in Karachi, Pakistan. Lancet Glob. Health 2020, 8, e1118–e1120. [Google Scholar] [CrossRef]
- Masresha, B.G.; Jr, R.L.; Shibeshi, M.E.; Ntsama, B.; Ndiaye, A.; Chakauya, J.; Poy, A.; Mihigo, R. The performance of routine immunization in selected African countries during the first six months of the COVID-19 pandemic. Pan Afr. Med J. 2020, 37. [Google Scholar] [CrossRef]
- Thysen, S.M.; Fisker, A.B.; Byberg, S.; Aaby, P.; Roy, P.; White, R.; Griffiths, U.; Harris, R.C. Disregarding the restrictive vial-opening policy for BCG vaccine in Guinea-Bissau: Impact and cost-effectiveness for tuberculosis mortality and all-cause mortality in children aged 0–4 years. BMJ Glob. Health 2021, 6, e006127. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. United Nations, Department of Economic and Social Affairs, Population, Division, UNICEF. Maternal and Newborn Health Coverage Database. 2021. Available online: https://data.unicef.org/topic/maternal-health/delivery-care/ (accessed on 19 June 2021).
- The BCG Atlas. A Database of Global BCG Vaccination Policies and Practices. The BCG World Atlas. 2020. Available online: http://www.bcgatlas.org/ (accessed on 24 June 2021).
- United Nations, Department of Economic and Social Affairs, Population. World Population Prospects: The 2019 Revision. 2019. Available online: https://esa.un.org/unpd/wpp/Download/Standard/Mortality/ (accessed on 24 June 2021).
- World Health Organisation. Global Tuberculosis Report 2020. Geneva. 2020. Available online: https://www.who.int/publications-detail-redirect/9789240013131 (accessed on 20 October 2021).
- Dodd, P.J.; Yuen, C.; Sismanidis, C.; Seddon, J.; E Jenkins, H. The global burden of tuberculosis mortality in children: A mathematical modelling study. Lancet Glob. Heal. 2017, 5, e898–e906. [Google Scholar] [CrossRef] [Green Version]
- Syakriah, A. Government Drives Vaccination to Prevent Rise in Childhood Diseases Amid COVID-19. The Jakarta Post. 2020. Available online: https://www.thejakartapost.com/news/2020/07/20/government-drives-vaccination-to-prevent-rise-in-childhood-diseases-amid-covid-19.html (accessed on 24 June 2021).
- Rukmini, S. COVID-19 Disrupted India’s Routine Health Services. IndiaSpend. 2020. Available online: https://www.indiaspend.com/covid-19-disrupted-indias-routine-health-services/ (accessed on 24 June 2021).
- Buonsenso, D.; Cinicola, B.; Kallon, M.N.; Iodice, F. Child Healthcare and Immunizations in Sub-Saharan Africa During the COVID-19 Pandemic. Front. Pediatr. 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. UNICEF Hails Immunization Progress in Bangladesh as Monthly Uptake Surpasses pre-COVID-19 Levels. Bangladesh. 2020. Available online: https://www.unicef.org/bangladesh/en/press-releases/unicef-hails-immunization-progress-bangladesh-monthly-uptake-surpasses-pre-covid-19 (accessed on 30 June 2021).
- Thysen, S.M.; Byberg, S.; Pedersen, M.; Rodrigues, A.; Ravn, H.; Martins, C.; Benn, C.S.; Aaby, P.; Fisker, A.B. BCG coverage and barriers to BCG vaccination in Guinea-Bissau: An observational study. BMC Public Health 2014, 14, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliori, G.B.; Thong, P.M.; Akkerman, O.; Alffenaar, J.-W.; Álvarez-Navascués, F.; Assao-Neino, M.M.; Bernard, P.V.; Biala, J.S.; Blanc, F.-X.; Bogorodskaya, E.M.; et al. Worldwide Effects of Coronavirus Disease Pandemic on Tuberculosis Services, January–April 2020. Emerg. Infect. Dis. 2020, 26, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Impact of the COVID-19 Pandemic on TB Detection and Mortality in 2020. 2021. Available online: https://www.who.int/publications/m/item/impact-of-the-covid-19-pandemic-on-tb-detection-and-mortality-in-2020 (accessed on 19 June 2021).
- McQuaid, C.F.; McCreesh, N.; Read, J.; Sumner, T.; Houben, R.M.G.J.; White, R.G.; Harris, R.C. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur. Respir. J. 2020, 56, 2001718. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Soares-Weiser, K.; López, J.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, i5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prentice, S.; Nassanga, B.; Webb, E.L.; Akello, F.; Kiwudhu, F.; Akurut, H.; Elliott, A.M.; Arts, R.J.W.; Netea, M.G.; Dockrell, H.M.; et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: An investigator-blind randomised controlled trial. Lancet Infect. Dis. 2021, 21, 993–1003. [Google Scholar] [CrossRef]
- Lazzerini, M.; Barbi, E.; Apicella, A.; Marchetti, F.; Cardinale, F.; Trobia, G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health 2020, 4, e10–e11. [Google Scholar] [CrossRef]
- Beaumont, P. Delta Variant of Covid Spreading Rapidly and Detected in 74 Countries. The Guardian. 2021. Available online: https://www.theguardian.com/world/2021/jun/14/delta-variant-of-covid-spreading-rapidly-and-detected-in-74-countries (accessed on 19 June 2021).
- Ritchie, H.; Ortiz-Ospina, E.; Beltekian, D.; Mathieu, E.; Hasell, J.; Macdonald, B. Coronavirus Pandemic (COVID-19). OurWorldInData.org. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 24 June 2021).
- Durrheim, D.N.; Andrus, J.K.; Tabassum, S.; Bashour, H.; Githanga, D.; Pfaff, G. A dangerous measles future looms beyond the COVID-19 pandemic. Nat. Med. 2021, 27, 360–361. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. COVID-19 Impact Assessment and Outlook on Global Logistics. 2020. Available online: https://www.unicef.org/supply/documents/covid-19-impact-assessment-and-outlook-global-logistics (accessed on 24 June 2021).
- UNICEF. Supply Division. Bacillus Calmette-Guérin (BCG) Supply and Demand Update. August 2019. Available online: https://www.unicef.org/supply/reports/bacillus-calmette-gu%C3%A9rin-bcg-supply-and-demand-update (accessed on 24 June 2021).
- UNICEF. Supply Division. Bacillus Calmette-Guérin (BCG): Supply Alert. December 2019. Available online: https://www.unicef.org/supply/reports/bacillus-calmette-gu%C3%A9rin-bcg-supply-and-demand-update (accessed on 24 June 2021).
- Dunleavy, K. ‘Very stressed’ Serum Institute Asks Government for $400M Vaccine Production Boost. Fierce Pharma. 2021. Available online: https://www.fiercepharma.com/manufacturing/very-stressed-serum-institute-india-asks-government-for-vaccine-production-boost (accessed on 24 June 2021).
- Safi, M. Indian Expansion of Covid Vaccine Drive May Further Strain Supplies. The Guardian. 2021. Available online: https://www.theguardian.com/world/2021/apr/19/indian-expansion-of-covid-vaccine-drive-may-further-strain-supplies (accessed on 24 June 2021).
- Curtis, N.; Sparrow, A.; A Ghebreyesus, T.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
- Tsilika, M.; Taks, E.; Dolianitis, K.; Kotsaki, A.; Leventogiannis, K.; Damoulari, C. Activate-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. medRxiv 2021. [CrossRef]
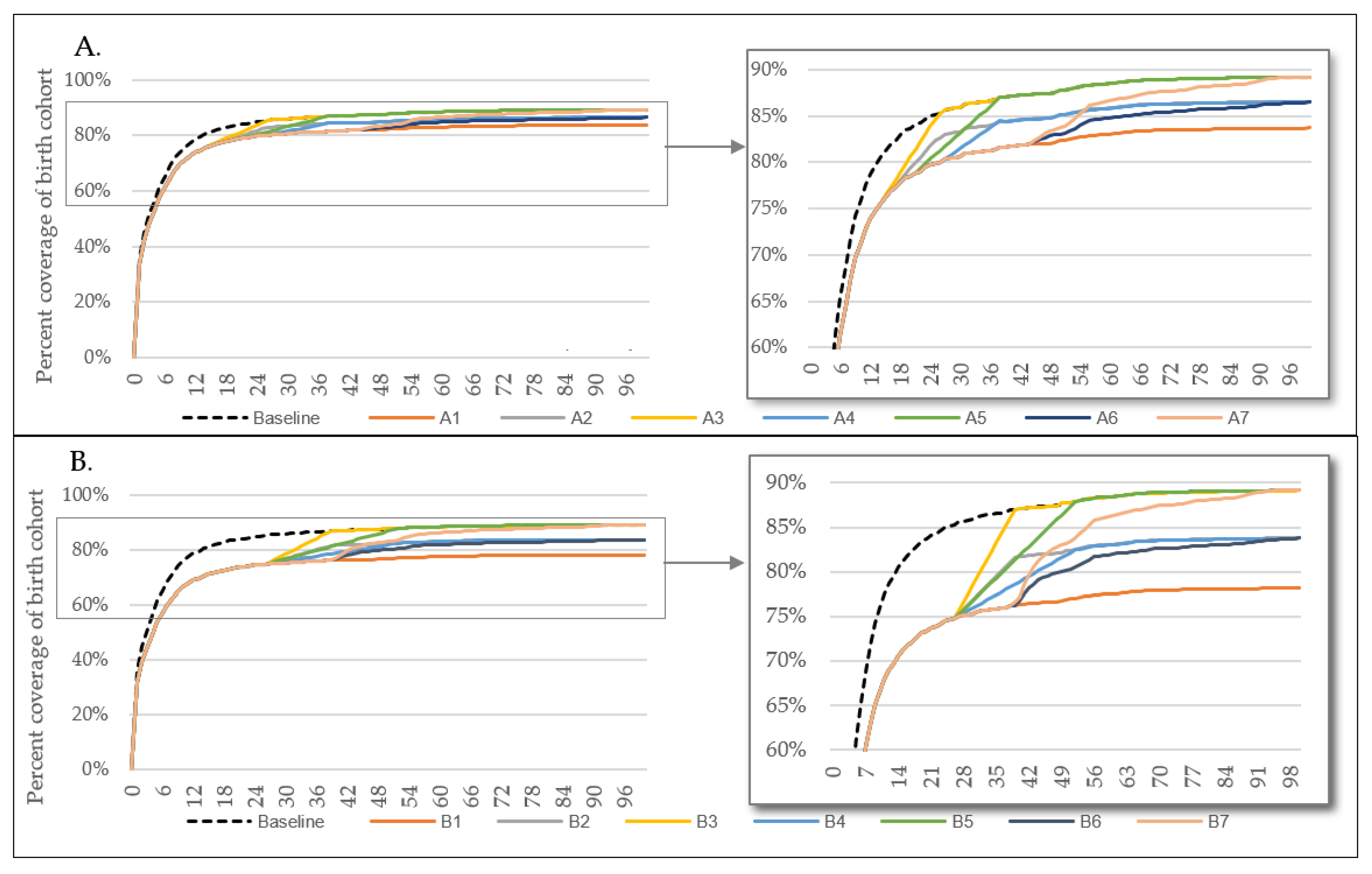
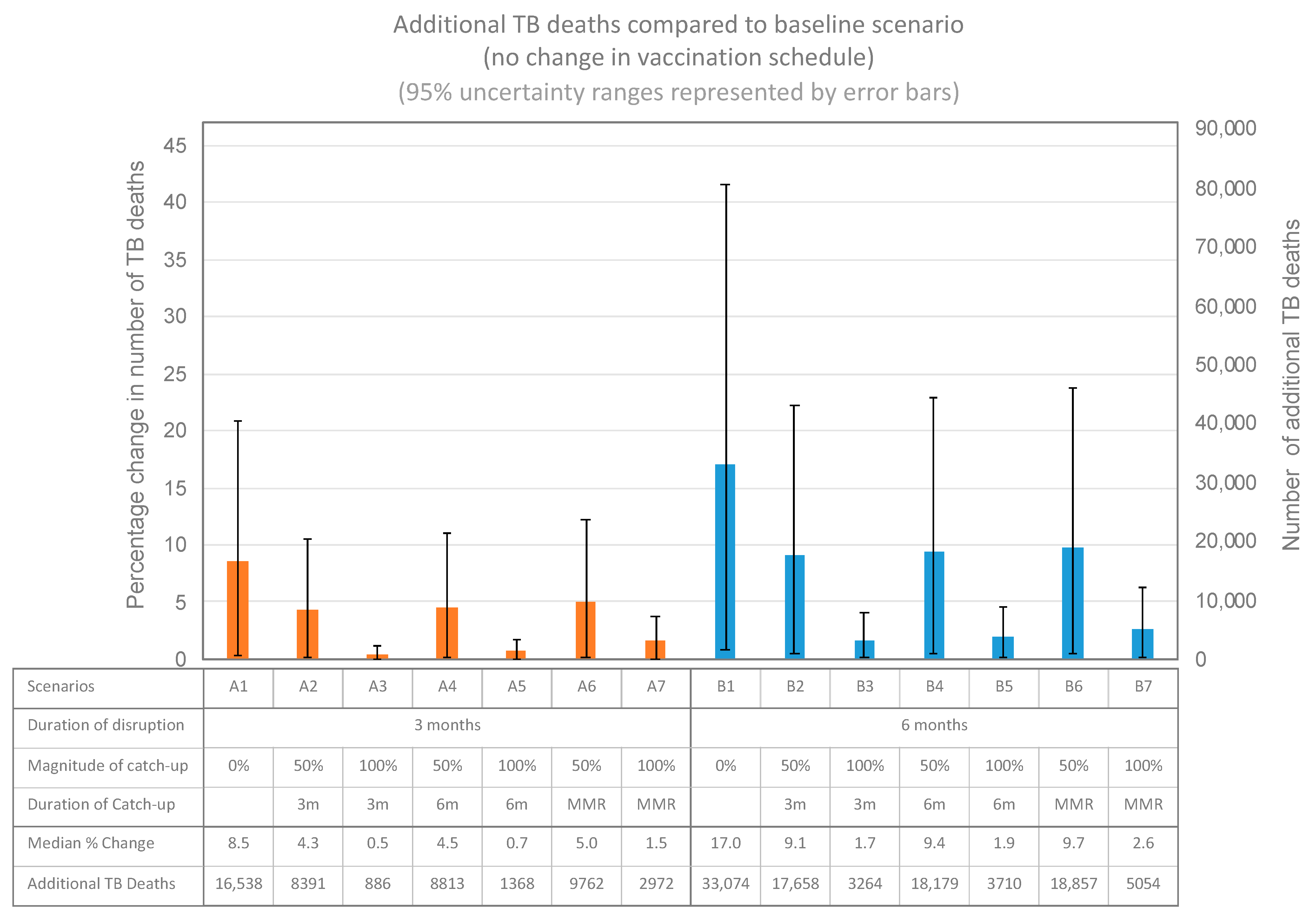
| Scenario | A | B |
|---|---|---|
| Disruption period | Disruption period: 3 m (e.g., April 2020 to June 2020) | Disruption period: 6 m (e.g., April 2020 to September 2020) |
| Catch-up strategy | 1. None 2. Catch-up: 50% of deficit–in 3 months 3. Catch-up: 100% of deficit–in 3 months 4. Catch-up: 50% of deficit–in 6 months 5. Catch-up: 100% of deficit–in 6 months 6. Catch-up: 50% of deficit–timed with MMR 1 7. Catch-up: 100% of deficit–timed with MMR 1 | 1. None 2. Catch-up: 50% of deficit–in 3 months 3. Catch-up: 100% of deficit–in 3 months 4. Catch-up: 50% of deficit–in 6 months 5. Catch-up: 100% of deficit–in 6 months 6. Catch-up: 50% of deficit–timed with MMR 1 7. Catch-up: 100% of deficit–timed with MMR 1 |
| Parameters for Uncertainty Analysis | Point Estimate | Uncertainty Interval | Reference |
|---|---|---|---|
| Vaccine efficacy against tuberculosis death | 0.66 | 0.08–0.88 | [4] |
| HIV-negative paediatric male tuberculosis deaths in 2019 | 104,000 | 93,000–115,000 | [18] |
| HIV-negative paediatric female tuberculosis deaths in 2019 | 90,000 | 80,000–99,000 | [18] |
| Tuberculosis deaths in children younger than 5 years who had not received tuberculosis treatment in 2015 (HIV negative) | 161,000 | 108,000–223,000 | [19] |
| Tuberculosis deaths in children younger than 5 years who had received tuberculosis treatment in 2015 (HIV negative) | 2690 | 1850–4180 | [19] |
| Tuberculosis deaths in children aged 5–15 years who had not received tuberculosis treatment in 2015 (HIV negative) | 31,500 | 18,600–51,400 | [19] |
| Tuberculosis deaths in children aged 5–15 years who had received tuberculosis treatment in 2015 (HIV negative) | 2050 | 1510–3100 | [19] |
| Country | Percentage of Global BCG 1 Vaccine Coverage Delivered by Each Country (2019) | Reduction in BCG 1 Coverage during Disruption (2020 vs. 2019) | Reduction in Coverage of Routine Paediatric Immunisations during Disruption (2020 vs. 2019) | References |
|---|---|---|---|---|
| India | 19.6% | 50% | [21] | |
| China | 14.5% | 0% | [10] | |
| Pakistan | 4.6% | 41% | [12] | |
| Indonesia | 3.8% | 26% | [20] | |
| Bangladesh | 2.5% | 96% | Country source | |
| Democratic Republic of Congo | 2.2% | 0% | [13] | |
| Brazil | 2.0% | 0% | Country source | |
| Philippines | 1.4% | 0% | [10] | |
| Other countries (n = 21) 2 | 13.2% | 8% 3 | ||
| WEIGHTED AVERAGE: | 25% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, N.; Pelzer, P.T.; Thysen, S.M.; Roy, P.; Harris, R.C.; White, R.G. Impact of COVID-19 Disruptions on Global BCG Coverage and Paediatric TB Mortality: A Modelling Study. Vaccines 2021, 9, 1228. https://doi.org/10.3390/vaccines9111228
Shaikh N, Pelzer PT, Thysen SM, Roy P, Harris RC, White RG. Impact of COVID-19 Disruptions on Global BCG Coverage and Paediatric TB Mortality: A Modelling Study. Vaccines. 2021; 9(11):1228. https://doi.org/10.3390/vaccines9111228
Chicago/Turabian StyleShaikh, Nabila, Puck T. Pelzer, Sanne M. Thysen, Partho Roy, Rebecca C. Harris, and Richard G. White. 2021. "Impact of COVID-19 Disruptions on Global BCG Coverage and Paediatric TB Mortality: A Modelling Study" Vaccines 9, no. 11: 1228. https://doi.org/10.3390/vaccines9111228
APA StyleShaikh, N., Pelzer, P. T., Thysen, S. M., Roy, P., Harris, R. C., & White, R. G. (2021). Impact of COVID-19 Disruptions on Global BCG Coverage and Paediatric TB Mortality: A Modelling Study. Vaccines, 9(11), 1228. https://doi.org/10.3390/vaccines9111228







