Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer
Abstract
1. Introduction
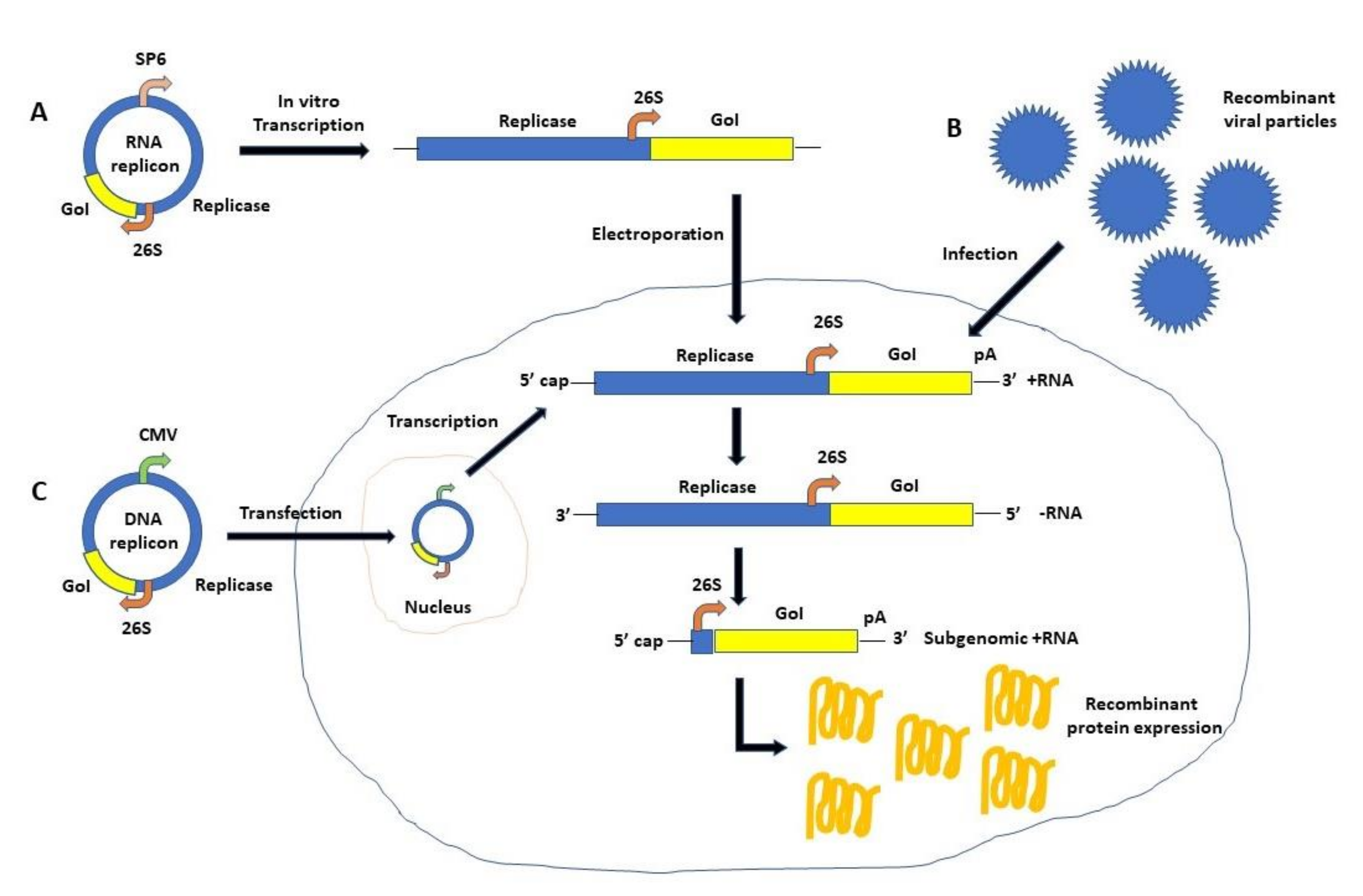
2. Self-Replicating RNA Viruses
3. Infectious Diseases
| Virus/Disease | Antigen | Vector | Findings | Ref. |
|---|---|---|---|---|
| Alphaviruses | ||||
| CHIKV/CHIK | C, Env | MV-CHIKV VLPs | Phase I: 100% seroconversion after two doses | [96] |
| CHIKV/CHIK | C, Env | MV-CHIKV VLPs | Phase II: good safety, strong immunogenicity | [97] |
| Filoviruses | ||||
| EBOV/EVD | GP (Zaire strain) | VSV-ZEBOV | Phase III: high vaccine efficacy, protection | [99,100] |
| EBOV/EVD | GP (Zaire strain) | VSV-ZEBOV | Phase III: high vaccine efficacy | [100] |
| EBOV/EVD | GP (Zaire strain) | VSV-ZEBOV | Ervebo approval by the FDA, EMA | [101] |
| Flaviviruses | ||||
| ZIKV/ZVD | E | MV-ZIKA-E | Phase I: study completed; no results available | [102] |
| ZIKV/ZVD | E | MV-ZIKA-RSP-E | Phase I: study in progress | [103] |
| Lentiviruses | ||||
| HIV/AIDS | HIV Gag | VEEV-Gag | Phase I: trials halted, stability & documentation | [104] |
| Coronaviruses | ||||
| SARS-CoV-2/COV | S | MV (TMV-083) | Phase I: weak immunogenicity, trial discontinued | [75,76] |
| SARS-CoV-2/COV | S | VSV (V590) | Phase I: weak immunogenicity, trial discontinued | [78,79] |
| SARS-CoV-2/COV | S | VSVΔG-S | Phase I/II: study in progress | [81,82] |
| SARS-CoV-2/COV | S | LNP-VEEV-S RNA | Phase I/II: study in progress | [85] |
4. Cancer
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lundstrom, K. Virus vectors for COVID-19 vaccine development. Viruses 2021, 13, 317. [Google Scholar] [CrossRef]
- Regulatory Approval of COVID-19 Vaccine AstraZeneca—GOV.UK. Available online: www.gov.uk (accessed on 1 July 2021).
- Ad26.COV2-S FDA Approval Status. Available online: drugs.com/history/ad26-cov2-s.html (accessed on 10 June 2021).
- Callaway, E. Russia’s fast-track coronavirus vaccine draws outrage over safety. Nature 2020, 584, 334–335. [Google Scholar] [CrossRef]
- Lundstrom, K. Application of viral vectors for vaccine development with a special emphasis on COVID-19. Viruses 2020, 12, 1324. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Frolov, I.; Hoffman, T.A.; Pragal, B.M.; Dryga, S.A.; Huang, H.; Schlesinger, S.; Rice, C.M. Alphavirus-based expression vectors: Strategies and applications. Proc. Natl. Acad. Sci. USA 1996, 93, 11371–11377. [Google Scholar] [CrossRef]
- Harvey, T.J.; Anraku, I.; Linedale, R.; Harrich, D.; MacKenzie, J.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicon vectors for human immunedefieciency virus vaccine development. J. Virol. 2003, 77, 7796–7803. [Google Scholar] [CrossRef]
- Mühlebach, M.D.; Hutzler, S. Development of Recombinant Measles Virus-Based Vaccines. Methods Mol. Biol. 2017, 1581, 151–168. [Google Scholar] [PubMed]
- Lyles, D.S.; Rupprecht, C.E. Rhabdoviridiae. In Fields’ Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1364–1408. [Google Scholar]
- Liljestrom, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991, 9, 1356–1361. [Google Scholar] [CrossRef]
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.L.; Willis, L.V.; Smith, J.F.; Johnston, R.F. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: Analysis of a viable deletion mutant. Virology 1989, 171, 189–204. [Google Scholar] [CrossRef]
- Pijlman, G.P.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicons: An RNA-based non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Expert Opin. Biol. Ther. 2006, 6, 134–145. [Google Scholar] [CrossRef] [PubMed]
- De Felipe, F. Skipping the co-expression problem: The new 2A ‘CHYSEL’ technology. Genet. Vaccines Ther. 2004, 2, 13. [Google Scholar] [CrossRef]
- Khromykh, A.A.; Varnavski, A.N.; Westaway, E.G. Encapsidation of the flavivirus Kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 1998, 72, 5967–5977. [Google Scholar] [CrossRef]
- Shi, P.Y.; Tilgner, M.; Lo, M.K. Construction and characterization of subgenomic replicons of New York strain of West Nile virus. Virology 2002, 296, 219–233. [Google Scholar] [CrossRef]
- Molenkamp, R.; Kooi, E.A.; Lucassen, M.A.; Greve, S.; Thijssen, J.C.; Spaan, W.J.; Bredenbeek, P.J. Yellow fever virus replicons as an expression system for hepatitis C virus structural proteins. J. Virol. 2003, 77, 1644–1648. [Google Scholar] [CrossRef]
- Jones, M.; Davidson, A.; Hibbert, L.; Gruenwald, P.; Schlaak, J.; Ball, S.; Foster, G.R.; Jacobs, M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 2005, 79, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Gherke, R.; Ecker, M.; Aberle, S.W.; Allison, S.L.; Heinz, F.X.; Mandi, C.W. Incorporation of tick-borne encephalitis virus replicons into virus-like particles by a packaging cell line. J. Virol. 2003, 77, 8924–8933. [Google Scholar] [CrossRef]
- Fan, Z.-C.; Dennis, J.C.; Bird, R.C. Bovine viral diarrhea virus is a suitable viral vector for stable expression of heterologous gene when inserted between N(pro) and C genes. Virus Res. 2008, 138, 97–104. [Google Scholar] [CrossRef]
- Stetter, P.; Devos, R.; Moser, C.; Tratschin, J.-D.; Hofmann, M. Establishment and application of bicistronic classical swine fever virus genomes for foreign gene expression and complementation of E2 deletion mutants. Virus Res. 2002, 85, 173–185. [Google Scholar] [CrossRef]
- Schnell, M.J.; Buonocore, L.; Kretzschmar, E.; Johnson, E.; Rose, J.K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 1996, 93, 11359–11365. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Morikawa, S.; Matsuura, Y. Development and applications of VSV vectors based on cell tropism. Front. Microbiol. 2012, 2, 272. [Google Scholar] [CrossRef]
- Harty, R.N.; Brown, M.E.; Hayes, F.P.; Wright, N.T.; Schnell, M.J. Vaccinia virus-free recovery of vesicular stomatitis virus. J. Mol. Microbiol. Biotechnol. 2001, 3, 513–517. [Google Scholar]
- Singh, M.; Cattaneo, R.; Billeter, M.A. A recombinant measles virus expressing hepatitis B surface antigen induces humoral responses in genetically modified mice. J. Virol. 1999, 73, 4823–4828. [Google Scholar] [CrossRef]
- Radecke, F.; Spielhofer, P.; Schneider, H.; Kaelin, K.; Huber, M.; Dötsch, C.; Christiansen, G.; Billeter, M.A. Rescue of measles viruses from cloned DNA. EMBO J. 1995, 14, 5773–5784. [Google Scholar] [CrossRef]
- Osakada, F.; Callaway, E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 2013, 8, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Inoue, K.; Yamada, M.; Yamawaki, T.; Koganezawa, N.; Tsuttsui, K.; Witter, M.P.; Iijima, T. Dual transneural tracing in the rat entorhoinal-hippocampal circuit by intracerebral injection of recombinant rabies virus vectors. Front. Neuroanat. 2009, 3, 1–11. [Google Scholar] [CrossRef]
- Ito, N.; Takayama-Ito, M.; Yamada, K.; Hosokawa, J.; Sugiyama, M.; Minamoto, N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol. Immunol. 2003, 47, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Self-amplifying RNA viruses as RNA vaccines. Int. J. Mol. Sci. 2020, 21, 5130. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Aquilar, P.V.; Bopp, N.E.; Yarovinsky, T.O.; Weaver, S.C.; Rose, J.K. A recombinant virus vaccine that protects both against Chikungunya and Zika virus infections. Vaccine 2018, 36, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.S.; Glass, P.J.; Bakken, R.R.; Barth, J.F.; Lind, C.M.; da Silva, L.; Hart, M.K.; Rayner, J.; Alterson, K.; Custer, M.; et al. Combined alphavirus replicon particle vaccine induces durable and cross-protective immune responses against equine encephalitis virus. J. Virol. 2014, 88, 12077–12086. [Google Scholar] [CrossRef]
- Tretyakova, I.; Tibbens, A.; Jokinen, J.D.; Johnson, D.M.; Lukashevich, J.S.; Pushko, P. Novel DNA-launched Venezuelan equine encephalitis virus vaccine with rearranged genome. Vaccine 2019, 37, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, I.; Plante, K.S.; Rossi, S.L.; Lawrence, W.S.; Peel, J.E.; Gudjohnsen, S.; Wang, E.; Mirchandani, D.; Tibbens, A.; Lamichhane, T.N. Venezuelan equine encephalitis vaccine with rearranged genome resists reversion and protects non-human primates from viremia after aerosol challenge. Vaccine 2020, 38, 3378–3386. [Google Scholar] [CrossRef]
- Rossi, S.L.; Comer, J.E.; Wang, E.; Azar, S.R.; Lawrence, W.S.; Plante, J.A.; Ramsauer, K.; Schrauf, S.; Weaver, S.C. Immunogenicity and efficacy of a measles virus-vectored chikungunya vaccine in nonhuman primates. J. Infect. Dis. 2019, 220, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Safronetz, D.; Mire, C.; Rosenke, K.; Feldmann, F.; Haddock, E.; Geissbert, T.; Feldmann, H. A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl. Trop. Dis. 2015, 9, e0003736. [Google Scholar] [CrossRef] [PubMed]
- Mateo, M.; Reynard, S.; Carnec, X.; Journeaux, A.; Baillet, N.; Schaeffer, J.; Picard, C.; Legras-Lachuer, C.; Allan, R.; Perthame, E.; et al. Vaccines inducing immunity to Lassa fever glycoprotein and nucleoprotein protect macaques after a single shot. Sci. Transl. Med. 2019, 11, eaaw3163. [Google Scholar] [CrossRef] [PubMed]
- Bredenbeck, P.J.; Molenkamp, R.; Spaan, W.J.M.; Deubel, V.; Marianneu, P.; Salvato, M.S.; Moshkoff, D.; Zapata, J.; Tikhonov, I.; Patterson, J.; et al. A recombinant Yellow Fever 17D expressing Lassa virus glycoproteins. Virology 2006, 345, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dalebout, T.J.; Bredenbeck, P.J.; Carrion, R.J.; Brasky, K.; Patterson, J.; Goicochea, M.; Bryant, J.; Salvato, M.S.; Lukashevich, I.S. Yellow fever 17D-vectored vaccines expressing LASV GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine 2011, 29, 1248–1257. [Google Scholar] [CrossRef]
- Lukashevich, I.S.; Pushko, P. Vaccine platforms to control Lassa fever. Exp. Rev. Vaccines 2016, 15, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Geisbert, J.; Parker, M.; Jahrling, P.; Smith, J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola virus. J. Virol. 2001, 75, 11677–11685. [Google Scholar] [CrossRef]
- Thompson, J.M.; Whitmore, A.C.; Staats, H.F.; Johnston, R.E. Alphavirus replicon particles acting as adjuvants promote CD8+ T cell responses to co-delivered antigen. Vaccine 2008, 26, 4267–4275. [Google Scholar] [CrossRef][Green Version]
- Johnson, D.M.; Jokinen, J.D.; Wang, M.; Pfeiffer, T.; Tretyakova, I.; Carrion, R., Jr.; Griffiths, A.; Pushko, P.; Lukashevich, I.S. Bivalent Junin and Machupo experimental vaccine based on alphavirus RNA replicon vector. Vaccine 2020, 38, 2949–2959. [Google Scholar] [CrossRef]
- Pyankov, O.V.; Bodnev, S.A.; Pyankova, O.G.; Solodkyi, V.V.; Pyankov, S.A.; Setoh, Y.X.; Volchokova, V.A.; Suhrbier, A.; Volchikov, V.V.; Agafonov, A.A.; et al. A Kunjin replicon virus-like vaccine provides protection against Ebola virus infection in nonhuman primates. J. Infect. Dis. 2015, 212, S368–S371. [Google Scholar] [CrossRef]
- Marzi, A.; Robertson, S.J.; Haddock, E.; Feldmann, F.; Hanley, P.W.; Scott, D.-P.; Strong, J.E.; Kobinger, G.; Best, S.M.; Feldmann, H. VSV-EBOV rapidly protects macaques against infection with the 2014/2015 Ebola virus outbreak strain. Science 2015, 349, 739–742. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Feldmann, H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg infections. J. Infect. Dis. 2011, 204, S1075–S1081. [Google Scholar] [CrossRef]
- Herbert, A.S.; Kuehne, A.I.; Barth, J.F.; Ortiz, R.A.; Nichols, D.K.; Zak, S.E.; Stonier, S.W.; Muhammad, M.A.; Bakken, R.R.; Prugar, L.I.; et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J. Virol. 2013, 87, 4852–4964. [Google Scholar] [CrossRef]
- Khalil, S.M.; Tonkin, D.R.; Mattocks, M.D.; Snead, A.T.; Johnston, R.E.; White, L.J. A tetravalent alphavirus-vector based dengue vaccine provides effective immunity in an early life mouse model. Vaccine 2014, 32, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.M.; Chen, H.W.; Hsiao, Y.; Wu, S.H.; Chung, H.H.; Hsieh, C.H.; Chong, P.; Leng, C.H.; Pan, C.H. The successful induction of T-cell and antibody responses by a recombinant measles virus-vectored tetravalent dengue vaccine provides partial protection against dengue-2 infection. Hum. Vaccines Immunother. 2016, 12, 1678–1689. [Google Scholar] [CrossRef]
- Guy, B.; Saville, M.; Lang, J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum. Vaccines 2010, 6, 696–705. [Google Scholar] [CrossRef]
- Ravel, G.; Mantel, N.; Silvano, J.; Rogue, A.; Guy, B.; Jackson, N.; Burdin, N. Biodistribution and safety of a live and attenuated tetravalent dengue vaccine in the cynomolgus monkey. Vaccine 2017, 35, 5918–5923. [Google Scholar] [CrossRef] [PubMed]
- Torresi, J.; Ebert, G.; Pellegrini, M. Vaccines licensed and in clinical trials for the prevention of dengue. Hum. Vaccines Immunother. 2017, 13, 1059–1072. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; Guderian, J.; Granger, B.; Archer, J.; Archer, M.; Cage, E.; Fuerte-Stone, J.; Larson, E.; Lin, S.; et al. A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol. Ther. 2018, 26, 2507–2522. [Google Scholar] [CrossRef]
- Kurup, D.; Wirblich, C.; Schnell, M.J. Measles-based Zika vaccine induces long-term immunity and requires NS1 antibodies to protect the female reproductive tract in the mouse model of Zika. bioRxiv 2020. [Google Scholar] [CrossRef]
- Del Valle, J.R.; Devaux, P.; Hodge, G.; Wegner, N.J.; McChesney, M.B.; Cattaneo, R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against virus challenge. J. Virol. 2007, 81, 10597–10605. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.D.; Buonocore, L.; Rose, N.F.; Rose, J.K.; Robek, M.D. Virus-like vesicle-based therapeutic vaccine vectors for chronic hepatis B virus infection. J. Virol. 2015, 89, 10407–10415. [Google Scholar] [CrossRef] [PubMed]
- Lorin, C.; Mollet, L.; Delebecque, F.; Combredet, C.; Hurtrel, B.; Charneau, P.; Brahic, M.; Tangy, F. A single injection of recombinant measles virus vaccine expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 2004, 78, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.; Lemiale, F.; Turbica, I.; Buzelay, L.; Brunet, S.; Barin, F. Comparative analysis of humoral immune responses to HIV type 1 envelope glycoproteins in mice immunized with a DNA vaccine, recombinant Semliki Forest virus RNA, or recombinant Semliki Forest virus particles. AIDS Res. Hum. Retrovir. 1998, 14, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.; Ataman-Onal, Y.; Battail, N. Generation of monoclonal antibodies to native human immunodeficiency virus type 1 envelope glycoprotein by immunization of mice with naked RNA. J. Virol. Methods 1999, 79, 75–84. [Google Scholar] [CrossRef]
- Ajbani, S.P.; Velhal, S.M.; Kadam, R.B.; Patel, V.V.; Lundstrom, K.; Bandivdekar, A.H. Immunogenicity of virus-like Semliki Forest virus replicon particles expressing Indian HIV-1C gag, env and pol RT genes. Immunol. Lett. 2017, 190, 221–232. [Google Scholar] [CrossRef]
- Knudsen, M.L.; Ljungberg, K.; Tatoud, R.; Weber, J.; Esteban, M.; Liljestrom, P. Alphavirus replicon DNA expressing HIV antigens is an excellent prime for boosting with recombinant modified vaccinia Ankara (MVA) or with HIV gp140 protein antigen. PLoS ONE 2015, 10, e0117042. [Google Scholar] [CrossRef]
- Bogers, W.M.; Oostermeijer, H.; Mooij, P.; Koopman, G.; Verschoor, E.J.; Davis, D.; Ulmer, J.B.; Brito, L.A.; Cu, Y.; Bannerjee, K.; et al. Potent immune responses in rhesus macaques induced by nonviral delivery of self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic emulsion. J. Infect. Dis. 2015, 211, 947–955. [Google Scholar] [CrossRef]
- Ito, T.; Kumagai, T.; Yamaji, Y.; Sawada, A.; Nakayama, T. Recombinant measles AIK-C vaccine strain expressing influenza HA protein. Vaccines 2020, 8, 149. [Google Scholar] [CrossRef]
- Ryder, A.B.; Buonocore, L.; Vogel, L.; Nachbagauer, R.; Krammer, F.; Rose, J.K. A viable recombinant rhabdovirus lacking its glycoprotein gene and expressing influenza virus hemagglutinin and neuraminidase is a potent influenza vaccine. J. Virol. 2015, 89, 2820–2830. [Google Scholar] [CrossRef]
- Furuyama, W.; Reynolds, P.; Haddock, E.; Meade-White, K.; Le, M.Q.; Kawaoka, Y.; Feldmann, H.; Marzi, A. A single dose of a vesicular stomatitis virus-based influenza vaccine confers rapid protection against H5 viruses from different clades. NPJ Vaccines 2020, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Cherry, S.; Dybing, J.K.; Davis, N.L.; Williamson, C.; Suarez, D.L.; Johnston, R.; Perdue, M.L. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects against lethal infection with Hong Kong-origin H5N1 viruses. Virology 2000, 278, 55–59. [Google Scholar] [CrossRef]
- Fleeton, M.N.; Chen, M.; Berglund, P.; Rhodes, G.; Parker, S.E.; Murphy, M.; Atkins, G.J.; Liljestrom, P. Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J. Infect. Dis. 2001, 183, 1395–1398. [Google Scholar] [CrossRef]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reufer, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Krishnavajhala, H.R.; Willimas, J.; Heidner, H. An Influenza A virus vaccine based on an M2e-modified alphavirus. Arch. Virol. 2018, 163, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Démoulins, T.; Ruggli, N.; Gerber, M.; Thomann-Harwood, L.J.; Ebensen, T.; Schulze, K.; Guzman, C.A.; McCullough, K.C. Self-amplifying pestivirus replicon RNA encoding influenza virus nucleoprotein and hemagglutinin promote humoral and cellular immune responses in pigs. Front. Immunol. 2021, 11, 622385. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.; Whitmore, A.; Long, K.; Ferris, M.; Rockx, B.; Funkhouser, B.; Donaldson, E.; Gralinski, L.; Collier, M.; Heise, M.; et al. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J. Virol. 2011, 85, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Shao, Y.; Wang, X.; Zhang, H.; Shuai, L.; Ge, J.; Wen, Z.; Bu, Z. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res. 2018, 150, 30–38. [Google Scholar] [CrossRef]
- Hörner, C.; Schürmann, C.; Auste, A.; Ebenig, A.; Muraleedharan, S.; Dinnon, K.H., III; Scholz, T.; Herrmann, M.; Schnierle, B.S.; Baric, R.S.; et al. A highly immunogenic and effective measles virus-based Th1-biased COVID-19 vaccine. Proc. Natl. Acad. Sci. USA 2020, 117, 32657–32666. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial to Evaluate the Safety and Immunogenicity of the COVID-19 Vaccine (COVID-19-101). Available online: https://clinicaltrials.gov/ct2/show/NCT04497298 (accessed on 30 June 2021).
- Merck Discontinues Development of SARS-CoV-2/COVID-19 Vaccine Candidates; Continues Development of Two Investigational Therapeutic Candidates. Available online: www.merck.com/news/merck-discontinues-development-of-sars-cov-2-covid-19-vaccine-candidates-continues-development-of-two-investigational-therapeutic-candidates/ (accessed on 30 June 2021).
- Case, J.B.; Rothlauf, P.W.; Chen, R.E.; Kafai, N.M.; Fox, J.M.; Smith, B.K.; Shrihari, S.; McCune, B.T.; Harvey, I.B.; Keeler, S.P.; et al. Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis in mice. Cell Host Microbe 2020, 28, 465–474. [Google Scholar] [CrossRef]
- Dose Ranging Trial to Assess Safety and Immunogenicity of V590 (COVID-19 Vaccine) in Healthy Adults (V590-001). Available online: https://clinicaltrials.gov/ct2/show/NCT04569786 (accessed on 30 June 2021).
- Merck and IAVI Discontinue Development of COVID-19 Vaccine Candidate V590. Available online: www.iavi.org/newsresources/press-releases/2021/merck-and-iavi-discontinue-development-of-covid-19-vaccine-candidate-v590 (accessed on 30 June 2021).
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-∆G-spike provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef]
- Evaluate the Safety, Immunogenicity and Potential Efficacy of an rVSV-SARS-CoV-2-S Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04608305 (accessed on 30 June 2021).
- Levin, Y.; Balakirski, N.M.; Caraco, Y.; Ben-Ami, E.; Atsmon, J.; Marcus, H. Ethics and execution of developing a 2nd wave COVID vaccine–Our interim phase I/II VSV-SARS-CoV2 vaccine experience. Vaccine 2021, 39, 2821–2823. [Google Scholar] [CrossRef]
- Tao, J.; Li, B.; Shi, Y.; Chen, J.; Zhu, G.; Shen, X.; Liu, H. Attenuated porcine-derived type 2 bovine viral diarrhea virus as vector stably expressing viral gene. J. Virol. Methods 2020, 279, 113842. [Google Scholar] [CrossRef]
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibodies in mice. Nat. Commun. 2020, 11, 3523. [Google Scholar] [CrossRef]
- Clinical Trial to Assess the Safety of a Coronavirus Vaccine in Healthy Men and Women. Available online: https://doi.org/10.1186/ISRCTN17072692 (accessed on 30 June 2021).
- De Alwis, R.; Gan, E.S.; Chen, S.; Leong, Y.S.; Tan, H.C.; Zhang, S.L.; Yau, C.; Low, J.G.H.; Kalimuddin, S.; Matsuda, D.; et al. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 2021, 29, 1970–1983. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, A.; Opp, S.; Hurtado, A.; Lin, Z.; Pampeno, C.; Noval, M.G.; Thannickal, S.A.; Stappleford, K.A.; Meruelo, D. Combination of a Sindbis-SARS-CoV-2 spike vaccine and αOX40 antibody elicits protective immunity against SARS-CoV-2 induced disease and potentiates long-term SARS-CoV-2-specific humoral and T-cell immunity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Thomas, J.M.; Moen, S.T.; Gnade, B.T.; Vargas-Inchaustegui, D.A.; Foitz, S.M.; Suarez, G.; Heidner, H.W.; König, R.; Chopra, A.K.; Peterson, J.W. Recombinant Sindbis virus vectors designed to express protective antigen of Bacillus anthracis protect animals from anthrax and display synergy with ciprofloxacin. Clin. Vaccine Immunol. 2009, 16, 1696–1699. [Google Scholar] [CrossRef]
- Cabrera, A.; Saez, D.; Cespedes, S.; Andrews, E.; Onate, A. Vaccination with recombinant Semliki Forest virus particles expressing translation initiation factor 3 of Brucella abortus induces protective immunity in BALB/c mice. Immunobiology 2009, 214, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasconcelos, N.M.; Sievertzon, M.; Haddad, D.; Liljeqvist, S.; Berglund, P.; Liljestrom, P.; Ahlborg, N.; Ståhl, S.; Berzins, K. Comparative immunization study using RNA and DNA constructs encoding a part of the Plasmodium falciparum antigen Pf332. Scand. J. Immunol. 2001, 54, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Bergmann, C.C.; Takita-Sonoda, Y.; Murata, K.; Rodrigues, E.G.; Nussenzweig, R.S.; Zavala, F. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J. Virol. 1998, 72, 6907–6910. [Google Scholar] [CrossRef]
- Savar, N.S.; Vallet, T.; Azizi, M.; Arashkia, A.; Lundstrom, K.; Vignuzzi, M.; Niknam, H.M. Quantitative evaluation of PpSP15-LmSTI1 fusion gene expression following transfection with an alphavirus-derived self-amplifying mRNA and conventional DNA vaccine platforms. Mol. Cell. Probes 2021, 59, 101749. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, A.A. Outbreak of Chikungunya in the Republic of Congo and the global picture. J. Infect. Dev. Ctries. 2011, 5, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.A. The 2005–2007 Chikungunya epidemic in Reunion: Ambiguous etiologies, memories, and meaning-making. Med. Anthropol. 2013, 32, 174–189. [Google Scholar] [CrossRef]
- Weaver, S.C.; Salas, R.; Rico-Hesse, R.; Ludwig, G.V.; Oberste, M.S.; Boshell, J.; Tesh, R.B. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet 1996, 348, 436–440. [Google Scholar] [CrossRef]
- Ramsauer, K.; Schwameis, M.; Firbas, C.; Mullner, M.; Putnak, R.J.; Thomas, S.J.; Despres, P.; Tauber, E.; Jilma, B.; Tangy, F. Immunogenicity, safety, and tolerability of a recombinant measlesvirus-based chikungunya vaccine: A randomised, double-blind, placebo controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 2015, 15, 519–527. [Google Scholar] [CrossRef]
- Reisinger, E.C.; Tschismarov, R.; Beubler, E.; Wiedermann, U.; Firbas, C.; Loebermann, M.; Pfeiffer, A.; Muellner, M.; Tauber, E.; Ramsauer, K. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: A double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet 2019, 392, 2718–2727. [Google Scholar] [CrossRef]
- A Trial to Evaluate the Optimal Dose of MV-LASV. Available online: https://clinicaltriasl.gov/ct2/show/NCT4055454 (accessed on 24 June 2021).
- Henao-Restrepo, A.M.; Longini, I.M.; Egger, M.; Dean, N.E.; Edmunds, W.J.; Camacho, A.; Carroll, M.W.; Doumbia, M.; Draguez, B.; Duraffour, S. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: Interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015, 386, 857–866. [Google Scholar] [CrossRef]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Maxmen, A. Ebola vaccine approved for use in ongoing outbreak. Nature 2017. [Google Scholar] [CrossRef]
- Zika-Vaccine Dose Finding Study Regarding Safety, Immunogenicity and Tolerability (V186-001). Available online: https://clinicaltrials.gov/ct2/show/NCT02996890 (accessed on 25 June 2021).
- Safety and Immunogenicity of a Novel Vaccine Formulation. Available online: https://clinicaltrials.gov/ct2/show/NCT04033068 (accessed on 25 June 2021).
- Wecker, M.; Gilbert, P.; Russell, N.; Hural, J.; Allen, M.; Pensiero, M.; Chulay, J.; Chiu, Y.-L.; Karim, S.S.A.; Burke, D.S.; et al. Phase I safety and immunogenicity evaluations of an alphavirus replicon HIV-1 subtype C gag vaccine in healthy HIV-1-uninfected adults. Clin. Vaccine Immunol. 2012, 19, 1651–1660. [Google Scholar] [CrossRef]
- Subissi, L.; Keita, M.; Mesfin, S.; Rezza, G.; Diallo, B.; Van Gucht, S.; Musa, E.O.; Yoti, Z.; Keita, S.; Djingarey, M.H.; et al. Ebola virus transmission caused by persistently infected survivors of the 2014–2016 outbreak in West Africa. J. Infect. Dis. 2018, 218, S287–S291. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Marques, E.T.A. A glimmer of hope: Recent updates and future challenges in Zika vaccine development. Viruses 2020, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, M.; Ge, J.; Ren, S.; Zhou, T.; Qi, F.; Pu, X.; Dou, J. Research progress of therapeutic vaccines for treating chronic hepatitis B. Hum. Vaccines Immunother. 2017, 13, 986–997. [Google Scholar] [CrossRef]
- Zoulim, F.; Fournier, C.; Habersetzer, F.; Sprinzl, M.; Pol, S.; Coffin, C.S.; Leroy, V.; Ma, M.; Wedemeyer, H.; Lohse, A.W.; et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: A phase 1b placebo-controlled trial. Hum. Vaccines Immunother. 2020, 16, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; Porter, E.; Zhang, Y.; Silva, M.; Li, N.; Dobosh, B.; Liquori, A.; Skog, P.; Landais, E.; Menis, S. Immunogenicity of RNA Replicons Encoding HIV Env Immunogens Designed for Self-Assembly into Nanoparticles. Mol. Ther. 2019, 27, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, L.; et al. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine–United States, December 2020. MMWR Morb. Mortal. Wkly Rep. 2021, 69, 1653–1656. [Google Scholar] [CrossRef]
- Tseng, J.-C.; Levin, B.; Hurtado, A.; Yee, H.; De Castro, I.P.; Jimenez, M.; Shamamian, P.; Jin, R.; Novick, R.; Pellicer, A.; et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 2004, 22, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Palese, P. Oncolytic Newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012, 7, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Aref, S.; Bailey, K.; Fielding, A. Measles to the rescue: A review of oncolytic measles virus. Viruses 2016, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Ebert, O.; Shinozaki, K.; Huang, T.-G.; Savontaus, M.J.; Garcia-Sastre, A.; Woo, S.L.C. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003, 63, 3605–3611. [Google Scholar] [PubMed]
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.-W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Lian, L.; et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene 2021, 40, 4783–4795. [Google Scholar] [CrossRef]
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Onodera, M.; Tanaka, R.; Blaese, M. Induction of therapeutic antitumor anti-angiogenesis by intratumoral injection of genetically engineered endostatin-producing Semliki Forest virus. Cancer Gene Ther. 2001, 8, 796–802. [Google Scholar] [CrossRef]
- Yamanaka, R.; Tsuchiya, N.; Yajima, N.; Honma, J.; Hasegawa, H.; Tanaka, R.; Ramsey, J.; Blaese, R.M.; Xanthopoulos, K.G. Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin-18 combined with the systemic administration of interleukin-12. J. Neurosurg. 2003, 99, 746–753. [Google Scholar] [CrossRef]
- Yamanaka, R.; Xanthopoulos, K.G. Induction of antigen-specific immune responses against malignant brain tumors by intramuscular injection of sindbis DNA encoding gp100 and IL-18. DNA Cell. Biol. 2005, 24, 317–324. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, G.; Van den Pol, A.N. Chikungunya-vesicular stomatitis chimeric virus targets and eliminates brain tumors. Virology 2018, 522, 244–259. [Google Scholar] [CrossRef]
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo, E.; Federspiel, M.J.; Galanis, E. Oncolytic measles virus strains have a significant antitumor activity against glioma stem cells. Gene Ther. 2013, 2, 444–449. [Google Scholar] [CrossRef]
- Heikkilä, J.E.; Vähä-Koskela, M.J.; Ruotsalainen, J.J.; Martikainen, M.W.; Stanford, M.M.; McCart, J.A.; Bell, J.C.; Hinkkanen, A.E. Intravenously administered alphavirus vector VA7 eradicates orthotopic human glioma xenografts in nude mice. PLoS ONE 2010, 5, e8603. [Google Scholar] [CrossRef]
- Martikainen, M.; Niittykoski, M.; von und zu Frauenberg, M.; Immonen, A.; Koponen, S. MicroRNA attenuated clone of virulent Semliki Forest virus overcomes antiviral type I interferon in resistant mouse CT-2A glioma. J. Virol. 2015, 89, 10637–10647. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.P.; Rao, X.M.; Price, J.E.; Zhou, H.S.; Lachman, L.B. Prime-boost vaccination with plasmid and adenovirus gene vaccines control HER2/neu+ metastatic breast cancer in mice. Breast Cancer Res. 2005, 7, R580–R588. [Google Scholar] [CrossRef]
- Lachman, L.B.; Rao, X.M.; Kremer, R.H.; Ozpolat, B.; Kirjakova, G.; Price, J.E. DNA vaccination against neu reduces breast cancer incidence and metastasis in mice. Cancer Gene Ther. 2001, 8, 259–268. [Google Scholar] [CrossRef]
- Kramer, M.G.; Masner, M.; Casales, E.; Moreno, M.; Smerdou, C.; Chabalgoity, J.A. Neoadjuvant administration of Semliki Forest virus expressing interleukin-12 combined with attenuated Salmonella eradicates breast cancer metastasis and achieves long-term survival in immunocompetent mice. BMC Cancer 2015, 15, 620. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate Concurrent VRP-HER2 Vaccination and Pembrolizumab for Patients with Breast Cancer. Available online: ClinicalTrials.govNCT03632941 (accessed on 2 July 2021).
- Sugiyama, T.; Yoneda, M.; Kuraishi, T.; Hattori, S.; Inoue, Y.; Sato, H.; Kai, C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013, 20, 338–347. [Google Scholar] [CrossRef]
- Cantarella, G.; Liniger, M.; Zuniga, A.; Schiller, J.T.; Billeter, M.; Naim, H.Y.; Glueck, R. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine 2009, 27, 3386–3390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, G.; Giannino, V.; Rishi, N.; Glueck, R. Immunogenicity of next-generation HPV vaccines in non-human primates: Measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine. Vaccine 2016, 34, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, J.L.; Shylankevich, M.; Su, Y.; Roberts, A.; Rose, J.K.; Zelterman, D.; Buonocore, L. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J. Virol. 2007, 81, 5749–5758. [Google Scholar] [CrossRef]
- Liao, J.B.; Publicover, J.; Rose, J.K.; DiMaio, D. Single-dose, therapeutic vaccination of mice with vesicular stomatitis virus expressing human papillomavirus type 16 E7 protein. Clin. Vaccine Immunol. 2008, 15, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.P.; McElhiney, S.; Cassetti, M.C.; Eiben, G.L.; Higgins, T.; Kovacs, G.R. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001, 61, 7861–7867. [Google Scholar] [PubMed]
- Daemen, T.; Riezebos-Brilman, A.; Bungener, L.; Regts, J.; Dontje, B.; Wilschut, J. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine 2003, 21, 1082–1088. [Google Scholar] [CrossRef]
- Van de Wall, S.; Ljungberg, K.; Ip, P.P.; Boerma, A.; Knudsen, M.L.; Nijman, H.W.; Liljeström, P.; Daemen, T. Potent therapeutic efficacy of an alphavirus replicon DNA vaccine expressing human papilloma virus E6 and E7 antigens. Oncoimmunology 2018, 7, e1487913. [Google Scholar] [CrossRef]
- Hoang-Le, D.; Smeenk, L.; Anraku, I.; Pijlman, G.P.; Wang, X.P.; De Vrij, J. A Kunjin replicon vector encoding granulocyte macrophage colony-stimulating factor for intra-tumoral gene therapy. Gene Ther. 2009, 16, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.A.; Sheahan, B.J.; Galbraith, S.E. Inhibition of angiogenesis by a Semliki Forest virus vector expressing VEGFR-2 reduces tumour growth and metastasis in mice. Gene Ther. 2007, 14, 503–513. [Google Scholar] [CrossRef]
- Ying, H.; Zaks, T.Z.; Wang, R.-F.; Irvine, K.R.; Kammula, U.S.; Marincola, F.M. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 1999, 5, 823–827. [Google Scholar] [CrossRef]
- Grossardt, C.; Engeland, C.E.; Bossow, S.; Halama, N.; Zaoui, K.; Leber, M.F.; Springfeld, C.; Jaeger, D.; Von Kalle, C.; Ungerechts, G. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 2013, 24, 644–654. [Google Scholar] [CrossRef]
- Murphy, A.M.; Morris-Downes, M.M.; Sheahan, B.J.; Atkins, G.J. Inhibition of human lung carcinoma cell growth by apoptosis induction using Semliki Forest virus recombinant particles. Gene Ther. 2000, 7, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Määttä, A.M.; Mäkinen, K.; Ketola, A.; Liimatainen, T.; Yongabi, F.N.; Vähä-Koskela, M. Replication competent Semliki Forest virus prolongs survival in experimental lung cancer. Int. J. Cancer 2008, 123, 1704–1711. [Google Scholar] [CrossRef]
- Granot, T.; Yamanashi, Y.; Meruelo, D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol. Ther. 2014, 22, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, P.; Yang, H.; Wu, Y.; Zeng, X.; Zhao, Y.; Wen, Y.; Zhao, X.; Liu, X.; Wei, Y.; et al. Live attenuated measles virus vaccine induces apoptosis and promotes tumor regression in lung cancer. Oncol. Rep. 2013, 29, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Boisgerault, N.; Guillerme, J.B.; Pouliquen, D.; Mesel-Lemoine, M.; Achard, C.; Combredet, C.; Fonteneau, J.-F.; Tangy, F.; Grégoire, M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed. Res. Int. 2013, 2013, 387362. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Jacobson, B.A.; Belgum, H.; Raza, A.; Sadiq, A.; Drees, J.; Wang, H.; Jay-Dixon, J.; Etchison, R.; Federspiel, M.J.; et al. Measles vaccine strains for virotherapy of non-small cell lung carcinoma. J. Thorac. Oncol. 2014, 9, 1101–1110. [Google Scholar] [CrossRef]
- Patel, M.R.; Jacobson, B.A.; Ji, Y.; Drees, J.; Tang, S.; Xiong, K. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget 2015, 6, 33165–33177. [Google Scholar] [CrossRef]
- McAllister, A.; Arbetman, A.E.; Mandl, S.; Pena-Rossi, C.; Andino, R. Recombinant yellow fever viruses are effective therapeutic vaccines for treatment of murine solid tumors and pulmonary metastases. J. Virol. 2000, 74, 9197–9205. [Google Scholar] [CrossRef]
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS ONE 2010, 5, e12670. [Google Scholar] [CrossRef]
- Avogadri, F.; Zappasodi, R.; Yang, A.; Budhu, S.; Malandro, N.; Hisrchhorn-Cymerman, D. Combination of alphavirus replicon particle-based vaccination with immunomodulatory antibodies: Therapeutic activity in the B16 melanoma mouse model and immune correlates. Cancer Immunol. Res. 2014, 2, 448–458. [Google Scholar] [CrossRef]
- Yin, X.; Wang, W.; Zhu, X.; Wang, Y.; Wu, S.; Wang, Z. Synergistic antitumor efficacy of combined DNA vaccines targeting tumor cells and angiogenesis. Biochem. Biophys. Res. Comm. 2015, 465, 239–244. [Google Scholar] [CrossRef]
- Ammour, Y.; Ryabaya, O.; Shchetinina, Y.; Prokofeva, E.; Gavrilova, M.; Khochenkov, D.; Vorobyev, D.; Faizuloev, E.; Shohin, I.; Zverev, V.V.; et al. The susceptibility of human melanoma cells to infection with the Leningrad-16 vaccine strain of measles virus. Viruses 2020, 12, 173. [Google Scholar] [CrossRef]
- Kimpel, J.; Urbiola, C.; Koske, I.; Tober, R.; Banki, Z.; Wollmann, G. The Oncolytic virus VSV-GP is effective against malignant melanoma. Viruses 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Dold, C.; Rodriguez Urbiola, C.; Wollmann, G.; Egerer, L.; Muik, A.; Bellmann, L.; Fiegl, H.; Marth, C.; Kimpel, J.; Von Laer, D. Application of interferon modulators to overcome partial resistance to ovarian cancers to VSV-GP oncolytic viral therapy. Mol. Ther. Oncolytics 2016, 3, 16021. [Google Scholar] [CrossRef]
- Hasegawa, K.; Nakamura, T.; Harvey, M.; Ikeda, Y.; Oberg, A.; Figini, M.; Canevari, S.; Hartmann, L.C.; Peng, K.-W. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin. Cancer Res. 2006, 12, 6170–6178. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Pham, L.; O’Connor, M.K.; Federspiel, M.J.; Russel, S.J.; Peng, K.-W. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin. Cancer Res. 2006, 12, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Granot, T.; Meruelo, D. The role of natural killer cells in combinatorial anti-cancer therapy using Sindbis viral vector and irinotecan. Cancer Gene Ther. 2012, 19, 588–591. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Wu, T.C.; Hung, C.F. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol. Ther. 2010, 18, 692–699. [Google Scholar] [CrossRef]
- Murphy, A.M.; Besmer, D.M.; Moerdyk-Schauwecker, M.; Moestl, N.; Ornelles, D.A.; Mukherjee, P. Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J. Virol. 2012, 86, 3073–3087. [Google Scholar] [CrossRef]
- Hastle, E.; Besmer, D.M.; Shah, N.R.; Murphy, A.M.; Moredyk-Schauwecker, M.; Molestina, C.; Roy, L.R.; Curry, J.M.; Mukherjee, P.; Grdzelishvili, V.Z. Oncolytic vesicular stomatitis virus in an immunocompetent model of MUC1-positive or MUC1-nulll pancreatic ductal adenocarcinoma. J. Virol. 2013, 87, 10283–10294. [Google Scholar] [CrossRef]
- Awano, M.; Fuijyki, T.; Shoji, K.; Amagai, Y.; Murakami, Y.; Furukawa, Y.; Sato, H.; Yoneda, M.; Kai, C. Measles virus selectively blind to signaling lymphocyte activity molecule has oncolytic efficacy against nectin-4 expressing pancreatic cells. Cancer Sci. 2016, 107, 1647–1652. [Google Scholar] [CrossRef]
- Msaouel, P.; Iankov, I.D.; Allen, C.; Morris, J.C.; von Messling, V.; Cattaneo, R. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009, 69, 82–91. [Google Scholar] [CrossRef]
- Son, H.A.; Zhang, L.; Cuong, B.K.; Van Tong, H.; Cuong, L.D.; Hang, N.T.; Nhung, H.T.M.; Yamamoto, N.; Toan, N.L. Combination of vaccine-strain measles and mumps viruses enhances oncolytic activity against human solid malignancies. Cancer Investig. 2018, 7, 106–117. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, S.; Luo, H.; Wan, X.; Gui, Y.; Li, J.; Wu, D. Evaluation of vesicular stomatitis virus mutant as an oncolytic agent against prostate cancer. Int. J. Clin. Exp. Med. 2014, 7, 1204–1213. [Google Scholar]
- Urbiola, C.; Santer, F.R.; Petersson, M.; van der Pluijm, G.; Horninger, W.; Erlmann, P. Oncolytic activity of the rhabdovirus VSV-GP against prostate cancer. Int. J. Cancer 2018, 143, 1786–1796. [Google Scholar] [CrossRef]
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2007, 13, 3999–4008. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: A candidate antigen for treating prostate cancer. Cancer Res. 2007, 67, 1344–1351. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Klinger, O.J.; Kast, W.M. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008, 68, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.J.; Gwin, W.; Blackwell, K.; Marcom, P.K.; Chang, S.; Maecker, H.T.; Broadwater, G.; Hyslop, T.M.; Kim, S.; Rogatko, A.; et al. Vaccine-induced memory CD8(+) T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clin. Cancer Res. 2019, 25, 2725–2736. [Google Scholar] [CrossRef]
- Komdeur, F.L.; Singh, A.; Van de Wall, S.; Meulenberg, J.J.M.; Boerma, A.; Hoogeboom, B.N.; Paijens, S.T.; Oyarce, C.; de Bruyn, M.; Schuuring, E.; et al. First-in-human phase I clinical trial of an SFVBased RNA replicon cancer vaccine against HPV-induced cancers. Mol. Ther. 2021, 29, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.J.; Hobeika, A.C.; Niedzweicki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Gwin, W.R., III; Hartman, Z.C.; et al. Long-term survival of patients with stage III colon cancer treated with VRP-CEA(6D), an alphavirus vector that increases the CD8+ effector memory T cell to Treg ration. J. Immunother. Cancer 2020, 8, e001662. [Google Scholar] [CrossRef]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef]
- Morse, M.A.; Hobelka, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Investig. 2010, 120, 3234–3241. [Google Scholar] [CrossRef]
- Griifin, D.E. Neurotropic alphaviruses. In Neurotropic Viral Infections; Reiss, C., Ed.; Springer: Cham, Switzerland, 2016; pp. 175–204. [Google Scholar]
- Vasilevska, J.; Skrastina, D.; Spunde, K.; Garoff, H.; Kozlovska, T.; Zajakina, A. Semliki Forest virus biodistribution in tumor-free and 4T1 mammary tumor-bearing mice: A comparison of transgene delivery by recombinant virus particles and naked RNA replicon. Cancer Gene Ther. 2012, 19, 579–587. [Google Scholar] [CrossRef] [PubMed]
- FDA Licenses New Vaccine for Prevention of Cervical Cancer and Other Diseases in Females Caused by Human Papillomavirus (Press release). U.S. Food and Drug Administration (FDA). 8 June 2006. Archived from the Original on 19 October 2009. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108666.htm (accessed on 2 July 2021).
- Hromic-Jahjefendic, A.; Lundstrom, K. Viral vector-based melanoma gene therapy. Biomedicines 2020, 8, 60. [Google Scholar] [CrossRef]
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine 2013, 31, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Berglund, P.; Sjöberg, M.; Garoff, H.; Atkins, G.J.; Sheahan, B.J.; Liljeström, P. Semliki Forest virus expression system: Production of conditionally infectious recombinant particles. Biotechnology 1993, 11, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Smerdou, C.; Liljeström, P. Two-helper system for production of recombinant Semliki Forest virus particles. J. Virol. 1999, 73, 1092–1098. [Google Scholar] [CrossRef] [PubMed]

| Virus/Disease | Antigen | Vector | Findings | Ref. |
|---|---|---|---|---|
| Alphaviruses | ||||
| CHIKV/CHIK | E3-E2-6K-E1 | Chimeric VSV-Env | Protection against CHIKV in mice | [32] |
| VEEV/VEE | E3-E2-6K-E1 | VEEV-Env | Protection against VEE in mice, macaques | [33] |
| WEEV/WEE | E3-E2-6K-E1 | WEEV-Env | Partial protection in macaques, strong in mice | [33] |
| EEEV/EEE | E3-E2-6K-E1 | EEEV-Env | Protection against EEE in mice, macaques | [33] |
| VEEV/VEE | V4020 strain | VEEV DNA | Protection against VEE in mice | [34] |
| VEEV/VEE | V4020 strain | VEEV DNA | Protection against VEE in macaques | [35] |
| CHIKV/CHIK | C, Env | MV-CHIKV VLPs | Protection against CHIKV in macaques | [36] |
| Arenaviruses | ||||
| LASV/LHF | GPC | VSV-GPC | Protection in guinea pigs and macaques | [37] |
| LASV/LHF | GPC | MV-LASV-GPC | Protection against LASV in macaques | [38] |
| LASV/LHF | GPC | YFV-LASV GPC | 80% protection in guinea pigs, vector instability | [39] |
| LASV/LHF | GPC G1/G2 | YFV-LASV G1 + G2 | 83% protection in guinea pigs, stable vector | [40] |
| LASV/LHF | GPC G1/G2 | YFV-LASV G1 + G2 | No protection in marmosets | [41] |
| LASV/LHF | GPC or NP | VEEV-GPC/NP | Protection in guinea pigs after 3 immunizations | [42] |
| LASV/LHF | GPC | Multivalent VEEV | Protection in inbred CBA/J mice | [43] |
| JUNV/AHF | GPC | VEEV-GPC | Protection against JUNV in mice | [44] |
| MACV/BHF | GPC | VEEV-GPC | Protection against MACV in mice | [44] |
| Filoviruses | ||||
| EBOV/EVD | GP D637L | KUN-GP D637L | Protection in 75% of nonhuman primates | [45] |
| EBOV/EVD | GP | VSV-GP | Protection against two EBOV strain in macaques | [46,47] |
| MARV/MHF | GP | VSV-GP | Protection against MARV in macaques | [48] |
| SUDV/EVD | GP | VEEV-GP | Protection against SUDV and EBOV in macaques | [48] |
| Flaviviruses | ||||
| DENV/DF | E85 | VEEV-E85 | Protection against DENV in mice | [49] |
| DENV/DF | ED3 | MV-ED3 | Strong immunogenicity, partial protection in mice | [50] |
| DENV/DF | Tetravalent DENV | YFV (CYD-TDV) | Good safety, immunogenicity in rodents, primates | [51,52] |
| DENV/DF | Tetravalent DENV | YFV (CYD-TDV) | Approved vaccine for endemic populations | [53] |
| ZIKV/ZVD | prME | VEEV-NLC RNA | Protection in mice with 10 ng of RNA | [54] |
| ZIKV/ZVD | prME | Chimeric VSV-prME | Protection against ZIKV in mice | [32] |
| ZIKV/ZVD | E-NS1 | VSV-E-NS1 | Protection against ZIKV in mice | [55] |
| Hepatotropic | ||||
| HBV/Hepatitis | HBsAg | MV-HBsAg | Protection against HBV in 50% of rhesus monkeys | [56] |
| HBV/Hepatitis | MHB | SFV-MHB | Protection against HBV in mice | [57] |
| HBV/Hepatitis | HBcAg | SFV-HBcAg | No protection against HBV in mice | [57] |
| Lentiviruses | ||||
| HIV/AIDS | HIV gp160 Env | MV-gp160 Env | Humoral and cellular immune responses in mice | [58] |
| HIV/AIDS | HIV Env | SFV-Env | Superior immunogenicity compared to immunization with DNA and Env protein | [59] |
| HIV/AIDS | HIV Env | SFV-Env RNA | Immune response in 75% of mice | [60] |
| HIV/AIDS | HIV Env/Gag/PolRT | SFV RPs/RNA | VLPs superior immunogenicity to RNA in mice | [61] |
| HIV/AIDS | HIV Env, Gag/Pol/Nef | SFV DNA | Robust immune responses in mice | [62] |
| HIV/AIDS | HIV gp140 | VEEV-RNA-CNE | Superior Ab response compared to VLPS in primates | [63] |
| Influenza Viruses | ||||
| IFVA/Influenza | HA | MV AIK-C-HA | Protection against influenza virus in cotton rats | [64] |
| IFVA/Influenza | HA, NA | VSVΔG-HA/NA | Protection against influenza virus in mice | [65] |
| IFVA/Influenza | HAfl | VSV-HAfl | Protection against influenza virus in mice | [66] |
| IFVA/Influenza | HA | VEEV-HA | Protection in chickens | [67] |
| IFVA/Influenza | HA | SFV-HA RNA | Protection in 90% of mice | [68] |
| IFVA/Influenza | HA | VEEV-HA RNA | Protection in mice with 64-fold less RNA * | [69] |
| IFVA/Influenza | M2e | SIN E2S1-M2e | Protection in mice | [70] |
| IFVA/Influenza | HA, NP | CSFV-HA/NP VRPs | Strong humoral and cellular response in pigs | [71] |
| Coronaviruses | ||||
| SARS-CoV/SARS | S | VEEV-S | Protection against SARS-CoV in mice | [72] |
| MERSCoV/MERS | S | VSVΔG-S | Neutralizing Abs and T cell responses in monkeys | [73] |
| SARS-CoV-2/COV | S | MV-S | Th1-biased Ab and T cell responses in mice | [74] |
| SARS-CoV-2/COV | S | MV (TMV-083) | Phase I: weak immunogenicity, trial discontinued | [75,76] |
| SARS-CoV-2/COV | S | VSV-S | Neutralizing Abs, protection in mice | [77] |
| SARS-CoV-2/COV | S | VSV (V590) | Phase I: weak immunogenicity, trial discontinued | [78,79] |
| SARS-CoV-2/COV | S | VSVΔG-S | Protection against SARS-CoV-2 in hamsters | [80] |
| SARS-CoV-2/COV | S | VSVΔG-S | Phase I/II: study in progress | [81,82] |
| PEDV/PED | S fragment | BVDV | Neutralization of BVDV and PEDV in mice | [83] |
| SARS-CoV-2/COV | S | LNP-VEEV-S RNA | Robust Ab responses in mice | [84] |
| SARS-CoV-2/COV | S | LNP-VEEV-S RNA | Phase I/II: study in progress | [85] |
| SARS-CoV-2/COV | S | LUNAR-VEEV RNA | Protection in mice after single dose | [86] |
| SARS-CoV-2/COV | S | SIN-S + αOX40 | Protection against SARS-CoV-2 in mice | [87] |
| Bacterial | ||||
| B. anthracis/Anthrax | PA | SIN-PA | Immune responses, some protection in mice | [88] |
| B. abortus/Brucellosis | B. abortus IF3 | SFV-CS | Immune responses, protection in mice | [89] |
| Parasitic | ||||
| Plasmodium/Malaria | Pf332 antigen | SFV-Pf332 | Robust Th1-type immune response in mice | [90] |
| Plasmodium/Malaria | P. yoelii CS epitope | SIN-CS | Protection against malaria in mice | [91] |
| Leishmania/Leishmaniasis | PpSP15-LmSTI1 | SFV-PpSP15-LmSTI1 | Superior expression from replicon RNA | [92] |
| Cancer | Antigen/Therapeutic | Vector | Findings | Ref. |
|---|---|---|---|---|
| Brain | ||||
| Glioblastoma | Endostatin | SFV | Complete tumor regression in mice | [119] |
| Glioblastoma | IL-18 | DC-SFV + IL-12 | Enhanced Th1-type response, anti-tumor immunity | [120] |
| Glioblastoma | gp100, IL-18 | SIN DNA | Therapeutic effect, prolonged survival in mice | [121] |
| Glioblastoma | CHIKV E3-E2-6K-E1 | VSVΔG-CHIKV | Selective infection, elimination of tumor cells | [122] |
| Glioblastoma | GFP, CEA, NIS | GSC-MV | Anti-tumor effect, prolonged survival in mice | [123] |
| Glioblastoma | EGFP | SFV VA | Tumor inhibition, prolonged survival in mice | [124] |
| CT-2A glioma | miRT124 | SFV4 | Replication in tumor cells, prolonged survival | [125] |
| Breast | ||||
| A2L2 | HER2/neu | Ad/SIN DNA | Prolonged survival in mice | [126] |
| A2L2 | HER2/neu | Ad + SIN DNA | Tumor protection in mice with 80% less DNA | [127] |
| HER2 | HER2 ECD, TMs | VEEV (VRP-HER2) | Preventive, therapeutic tumor growth control in mice | [128] |
| 4T1 | IL-12 | SFV + S. typhimurium | Inhibition of metastasis, long-term survival in mice | [129] |
| TNBC | M1 | M1 + Doxorubicin | Synergistic effect of M1 and Doxorubicin | [118] |
| MCF7 | SLAMblind | MV | Targeting and killing of breast cancer cells | [130] |
| Cervical | ||||
| HPV-16 | Capsid | MV | Humoral immune responses in mice | [131] |
| HPV-16 | Capsid | MV + HPV protein | IgG and neutralizing antibody responses | [132] |
| CRPV | E1, E2, E6, E7 | VSV | Reduced papilloma volumes, elimination of disease | [133] |
| HPV-16 | E7 | VSV | Tumor regression in mice | [134] |
| HPV-16 | E7 | VEEV | Immune response, protection against tumors in mice | [135] |
| HPV-16 | E6/E7 fusion | SFVEnh | Tumor regression, complete eradication | [136] |
| HPV | E6-E7 | SFV DNA + EP | 85% of immunized mice became tumor-free | [137] |
| Colon | ||||
| CT26 | GM-CSF | KUN | Tumor regression, cure in 50% mice | [138] |
| CT26 | VEGFR-2 | SFV | Inhibition of tumor growth, metastasis | [139] |
| CT26 | VEGFR-2 + IL-4 | SFV | Super immunogenicity, prolonged survival | [139] |
| CT26 | LacZ | SFV RNA | Tumor regression, protection against tumor cells | [140] |
| MC28cea | GM-CSF | MV | Tumor regression, prevention of re-engraftment | [141] |
| Lung | ||||
| H358cea | EGFP | SFV | Protection against HBV in 50% of rhesus monkeys | [142] |
| A549 | EGFP | SFV VA | Superior survival compared to adenovirus delivery | [143] |
| CT26 | LacZ | SIN | Complete tumor remission, prolonged survival | [144] |
| CL25 | oMV | MV Hu-191 | Suppressed tumor growth, prolonged survival | [145] |
| LLC | oMV | MV Schwarz | Suppression of uncontrollable tumor growth | [146] |
| Adenocarcinoma | CEA | MV | Tumor regression in mice | [147] |
| H2009, A549 | IFNβ | VSV | Tumor regression in mice | [148] |
| LM2 | IFNβ | VSV | Prolonged survival, cure in 30% of mice | [148] |
| Melanoma | ||||
| B16-OVA | GM-CSF | KUN | Tumor regression, cure of more than 50% of mice | [138] |
| B16-OVA, B16F0 | SIIINFEKL epitope | YFV | Immune response, protection in mice | [149] |
| B16 | TRP-2 | VEEV | Immune response, prolonged survival in mice | [150] |
| B16 | TRP-2 | VEEV + GITR mAb | Complete tumor regression in 90% of mice | [151] |
| B16 | TRP-2 | VEEV + CTLA-4 mAb | Complete tumor regression in 50% of mice | [151] |
| B16 | VEGFR-2/IL-12 + survivin/β-hCG | SFV DNA | Superior tumor growth inhibition, prolonged survival after combination therapy | [152] |
| mel Z | oMV | MV L-16 | Tumor cell killing, inhibition of tumor growth | [153] |
| B16-OVA | LCMV GP | VSV | Efficacy in subcutaneous tumor models | [154] |
| Ovarian | ||||
| A2780 | LCMV GP | VSV + ruxolitinib | Tumor regression in mice | [155] |
| SKOV3ip.1 | αFR scFV | MV | Tumor volume reduction, prolonged survival | [156] |
| SKOV3ip.1 | CEA, NIS | MV | Dual therapy superior in mice | [157] |
| ES2 | IL-12 | SIN + irinotecan | Long-term survival in mice | [158] |
| MOSEC | OVA | SFV + VV | Immune response, enhanced anti-tumor activity | [159] |
| Pancreatic | ||||
| PDAC | GFP | VSV | Superior oncolytic activity compared to Sendai, RSV | [160] |
| PDAC | GFP | VSV-ΔM51 | Anti-tumor activity enhanced by gemcitabine | [161] |
| KLM1 | SLAMBlind | MV | Suppression of tumor growth in mice | [162] |
| Capan-2 | SLAMBlind | MV | Suppression of tumor growth in mice | [162] |
| Prostate | ||||
| PC-3 | CEA | MV | Delay in tumor growth, prolonged survival in mice | [163] |
| PC-3 | oMv, oMuV | MV + MuV | Immune responses, protection in mice | [164] |
| DU145, PC-3 | GFP | VSV-ΔM51 | Apoptosis in tumor cells, prolonged survival | [165] |
| DU-145, 22Rv1 | LCMV GP | VSV | Long-term remission in mice | [166] |
| TRAMP-C | PSMA | VEEV | PSMA-specific immune response in mice | [167] |
| TRAMP | STEAP | VEEV | STEAP-specific immune response, prolonged survival | [168] |
| TRAMP | PSCA | VEEV | Long-term survival for 12 months in 90% of mice | [169] |
| Cancer | Antigen/Therapeutic | Vector | Findings | Ref. |
|---|---|---|---|---|
| Breast | ||||
| HER2 | HER2 ECD TMs | VEEV (VRP-HER2) | Phase I: immune response, PR and SD | [128] |
| HER2 | HER2 ECD TMs | VEEV (VRP-HER2) | Phase II: study in progress | [170] |
| Cervical | ||||
| HPV-16 | E6/E7 fusion | SFVEnh (Vvax001) | Phase I: safe, immune responses in all patients | [171] |
| Colon | ||||
| Stage III-IV | CEA | VEEV | Phase I: immune responses, prolonged survival | [172] |
| Ovarian | ||||
| RROC | CEA | MV | Phase I: well-tolerated, dose-dependent activity | [173] |
| Pancreatic | ||||
| Metastatic | CEA | VEEV | Phase I: immunogenicity, prolonged overall survival | [174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundstrom, K. Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer. Vaccines 2021, 9, 1187. https://doi.org/10.3390/vaccines9101187
Lundstrom K. Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer. Vaccines. 2021; 9(10):1187. https://doi.org/10.3390/vaccines9101187
Chicago/Turabian StyleLundstrom, Kenneth. 2021. "Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer" Vaccines 9, no. 10: 1187. https://doi.org/10.3390/vaccines9101187
APA StyleLundstrom, K. (2021). Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer. Vaccines, 9(10), 1187. https://doi.org/10.3390/vaccines9101187





