The Involvement of CXC Motif Chemokine Ligand 10 (CXCL10) and Its Related Chemokines in the Pathogenesis of Coronary Artery Disease and in the COVID-19 Vaccination: A Narrative Review
Abstract
1. Introduction
2. Biostructure and Functions of CXCL10
3. CXCL10 Signal Transduction
4. CXCL10 and CAD
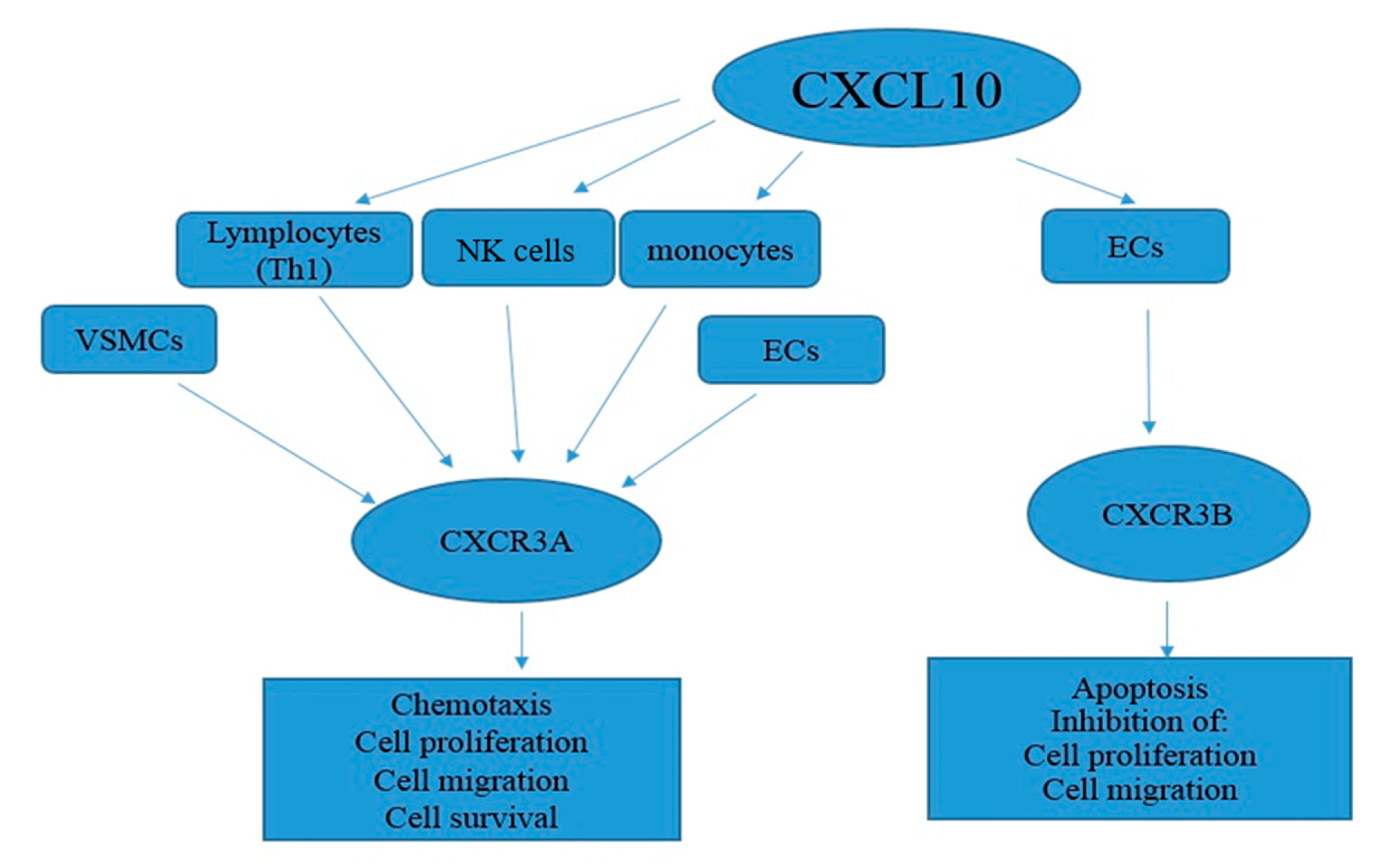
4.1. The Role of CXCL10-Expressing Cells in CAD
4.2. Mechanism of the CXCL10 Action in CAD
4.3. CXCL10 Implications in CAD
5. CXCL10 Targeting in Various Cardiovascular Diseases
6. CXCL10 and Immune Responses in the COVID-19 Vaccination
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| APOE | Apolipoprotein E |
| BMP | Bone morphogenetic protein |
| CVD | Cardiovascular disease |
| CCK8 | Cholecystokinin 8 |
| CD4 | Clusterof differentiation 4 |
| CAC | Coronary artery calcification |
| CAD | Coronary artery disease |
| CCL5 | C-C motif chemokine ligand 5 |
| CHD | Coronary heart disease |
| CTCs | Cytotoxic T cells |
| CTL | Cytotoxic T lymphocyte |
| CXCL10 | CXC motif chemokine ligand 10 |
| COVID-19 | Coronavirus disease |
| ELR | Enzyme-linked receptor |
| ECM | Extracellular matrix |
| FOXP3 | Fork head box P3 |
| GPCRs | G-protein-coupled receptors |
| HCASMCs | Human coronary artery smooth muscle cells |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IHG | Ischemic heart disease |
| IFN | Interferon |
| IL-4 | Interleukin 4 |
| IgG1 | Immunoglobulin G1 |
| IP-10/CXLC10 | Induced protein 10/CXLC10 |
| ISRE | Interferon-stimulated response element |
| INSRE | Interferon-stimulated response element |
| LPS | Lipopolysaccharides |
| LDL | Low density lipoprotein |
| mRNA | Messenger ribonucleic acid |
| NK | Natural killer |
| NF-κB | Nuclear factor-κB |
| PBMC | Peripheral blood mononuclear cells |
| pMHCII | Peptides bound to major histocompatibility complex class II |
| PTCA | Percutaneous transluminal coronary angioplasty |
| PPAR | Peroxisome proliferator-activated receptor |
| PDTC | Pyrrolidine dithiocarbamate |
| PKC | Protein kinase C |
| PD3K | Phosphatidylinositide 3-kinase |
| RANTES | Regulated upon activation, normal T cell expressed and secreted |
| RT-PCR | Reverse transcription polymerase chain reaction |
| ROC | Receiver operating characteristic |
| STAT1α | Signal transducer and activator of transcription 1α |
| Treg cells | Regulatory T cells |
| TIV | Trivalent influenza vaccine |
| ΤΡ1 | T regulatory type 1 |
| TNF-α | Tumor necrosis factor α |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VSMC | Vascular smooth muscle cells |
| WBC | White blood count |
References
- Dahlof, B. Cardiovascular disease risk factors: Epidemiology and risk assessment. Am. J. Cardiol. 2010, 105, 3a–9a. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M. Cardiovascular risk prediction: Basic concepts, current status, and future directions. Circulation 2010, 121, 1768–1777. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Benetos, G.; Buechel, R.R.; Goncalves, M.; Benz, D.C.; von Felten, E.; Rampidis, G.P.; Clerc, O.F.; Messerli, M.; Giannopoulos, A.A.; Gebhard, C.; et al. Coronary artery volume index: A novel CCTA-derived predictor for cardiovascular events. Int. J. Cardiovasc. Imaging 2020, 36, 713–722. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, B.; Zhao, Y.; Zhang, Z. Prognostic value of adiponectin level in patients with coronary artery disease: A systematic review and meta-analysis. Lipids Health Dis. 2019, 18, 227. [Google Scholar] [CrossRef]
- Ow, K.W.; Parker, W.A.E.; Porter, M.M.; Hanson, J.; Judge, H.M.; Briffa, N.P.; Thomas, M.R.; Storey, R.F. Offset of ticagrelor prior to coronary artery bypass graft surgery for acute coronary syndromes: Effects on platelet function and cellular adenosine uptake. Platelets 2020, 31, 945–951. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; Cannon, C.P.; Murphy, S.A.; Demopoulos, L.A.; DiBattiste, P.M.; McCabe, C.H.; Braunwald, E.; Gibson, C.M. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: A TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy- Thrombolysis in Myocardial Infarction 18 trial)substudy. J. Am. Coll. Cardiol. 2002, 40, 1761–1768. [Google Scholar]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.; Sciacca, R.; Boden-Albala, B.; Homma, S.; Di Tullio, M.R. Leukocyte count is associated with aortic arch plaque thickness. Stroke 2002, 33, 2587–2592. [Google Scholar] [CrossRef]
- Noroozi Karimabad, M.; Khanamani Falahati-Pour, S.; Hassanshahi, G. Significant Role(s) of CXCL12 and the SDF-1 3’A Genetic Variant in the Pathogenesis of Multiple Sclerosis. Neuroimmunomodulation 2016, 23, 197–208. [Google Scholar] [CrossRef]
- Darakhshan, S.; Fatehi, A.; Hassanshahi, G.; Mahmoodi, S.; Hashemi, M.S.; Karimabad, M.N. Serum concentration of angiogenic (CXCL1, CXCL12) and angiostasis (CXCL9, CXCL10) CXC chemokines are differentially altered in normal and gestational diabetes mellitus associated pregnancies. J. Diabetes Metab. Disord. 2019, 18, 371–378. [Google Scholar] [CrossRef]
- Karimabad, M.N.; Hassanshahi, G. Significance of CXCL12 in type 2 diabetes mellitus and its associated complications. Inflammation 2015, 38, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Karin, N.; Razon, H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Shields, P.L.; Morland, C.M.; Salmon, M.; Qin, S.; Hubscher, S.G.; Adams, D.H. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 1999, 163, 6236–6243. [Google Scholar]
- Ciesielski, C.J.; Andreakos, E.; Foxwell, B.M.; Feldmann, M. TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion. Eur. J. Immunol. 2002, 32, 2037–2045. [Google Scholar] [CrossRef]
- Darakhshan, S.; Hassanshahi, G.; Mofidifar, Z.; Soltani, B.; Karimabad, M.N. CXCL9/CXCL10 angiostasis CXC-chemokines in parallel with the CXCL12 as an angiogenesis CXC-chemokine are variously expressed in pre-eclamptic-women and their neonates. Pregnancy Hypertens. 2019, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.F., 3rd; McGary, K.L.; Schlager, J.J. An inhibitor of p38 MAP kinase downregulates cytokine release induced by sulfur mustard exposure in human epidermal keratinocytes. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2004, 18, 593–599. [Google Scholar] [CrossRef]
- Ohmori, Y.; Hamilton, T.A. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J. Immunol. 1995, 154, 5235–5244. [Google Scholar]
- Ge, M.Q.; Ho, A.W.; Tang, Y.; Wong, K.H.; Chua, B.Y.; Gasser, S.; Kemeny, D.M. NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza A infection by IFN-gamma and perforin-dependent mechanisms. J. Immunol. 2012, 189, 2099–2109. [Google Scholar] [CrossRef]
- Ohmori, Y.; Hamilton, T.A. Cell type and stimulus specific regulation of chemokine gene expression. Biochem. Biophys. Res. Commun. 1994, 198, 590–596. [Google Scholar] [CrossRef]
- Zeissig, S.; Murata, K.; Sweet, L.; Publicover, J.; Hu, Z.; Kaser, A.; Bosse, E.; Iqbal, J.; Hussain, M.M.; Balschun, K.; et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat. Med. 2012, 18, 1060–1068. [Google Scholar] [CrossRef]
- Treacy, O.; Ryan, A.E.; Heinzl, T.; O’Flynn, L.; Cregg, M.; Wilk, M.; Odoardi, F.; Lohan, P.; O’Brien, T.; Nosov, M.; et al. Adenoviral transduction of mesenchymal stem cells: In vitro responses and in vivo immune responses after cell transplantation. PLoS ONE 2012, 7, e42662. [Google Scholar] [CrossRef]
- Hassanshahi, G.; Patel, S.S.; Jafarzadeh, A.A.; Dickson, A.J. Expression of CXC chemokine IP-10/Mob-1 by primary hepatocytes following heat shock. Saudi Med. J. 2007, 28, 514–518. [Google Scholar]
- Rotondi, M.; Chiovato, L.; Romagnani, S.; Serio, M.; Romagnani, P. Role of chemokines in endocrine autoimmune diseases. Endocr. Rev. 2007, 28, 492–520. [Google Scholar] [CrossRef]
- Ha, Y.; Liu, H.; Zhu, S.; Yi, P.; Liu, W.; Nathanson, J.; Kayed, R.; Loucas, B.; Sun, J.; Frishman, L.J.; et al. Critical role of the CXCL10/CXC chemokine receptor 3 axis in promoting leukocyte recruitment and neuronal injury during traumatic optic neuropathy induced by optic nerve crush. Am. J. Pathol. 2017, 187, 352–365. [Google Scholar] [CrossRef] [PubMed]
- David, B.A.; Kubes, P. Exploring the complex role of chemokines and chemoattractants in vivo on leukocyte dynamics. Immunol. Rev. 2019, 289, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Varney, M.; Singh, R.K. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009, 69, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Johrer, K.; Pleyer, L.; Olivier, A.; Maizner, E.; Zelle-Rieser, C.; Greil, R. Tumour-immune cell interactions modulated by chemokines. Expert Opin. Biol. Ther. 2008, 8, 269–290. [Google Scholar] [CrossRef]
- Jamali, Z.; Nazari, M.; Khoramdelazad, H.; Hakimizadeh, E.; Mahmoodi, M.; Karimabad, M.N.; Hassanshahi, G.; Rezaeian, M.; Balaei, P.; Darakhshan, S.; et al. Expression of CC chemokines CCL2, CCL5, and CCL11 is associated with duration of disease and complications in type-1 diabetes: A study on Iranian diabetic patients. Clin. Lab. 2013, 59, 993–1001. [Google Scholar] [CrossRef]
- Derakhshan, R.; Arababadi, M.K.; Ahmadi, Z.; Karimabad, M.N.; Salehabadi, V.A.; Abedinzadeh, M.; Khorramdelazad, H.; Balaei, P.; Kennedy, D.; Hassanshahi, G. Increased circulating levels of SDF-1 (CXCL12) in type 2 diabetic patients are correlated to disease state but are unrelated to polymorphism of the SDF-1beta gene in the Iranian population. Inflammation 2012, 35, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, F.; Mollahosseini, M.; Hassanshahi, G.; Falahati-Pour, S.K.; Khorramdelazad, H.; Ahmadi, Z.; Farahmand, H. CC chemokines CCL2, CCL3, CCL4 and CCL5 are elevated in osteoporosis patients. J. Biomed. Res. 2017, 31, 468–470. [Google Scholar]
- Arababadi, M.K.; Aminzadeh, F.; Hassanshahi, G.; Khorramdelazad, H.; Norouzi, M.; Zarandi, E.R.; Rezayati, M.; Kennedy, D. Cytokines in preterm delivery. Lab. Med. 2012, 43, 27–30. [Google Scholar] [CrossRef]
- Ostadebrahimi, H.; Jamali, Z.; Nazari, M.; Bahri, M.; Farahmandnia, Z.; Khandany, B.K.; Taheri, M.; Khorramdelazad, H.; Hakimizadeh, E.; Zaker, F.; et al. CXC chemokines CXCL1, CXCL9, CXCL10 and CXCL12 are variably expressed in patients with sickle cell disease and carriers: Are they predictive tools for disease complications? Clin. Lab. 2014, 60, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Arababadi, M.K.; Hassanshahi, G. CXCL10 activities, biological structure, and source along with its significant role played in pathophysiology of type I diabetes mellitus. Inflammation 2013, 36, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Gniewkiewicz, M.S.; Czerwinska, M.; Gozdowska, J.; Czerwinska, K.; Sadowska, A.; Deborska-Materkowska, D.; Perkowska-Ptasinska, A.; Kosieradzki, M.; Durlik, M. Urinary levels of CCL2 and CXCL10 chemokines as potential biomarkers of ongoing pathological processes in kidney allograft: An association with BK virus nephropathy. Pol. Arch. Intern. Med. 2019, 129, 592–597. [Google Scholar] [CrossRef]
- Liang, P.; Averboukh, L.; Zhu, W.; Pardee, A.B. Ras activation of genes: Mob-1 as a model. Proc. Natl. Acad. Sci. USA 1994, 91, 12515–12519. [Google Scholar] [CrossRef]
- Kelsen, S.G.; Aksoy, M.O.; Yang, Y.; Shahabuddin, S.; Litvin, J.; Safadi, F.; Rogers, T.J. The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L584–L591. [Google Scholar] [CrossRef]
- Mordillo-Mateos, L.; Sanchez-Ramos, A.; Coperchini, F.; Bustos-Guadamillas, I.; Alonso-Bonilla, C.; Vargas-Baquero, E.; Rodriguez-Carrion, I.; Rotondi, M.; Oliviero, A. Development of chronic pain in males with traumatic spinal cord injury: Role of circulating levels of the chemokines CCL2 and CXCL10 in subacute stage. Spinal Cord 2019, 57, 953–959. [Google Scholar] [CrossRef]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D.; et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef]
- Deng, W.; Ohmori, Y.; Hamilton, T.A. Mechanisms of IL-4-mediated suppression of IP-10 gene expression in murine macrophages. J. Immunol. 1994, 153, 2130–2136. [Google Scholar] [PubMed]
- Ohmori, Y.; Hamilton, T.A. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 1993, 268, 6677–6688. [Google Scholar] [CrossRef]
- Taub, D.D.; Lloyd, A.R.; Conlon, K.; Wang, J.M.; Ortaldo, J.R.; Harada, A.; Matsushima, K.; Kelvin, D.J.; Oppenheim, J.J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med. 1993, 177, 1809–1814. [Google Scholar] [CrossRef]
- Taub, D.D.; Longo, D.L.; Murphy, W.J. Human interferon-inducible protein-10 induces mononuclear cell infiltration in mice and promotes the migration of human T lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood 1996, 87, 1423–1431. [Google Scholar] [CrossRef]
- Park, J.W.; Gruys, M.E.; McCormick, K.; Lee, J.K.; Subleski, J.; Wigginton, J.M.; Fenton, R.G.; Wang, J.M.; Wiltrout, R.H. Primary hepatocytes from mice treated with IL-2/IL-12 produce T cell chemoattractant activity that is dependent on monokine induced by IFN-gamma (Mig) and chemokine responsive to gamma-2 (Crg-2). J. Immunol. 2001, 166, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E.; et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Yates, C.C.; Rodgers, M.E.; Du, X.; Wells, A. IP-10 induces dissociation of newly formed blood vessels. J. Cell Sci. 2009, 122, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef]
- Wang, X.; Yue, T.L.; Ohlstein, E.H.; Sung, C.P.; Feuerstein, G.Z. Interferon-inducible protein-10 involves vascular smooth muscle cell migration, proliferation, and inflammatory response. J. Biol. Chem. 1996, 271, 24286–24293. [Google Scholar] [CrossRef] [PubMed]
- Neville, L.F.; Abdullah, F.; McDonnell, P.M.; Young, P.R.; Feuerstein, G.Z.; Rabinovici, R. Mob-1 expression in IL-2-induced ARDS: Regulation by TNF-alpha. Am. J. Physiol. 1995, 269, L884–L890. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.R.; Oppenheim, J.J.; Kelvin, D.J.; Taub, D.D. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J. Immunol. 1996, 156, 932–938. [Google Scholar]
- Kanda, N.; Watanabe, S. Histamine inhibits the production of interferon-induced protein of 10 kDa in human squamous cell carcinoma and melanoma. J. Investig. Dermatol. 2002, 119, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Zhou, L.Z.; Chaturvedi, P.; Babcock, G.; Aras, S.; Ransohoff, R.M. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J. Neurosci. Res. 1998, 54, 169–180. [Google Scholar] [CrossRef]
- Varley, C.L.; Armitage, S.; Hassanshahiraviz, G.; Dickson, A.J. Regulation of the C-X-C chemokine, mob-1, gene expression in primary rat hepatocytes. Cytokine 2003, 23, 64–75. [Google Scholar] [CrossRef]
- Han, B.; Logsdon, C.D. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-kappaB activation. Am. J. Physiol. 1999, 277, C74–C82. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Logsdon, C.D. CCK stimulates mob-1 expression and NF-kappaB activation via protein kinase C and intracellular Ca(2+). Am. J. Physiol. Cell Physiol. 2000, 278, C344–C351. [Google Scholar] [CrossRef]
- Kaplan, G.; Luster, A.D.; Hancock, G.; Cohn, Z.A. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J. Exp. Med. 1987, 166, 1098–1108. [Google Scholar] [CrossRef]
- Romagnani, P.; Lazzeri, E.; Lasagni, L.; Mavilia, C.; Beltrame, C.; Francalanci, M.; Rotondi, M.; Annunziato, F.; Maurenzig, L.; Cosmi, L.; et al. IP-10 and Mig production by glomerular cells in human proliferative glomerulonephritis and regulation by nitric oxide. J. Am. Soc. Nephrol. JASN 2002, 13, 53–64. [Google Scholar] [CrossRef]
- Schuh, E.; Berer, K.; Mulazzani, M. Features of Human CD3+CD20+ T Cells. J. Immunol. 2016, 197, 1111–1117. [Google Scholar] [CrossRef]
- Kopydlowski, K.M.; Salkowski, C.A.; Cody, M.J.; van Rooijen, N.; Major, J.; Hamilton, T.A.; Vogel, S.N. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 1999, 163, 1537–1544. [Google Scholar]
- Milanos, L.; Brox, R.; Frank, T.; Poklukar, G.; Palmisano, R.; Waibel, R.; Einsiedel, J.; Durr, M.; Ivanovic-Burmazovic, I.; Larsen, O.; et al. Discovery and Characterization of Biased Allosteric Agonists of the Chemokine Receptor CXCR3. J. Med. Chem. 2016, 59, 2222–2243. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Ammirati, E.; Moroni, F.; Magnoni, M.; Camici, P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015, 179, 173–187. [Google Scholar] [CrossRef]
- Groom, J.R.; Richmond, J.; Murooka, T.T.; Sorensen, E.W.; Sung, J.H.; Bankert, K.; von Andrian, U.H.; Moon, J.J.; Mempel, T.R.; Luster, A.D. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 2012, 37, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Sauty, A.; Iarossi, A.S.; Sukhova, G.K.; Neote, K.; Libby, P.; Luster, A.D. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Investig. 1999, 104, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; van Haperen, R.; de Boer, H.C.; Segers, D.; Huisman, M.; van Zonneveld, A.J.; Leenen, P.J.; van der Steen, A.; Serruys, P.W.; et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J. Clin. Investig. 2007, 117, 616–626. [Google Scholar] [CrossRef]
- Segers, D.; Lipton, J.A.; Leenen, P.J.; Cheng, C.; Tempel, D.; Pasterkamp, G.; Moll, F.L.; de Crom, R.; Krams, R. Atherosclerotic Plaque Stability Is Affected by the Chemokine CXCL10 in Both Mice and Humans. Int. J. Inflamm. 2011, 2011, 936109. [Google Scholar] [CrossRef][Green Version]
- Rohm, I.; Atiskova, Y.; Drobnik, S. Decreased regulatory T cells in vulnerable atherosclerotic lesions: Imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediat. Inflamm. 2015, 2015, 364710. [Google Scholar] [CrossRef]
- Emoto, T.; Sasaki, N.; Yamashita, T.; Kasahara, K.; Yodoi, K.; Sasaki, Y.; Matsumoto, T.; Mizoguchi, T.; Hirata, K. Regulatory/effector T-cell ratio is reduced in coronary artery disease. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 2935–2941. [Google Scholar] [CrossRef] [PubMed]
- Hasib, L.; Lundberg, A.K.; Zachrisson, H.; Ernerudh, J.; Jonasson, L. Functional and homeostatic defects of regulatory T cells in patients with coronary artery disease. J. Intern. Med. 2016, 279, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Heller, E.A.; Liu, E.; Tager, A.M.; Yuan, Q.; Lin, A.Y.; Ahluwalia, N.; Jones, K.; Koehn, S.L.; Lok, V.M.; Aikawa, E.; et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 2006, 113, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.N.; Liu, B.; Liu, W.; Liu, S. Interleukin-27 enhances TNF-alpha-mediated activation of human coronary artery endothelial cells. Mol. Cell Biochem. 2016, 411, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Moorman, J.R.; Liu, L.; Gimple, L.W.; Lipson, L.C.; Ragosta, M.; Taylor, A.M.; Lake, D.E.; Burdick, M.D.; Mehrad, B.; et al. Plasma chemokine levels are associated with the presence and extent of angiographic coronary collaterals in chronic ischemic heart disease. PLoS ONE 2011, 6, e21174. [Google Scholar] [CrossRef]
- de Oliveira, R.T.; Mamoni, R.L.; Souza, J.R.; Fernandes, J.L.; Rios, F.J.; Gidlund, M.; Coelho, O.R.; Blotta, M.H. Differential expression of cytokines, chemokines and chemokine receptors in patients with coronary artery disease. Int. J. Cardiol. 2009, 136, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, H.; Wang, Y.; Manes, T.D.; Yakimov, A.O.; Akhtar, S.; Kluger, M.S.; Pober, J.S.; Tellides, G. Heparin displaces interferon-gamma-inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation 2006, 114, 1293–1300. [Google Scholar] [CrossRef]
- Rothenbacher, D.; Muller-Scholze, S.; Herder, C.; Koenig, W.; Kolb, H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 194–199. [Google Scholar] [CrossRef]
- Zuojun, H.; Lingyu, H.; Wei, H.; Henghui, Y.; Chonggang, Z.; Jingsong, W.; Mian, W.; Yong, L.; Shenming, W. Interference of IP-10 expression inhibits vascular smooth muscle cell proliferation and intimal hyperplasia in carotid artery: A new insight in the prevention of restenosis. Cell Biochem. Biophys. 2012, 62, 125–135. [Google Scholar] [CrossRef]
- Kawamura, A.; Miura, S.-i.; Fujino, M.; Nishikawa, H.; Matsuo, Y.; Tanigawa, H.; Tomita, S.; Tsuchiya, Y.; Matsuo, K.; Saku, K. CXCR3 chemokine receptor-plasma IP10 interaction in patients with coronary artery disease. Circ. J. 2003, 67, 851–854. [Google Scholar] [CrossRef]
- Chang, S.-F.; Liu, S.-F.; Chen, C.-N.; Kuo, H.-C. Serum IP-10 and IL-17 from Kawasaki disease patients induce calcification-related genes and proteins in human coronary artery smooth muscle cells in vitro. Cell Biosci. 2020, 10, 36. [Google Scholar] [CrossRef]
- Lupieri, A.; Smirnova, N.F.; Solinhac, R.; Malet, N.; Benamar, M.; Saoudi, A.; Santos-Zas, I.; Zeboudj, L.; Ait-Oufella, H.; Hirsch, E.; et al. Smooth muscle cells-derived CXCL10 prevents endothelial healing through PI3Kγ-dependent T cells response. Cardiovasc. Res. 2020, 116, 438–449. [Google Scholar] [CrossRef]
- Niki, T.; Soeki, T.; Yamaguchi, K.; Taketani, Y.; Yagi, S.; Iwase, T.; Yamada, H.; Wakatsuki, T.; Shimabukuro, M.; Sata, M. Elevated concentration of interferon-inducible protein of 10 kD (IP-10) is associated with coronary atherosclerosis. Int. Heart J. 2015, 56, 269–272. [Google Scholar] [CrossRef][Green Version]
- Safa, A.; Rashidinejad, H.; Khalili, M.; Dabiri, S.; Nemati, M.; Mohammadi, M.; Jafarzadeh, A. Higher circulating levels of chemokines CXCL10, CCL20 and CCL22 in patients with ischemic heart disease. Cytokine 2016, 83, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ardigo, D.; Assimes, T.L.; Fortmann, S.P.; Go, A.S.; Hlatky, M.; Hytopoulos, E.; Iribarren, C.; Tsao, P.S.; Tabibiazar, R.; Quertermous, T. Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease. Physiol. Genom. 2007, 31, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ørn, S.; Breland, U.M.; Mollnes, T.E.; Manhenke, C.; Dickstein, K.; Aukrust, P.; Ueland, T. The chemokine network in relation to infarct size and left ventricular remodeling following acute myocardial infarction. Am. J. Cardiol. 2009, 104, 1179–1183. [Google Scholar] [CrossRef]
- Tavakolian Ferdousie, V.; Mohammadi, M.; Hassanshahi, G.; Khorramdelazad, H.; Khanamani Falahati-Pour, S.; Mirzaei, M.; Allah Tavakoli, M.; Kamiab, Z.; Ahmadi, Z.; Vazirinejad, R.; et al. Serum CXCL10 and CXCL12 chemokine levels are associated with the severity of coronary artery disease and coronary artery occlusion. Int. J. Cardiol. 2017, 233, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, P.F.; Boshuizen, M.C.; Hollander, M.R.; Biesbroek, P.S.; van der Hoeven, N.W.; Mol, J.Q.; Gijbels, M.J.; van der Velden, S.; van der Pouw Kraan, T.C.; Horrevoets, A.J.; et al. MAb therapy against the IFN-alpha/beta receptor subunit 1 stimulates arteriogenesis in a murine hindlimb ischaemia model without enhancing atherosclerotic burden. Cardiovasc. Res. 2015, 107, 255–266. [Google Scholar] [CrossRef]
- Klinghammer, L.; Urschel, K.; Cicha, I.; Lewczuk, P.; Raaz-Schrauder, D.; Achenbach, S.; Garlichs, C.D. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis--influence on IP-10, TNF-alpha and MCP-1. Cytokine 2013, 62, 290–296. [Google Scholar] [CrossRef]
- Altara, R.; Mallat, Z.; Booz, G.W.; Zouein, F.A. The CXCL10/CXCR3 axis and cardiac inflammation: Implications for immunotherapy to treat infectious and noninfectious diseases of the heart. J. Immunol. Res. 2016, 2016, 4396368. [Google Scholar] [CrossRef]
- Altara, R.; Manca, M.; Brandão, R.D.; Zeidan, A.; Booz, G.W.; Zouein, F.A. Emerging importance of chemokine receptor CXCR3 and its ligands in cardiovascular diseases. Clin. Sci. 2016, 130, 463–478. [Google Scholar] [CrossRef]
- Martina Ferrari, S.; Antonelli, A.; Di Domenicantonio, A.; Manfredi, A.; Ferri, C.; Fallahi, P. Modulatory effects of peroxisome proliferator-activated receptor-γ on CXCR3 chemokines. Recent Pat. Inflamm. Allergy Drug Discov. 2014, 8, 132–138. [Google Scholar] [CrossRef]
- Di Luigi, L.; Corinaldesi, C.; Colletti, M.; Scolletta, S.; Antinozzi, C.; Vannelli, G.B.; Giannetta, E.; Gianfrilli, D.; Isidori, A.M.; Migliaccio, S.; et al. Phosphodiesterase Type 5 Inhibitor Sildenafil Decreases the Proinflammatory Chemokine CXCL10 in Human Cardiomyocytes and in Subjects with Diabetic Cardiomyopathy. Inflammation 2016, 39, 1238–1252. [Google Scholar] [CrossRef]
- Das, A.; Durrant, D.; Salloum, F.N.; Xi, L.; Kukreja, R.C. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 2015, 147, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Karin, N.; Wildbaum, G.; Thelen, M. Biased signaling pathways via CXCR3 control the development and function of CD4+ T cell subsets. J. Leukoc. Biol. 2016, 99, 857–862. [Google Scholar] [CrossRef]
- Karin, N.; Wildbaum, G. The role of chemokines in adjusting the balance between CD4+ effector T cell subsets and FOXp3-negative regulatory T cells. Int. Immunopharmacol. 2015, 28, 829–835. [Google Scholar] [CrossRef]
- Standl, E.; Schnell, O.; McGuire, D.K. Heart failure considerations of antihyperglycemic medications for type 2 diabetes. Circ. Res. 2016, 118, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Alves, C.; Batel-Marques, F. Number needed to harm in the post-marketing safety evaluation: Results for rosiglitazone and pioglitazone. Pharmacoepidemiol. Drug Saf. 2015, 24, 1259–1270. [Google Scholar] [CrossRef]
- Erdmann, E.; Wilcox, R. Pioglitazone and mechanisms of CV protection. QJM Int. J. Med. 2009, 103, 213–228. [Google Scholar] [CrossRef]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; Le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S.; et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Circulation 2003, 108, 2941–2948. [Google Scholar] [CrossRef]
- Zohar, Y.; Wildbaum, G.; Novak, R.; Salzman, A.L.; Thelen, M.; Alon, R.; Barsheshet, Y.; Karp, C.L.; Karin, N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J. Clin. Investig. 2014, 124, 2009. [Google Scholar] [CrossRef] [PubMed]
- Sottili, M.; Cosmi, L.; Borgogni, E.; Sarchielli, E.; Maggi, L.; Francalanci, M.; Vannelli, G.; Ronconi, E.; Adorini, L.; Annunziato, F. Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp. Cell Res. 2009, 315, 264–273. [Google Scholar] [CrossRef]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating cells and biomolecules involved in its activation and inhibition. Adv. Protein Chem. Struct. Biol. 2020, 120, 85. [Google Scholar]
- Andersen-Nissen, E.; Fiore-Gartland, A.; Ballweber Fleming, L.; Carpp, L.N.; Naidoo, A.F.; Harper, M.S.; Voillet, V.; Grunenberg, N.; Laher, F.; Innes, C.; et al. Innate immune signa tures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS Pathog. 2021, 17, e1009363. [Google Scholar] [CrossRef] [PubMed]
- Rechtien, A.; Richert, L.; Lorenzo, H.; Martrus, G.; Hejblum, B.; Dahlke, C.; Kasonta, R.; Zinser, M.; Stubbe, H.; Matschl, U.; et al. Systems Vaccinology Identifies an Early Innate Immune Signature as a Correlate of Antibody Responses to the Ebola Vaccine rVSV-ZEBOV. Cell Rep. 2017, 20, 2251–2261. [Google Scholar] [CrossRef]
- Chang, J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw. 2021, 21, e6. [Google Scholar] [CrossRef] [PubMed]
- Azamor, T.; da Silva, A.M.V.; Melgaço, J.G.; Dos Santos, A.P.; Xavier-Carvalho, C.; Alvarado-Arnez, L.E.; Batista-Silva, L.R.; de Souza Matos, D.C.; Bayma, C.; Missailidis, S.; et al. Activation of an Effective Immune Response after Yellow Fever Vaccination Is Associated with the Genetic Background and Early Response of IFN-γ and CLEC5A. Viruses 2021, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, C.; Pandit, H.; Nagy, B.A.; Stellas, D.; Jensen, S.M.; Bear, J.; Cam, M.; Valentin, A.; Fox, B.A.; Felber, B.K.; et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J. Immunother. Cancer 2020, 8, e000599. [Google Scholar] [CrossRef]
- Gonçalves, E.; Bonduelle, O.; Soria, A.; Loulergue, P.; Rousseau, A.; Cachanado, M.; Bonnabau, H.; Thiebaut, R.; Tchitchek, N.; Behillil, S.; et al. Innate gene signature distinguishes humoral versus cytotoxic responses to influenza vaccination. J. Clin. Investig. 2019, 129, 1960–1971. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Gogos, C.; Assimakopoulos, S.F. Hypercoagulation and myocardial injury as risk factors for mortality in patients with COVID-19 pneumonia. Am. J. Emerg. Med. 2021, 47, 313–314. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Assimakopoulos, S.F.; Velissaris, D.; Hung, M.Y.; Mplani, V.; Saba, L.; Brinia, A.; Kouni, S.N.; et al. COVID-19 Disease, Women’s Predominant Non-Heparin Vaccine-Induced Thrombotic Thrombocytopenia and Kounis Syndrome: A Passepartout Cytokine Storm Interplay. Biomedicines 2021, 9, 959. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef] [PubMed]
- Tajstra, M.; Jaroszewicz, J.; Gąsior, M. Acute Coronary Tree Thrombosis After Vaccination for COVID-19. JACC Cardiovasc. Interv. 2021, 14, e103–e104. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ojha, U.K.; Vardhan, B.; Tiwari, A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab. Syndr. 2021, 15, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.N.; Sathyamurthy, I.; Neelagandan, M. Relation between COVID-19 vaccination and myocardial infarction e Casual or coincidental? IHJ Cardiovasc. Case Rep. 2021, 5, 71–74. [Google Scholar] [CrossRef]
- Özdemir, İ.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Bayturan, Ö. Type 1 Kounis Syndrome Induced by Inactivated SARS-CoV-2 Vaccine. J. Emerg. Med. 2021, 7. [Google Scholar] [CrossRef]
- Boivin, Z.; Martin, J. Untimely Myocardial Infarction or COVID-19 Vaccine Side Effect. Cureus 2021, 13, e13651. [Google Scholar] [CrossRef]
- Maadarani, O.; Bitar, Z.; Elzoueiry, M.; Nader, M.; Abdelfatah, M.; Zaalouk, T.; Mohsen, M.; Elhabibi, M. Myocardial infarction post COVID-19 vaccine—Coincidence, Kounis syndrome or other explanation—Time will tell. JRSM Open 2021, 12, 20542704211025259. [Google Scholar] [CrossRef]
- van den Borne, P.; Quax, P.H.; Hoefer, I.E.; Pasterkamp, G. The multifaceted functions of CXCL10 in cardiovascular disease. Biomed. Res. Int. 2014, 2014, 893106. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Ghidoni, A.; Ravera, A.; De Ferrari, G.M.; Calvillo, L. Chemokines and Heart Disease: A Network Connecting Cardiovascular Biology to Immune and Autonomic Nervous Systems. Mediat. Inflamm. 2016, 2016, 5902947. [Google Scholar] [CrossRef]
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dézsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and during Myocardial Infarction. Front. Immunol. 2018, 9, 1932. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C. COVID-19 and Kounis Syndrome: Deciphering Their Relationship. Balkan Med. J. 2021, 38, 145–149. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimabad, M.N.; Kounis, N.G.; Hassanshahi, G.; Hassanshahi, F.; Mplani, V.; Koniari, I.; Hung, M.-Y.; Nadimi, A.E. The Involvement of CXC Motif Chemokine Ligand 10 (CXCL10) and Its Related Chemokines in the Pathogenesis of Coronary Artery Disease and in the COVID-19 Vaccination: A Narrative Review. Vaccines 2021, 9, 1224. https://doi.org/10.3390/vaccines9111224
Karimabad MN, Kounis NG, Hassanshahi G, Hassanshahi F, Mplani V, Koniari I, Hung M-Y, Nadimi AE. The Involvement of CXC Motif Chemokine Ligand 10 (CXCL10) and Its Related Chemokines in the Pathogenesis of Coronary Artery Disease and in the COVID-19 Vaccination: A Narrative Review. Vaccines. 2021; 9(11):1224. https://doi.org/10.3390/vaccines9111224
Chicago/Turabian StyleKarimabad, Mojgan Noroozi, Nicholas G. Kounis, Gholamhossein Hassanshahi, Farzaneh Hassanshahi, Virginia Mplani, Ioanna Koniari, Ming-Yow Hung, and Ali Esmaeili Nadimi. 2021. "The Involvement of CXC Motif Chemokine Ligand 10 (CXCL10) and Its Related Chemokines in the Pathogenesis of Coronary Artery Disease and in the COVID-19 Vaccination: A Narrative Review" Vaccines 9, no. 11: 1224. https://doi.org/10.3390/vaccines9111224
APA StyleKarimabad, M. N., Kounis, N. G., Hassanshahi, G., Hassanshahi, F., Mplani, V., Koniari, I., Hung, M.-Y., & Nadimi, A. E. (2021). The Involvement of CXC Motif Chemokine Ligand 10 (CXCL10) and Its Related Chemokines in the Pathogenesis of Coronary Artery Disease and in the COVID-19 Vaccination: A Narrative Review. Vaccines, 9(11), 1224. https://doi.org/10.3390/vaccines9111224







