A Data-Driven Digital Application to Enhance the Capacity Planning of the COVID-19 Vaccination Process
Abstract
:1. Introduction
1.1. Contribution
1.2. Problem Description
x% of persons in priority class A have to wait y time units to be vaccinated.
- The number and locations of the medical hubs.
- The available vaccination capacity of a medical hub.
- The number of healthcare professionals (authorized to administer the vaccine).
- The number of available vaccines per medical hub.
2. Modelling Approach
- Determining the optimal locations of the medical hubs.
- Determining the optimal distribution of the medical capacity (i.e., the available vaccines and healthcare workers) over the medical hubs.
- Creating a simulation of the vaccination process.
2.1. Location of the Medical Hubs
- As we use heuristic Euclidean distances between the centroids of municipalities, the “true” travel distance, which can be calculated by using route planners (depending on the travel modality), may exceed the maximum travel distance.
- The heuristic does not take the costs concerned with opening a hub into account. This was a design decision, as these costs were unknown beforehand. However, as the algorithm aims to minimize the number of hubs, it does work in favor of lowering the hub opening costs.
| Algorithm 1. Compute locations of the hubs. | |
| 1: | every municipality has its own hub. |
| 2: | while not every hub is done do |
| 3: | select the smallest hub that is not done yet (e.g., hub A) |
| 4: | find the nearest hub to the smallest hub (e.g., hub B) |
| 5: | if the maximal distance constraint is violated then |
| 6: | hub A is done |
| 7: | else |
| 8: | if distance constraint between center hub A and all municipalities in hub B are not violated then |
| 9: | merge municipalities hub B in hub A |
| 10: | remove hub B from hub list |
| 11: | else if distance constraint between center hub B and all municipalities in hub A are not violated then |
| 12: | merge municipalities hub A in hub B |
| 13: | remove hub A from hub list |
| 14: | else |
| 15: | hub A is done |
| 16: | end if |
| 17: | end if |
| 18: | end while |
2.2. Distribution of Medical Capacity
- Equal distribution of vaccines across the hubs—The available number of vaccines per day is equally distributed across the hubs that are not finished with vaccinating yet. Thus, if on a given day, H hubs are not finished with vaccinating, and there are V vaccines available for that day, then each of those H hubs will get the same number of vaccines, namely:The advantage of this method is that each hub will receive the same number of vaccines, independent of its size. As a result, smaller hubs will be finished vaccinating earlier than larger hubs. Consequently, larger hubs will eventually get more vaccines per day once the number of finished hubs increases and are no longer considered in the division of vaccines. A major disadvantage of this method is that, during the early stage, densely populated areas will not receive more vaccines, which increases the risk of a local COVID-outbreak.
- Proportional allocation of vaccines over hubs—This strategy is based on the number of susceptible persons. For this strategy, the number of non-vaccinated persons (i.e., susceptible persons) in the whole country or region, , and the number of non-vaccinated persons for each hub, , are determined. The number of available vaccines per day is denoted as V. To calculate the number of vaccines that each hub gets per day, the following formula is used:Over time, every hub will get the same number of vaccines, as the proportional distribution has not been altered. As a result, every hub will be finished vaccinating almost simultaneously. An advantage of using this proportional allocation is that the areas that are more likely to endure local outbreaks (due to a high number of non-vaccinated persons) will receive more vaccines.As both approaches have been implemented for the allocation of medical capacity, which includes vaccines and health professionals, a short summary can be found below that discusses each separately.
- Vaccine allocation—The pseudo-code of the vaccine allocation algorithm is shown in Algorithm 2. The implementation consists of two parts: the allocation and the number of shots required. Being able to deal with both one- and two-shot vaccinations requires more advanced algorithms. For example, once a person is vaccinated for the first time, another shot from today’s supply should be reserved for the person to be given later to make sure it will be available when needed. To support this additional feature, the time period between the first and second shot has been added as an additional input parameter. In this way, it is ensured that there are enough vaccines available to provide persons with their second shot, independently of the timing when this would take place. The reason for choosing this approach is to make the model more realistic as it resembles the strategy of the Dutch government.
- Healthcare worker allocation—Algorithm 3 shows the pseudo-code of the healthcare worker allocation algorithm. Before applying the equal and proportional approach, which is similar to the vaccine allocation, a distinction should be made between a single- or double-shot vaccine. In case of the double shot vaccine, the algorithm reserves sufficient capacity for the second shot. Once the required “second shot capacity” has been determined, the remaining number of healthcare workers get divided over the hubs either proportionally or equally.
| Algorithm 2. Division of vaccines. | |
| 1: | With some divisions, it is necessary to floor (**) the resulting numbers. |
| 2: | for every hub do |
| 3: | if allocation is proportional then |
| 4: | Total left over: compute the national number of people that are left over from all priority classes |
| 5: | Hub left over: compute the number of people that are left over from all priority classes on a hub-level |
| 6: | if Hub left over is 0 then |
| 7: | Hub is done vaccinating new people. |
| 8: | else |
| 9: | (**) Amount of vaccines: hub left over / total left over * number of vaccines available |
| 10: | end if |
| 11: | else if allocation is equal then |
| 12: | Left over hubs: compute the number of hubs that are still busy vaccinating new people |
| 13: | if hub is still busy vaccinating new people then |
| 14: | (**) Amount of vaccines: number of vaccines available / left over hubs |
| 15: | else |
| 16: | Hub is done vaccinating new people. |
| 17: | end if |
| 18: | end if |
| 19: | end for |
| Algorithm 3. Division of healthcare workers. | |
| 1: | With some divisions, it is necessary to floor (**) the resulting numbers and with others to ceil (*). |
| 2: | if two shots are necessary then |
| 3: | for every hub do |
| 4: | Second shot: check how many people come for a second shot. |
| 5: | (*) Minimal amount of healthcare workers: second shot / (8 hours * vaccination speed per hour per healthcare worker) |
| 6: | end for |
| 7: | end if |
| 8: | Compute the remaining number of available healthcare workers. |
| 9: | for every hub do |
| 10: | if allocation is proportional then |
| 11: | Total left over: compute the national number of people that are left over from all priority classes |
| 12: | Hub left over: compute the number of people that are left over from all priority classes on a hub-level |
| 13: | (**) Amount of healthcare workers: hub left over / total left over * number of healthcare workers available |
| 14: | else if allocation is equal then |
| 15: | Left over hubs: compute the number of hubs that are still busy vaccinating new people |
| 16: | if hub is still busy vaccinating new people then |
| 17: | (**) Amount of healthcare workers: number of healthcare workers available / left over hubs |
| 18: | else |
| 19: | Amount of healthcare workers: 0 |
| 20: | end if |
| 21: | end if |
| 22: | end for |
2.3. The Vaccination Process
3. Decision Support System
3.1. Input Parameters
- The number of available vaccines per period: To include uncertainty regarding the number of available vaccines, one can dynamically fill in the number of available vaccines per day per period. Thus, different amounts can be used for various dates.
- The number of vaccines per hour per healthcare worker: The (average) number of vaccines that can be administered by one healthcare worker per hour, given that a working day is 8 h long.
- The number of healthcare workers per day: The total number of available healthcare workers per day. These healthcare workers will be distributed across the hubs according to the selected allocation strategy.
- The allocation of healthcare workers and vaccines: Two different allocation strategies can be chosen from: ‘equal’ and ‘proportional.’ By choosing the option ’equal’ the available number of healthcare workers and vaccines will be equally distributed across all hubs. On the other hand, by choosing ’proportional’ the available number of healthcare workers and vaccines will be divided between the hubs according to the proportion of inhabitants that still need to be vaccinated in that hub.
- The vaccination strategy prioritization: The Dutch government has defined priority classes. For this model specifically, only three have been considered: healthcare workers, non-vulnerable elderly (65+), and non-vulnerable adults (18–65). However, this input parameter has been implemented to account for the continuously changing order of vaccination prioritization. It enables the user to switch the order in which these three priority classes should be vaccinated.
- The number of vaccine shots: The user can select whether one or two shots (doses) are required. As the majority of available vaccines in the Netherlands require two shots, the latter option is set as the default value.
- The number of days between the shots: This is an essential input parameter, as the number of days between two shots can vary between vaccines. In addition, it should be mentioned that once a person receives the first shot (of a two-shot vaccine), the second shot is instantly saved and is not given to anyone else. This ensures that each person who is vaccinated once gets the other shot, regardless of the storage of available vaccines.
- The maximum acceptable travel distance: The maximum distance (in kilometers) that persons are willing to travel to receive their vaccine.
- The coverage ratio: The proportion of the Dutch population that should be vaccinated.
- The willingness to get vaccinated: The proportion of persons that is willing to get vaccinated (per priority class).
3.2. Output
- The total number and location of the (medical) hubs.
- The overall waiting time.
- The waiting times per priority class (healthcare workers, elderly, and adults).
3.2.1. Overview Tab
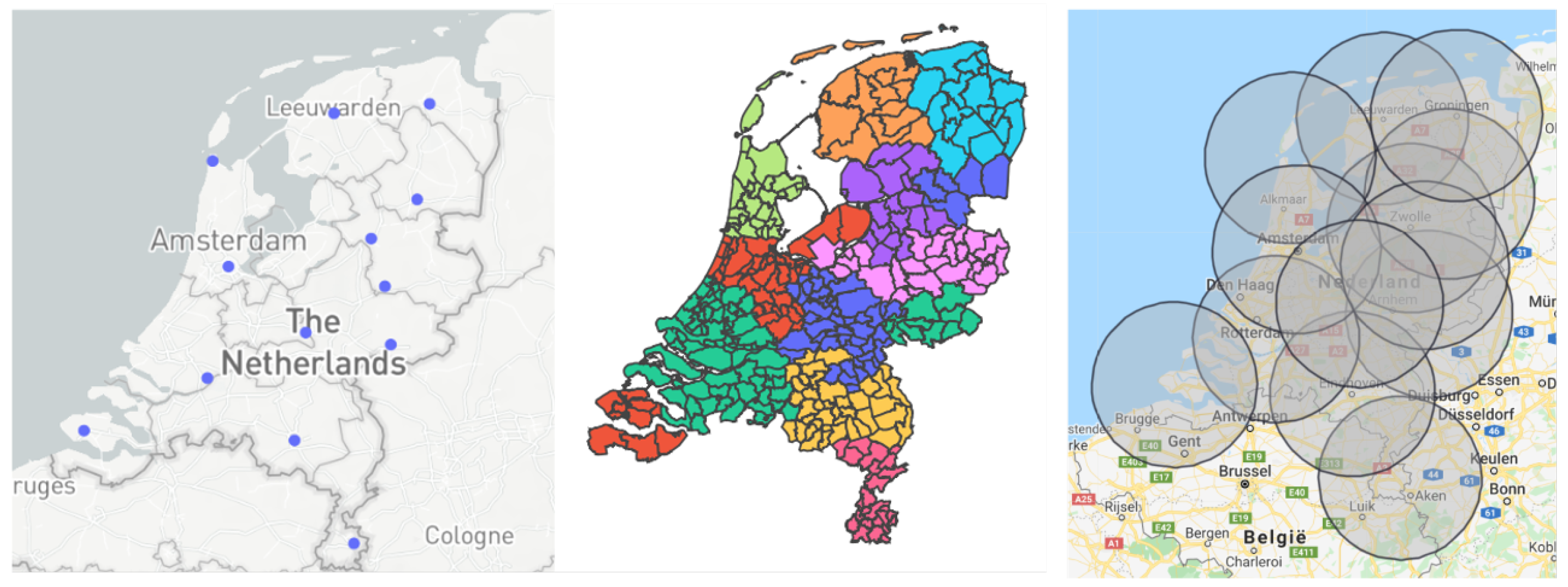
- Location of the hubs: This graph shows the placement of the medical hubs in the Netherlands, where each point denotes a single hub. The user can hover over the hubs, which will provide each medical hub’s location.
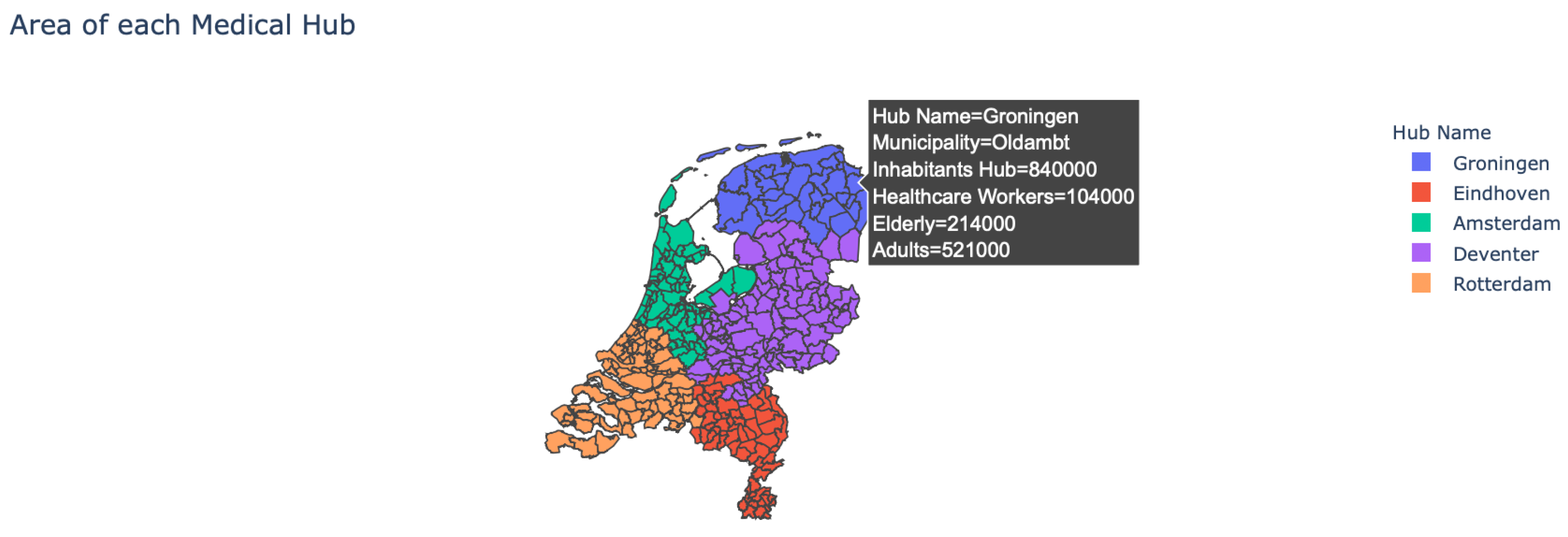
- Area of the hubs: The user is provided with the option to see which municipality belongs to which medical hub, as multiple municipalities can belong to a single hub (see Figure 2). In addition, by hovering over the map the user can see more detailed information about each municipality.
- Impact travel distance: The user can see the effect that the maximum travel distance has on the number and location of the medical hubs.
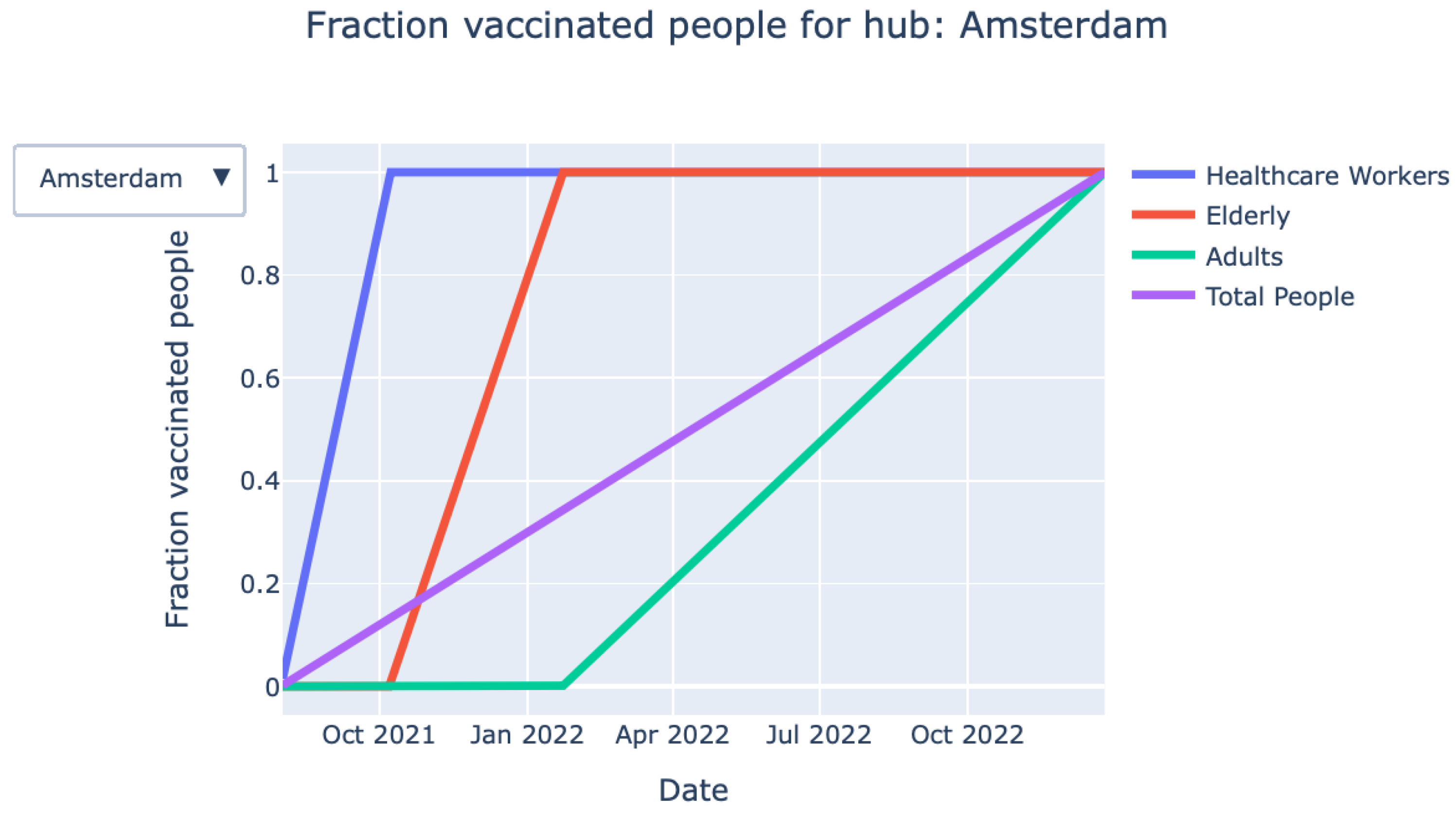
- Vaccination process over time (graph): The graph shown in Figure 3 provides detailed information on the fraction of vaccinated persons over time for each priority class. The exact information on when a particular priority class is finished with vaccinating is also given by the KPIs at the top of the page.
- Vaccination process over time (animation): Closely related to the graph mentioned above, an animation of the vaccination process over time can be found.
3.2.2. More Detailed Information Tab
- Division of healthcare workers/vaccines: By clicking the Select division button, the user can choose between healthcare workers and vaccines. The visual (see Figure 4) shows how the vaccines are allocated across the medical hubs over time.
- The fraction of vaccinated persons:Figure 4 shows the division of vaccines across hub over time on an aggregate (i.e., national) level. However, the dashboard also provides the user with in-depth information on hub level.
- The fraction of persons with the first shot (active): This graph, shown in Figure 5, will only appear when the option “two shots” is selected. It shows, for each priority class, the fraction of persons who have received their first vaccine shot over time on a national level.
4. Implementation in Practice
4.1. Assigning Inhabitants to Medical Hubs
5. Conclusions Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- KPMG. Five Ways to Optimize the COVID-19 Vaccine Supply Chain. Available online: https://assets.kpmg/content/dam/kpmg/xx/pdf/2020/12/five-ways-to-optimize-the-covid-19-vaccine-supply-chain.pdf (accessed on 1 April 2020).
- Goralnick, E.; Kaufmann, C.; Gawande, A.A. Mass-vaccination sites—An essential innovation to curb the covid-19 pandemic. N. Engl. J. Med. 2021, 384, e67. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.R.; Blumenstock, J. Lessons relearned? H1N1, COVID-19, and vaccination planning. J. Public Health Manag. Pract. 2021, 27, S106–S110. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, S.S.; Pudalov, N.J.; Fu, L.Y. Planning for a COVID-19 vaccination program. JAMA 2020, 323, 2458–2459. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, M.D.M.; Mendoza, A.J.M.; Gueco, G.E.A.; Maghinang, C.M.P.; Gopez, J.M.W. Transparency of the national government as key in promoting the rollout of COVID-19 vaccines. J. Public Health 2021, 43, e381–e382. [Google Scholar] [CrossRef]
- Asgary, A.; Najafabadi, M.M.; Karsseboom, R.; Wu, J. A Drive-through Simulation Tool for Mass Vaccination during COVID-19 Pandemic. Healthcare 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Tavana, M.; Govindan, K.; Nasr, A.K.; Heidary, M.S.; Mina, H. A mathematical programming approach for equitable COVID-19 vaccine distribution in developing countries. Ann. Oper. Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Leithaeuser, N.; Schneider, J.; Johann, S.; Krumke, S.; Streicher, M.; Schmidt, E.; Scholz, S. Quantifying Covid19-Vaccine Location Strategies For Germany. BMC Health Serv. Res. 2021, 21, 1–18. [Google Scholar]
- Krzysztofowicz, S.; Osińska-Skotak, K. The Use of GIS Technology to Optimize COVID-19 Vaccine Distribution: A Case Study of the City of Warsaw, Poland. Int. J. Environ. Res. Public Health 2021, 18, 5636. [Google Scholar] [CrossRef]
- Sun, X.; Andoh, E.A.; Yu, H. A simulation-based analysis for effective distribution of COVID-19 vaccines: A case study in Norway. Transp. Res. Interdiscip. Perspect. 2021, 11, 100453. [Google Scholar]
- Lemaitre, J.C.; Pasetto, D.; Zanon, M.; Bertuzzo, E.; Mari, L.; Miccoli, S.; Casagrandi, R.; Gatto, M.; Rinaldo, A. Optimizing the spatio-temporal allocation of COVID-19 vaccines: Italy as a case study. medRxiv 2021. [Google Scholar] [CrossRef]
- Jacobson, S.H.; Sewell, E.C.; Proano, R.A.; Jokela, J.A. Stockpile levels for pediatric vaccines: How much is enough? Vaccine 2006, 24, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Herrmann, J.W.; Jordan, C.S.; Treadwell, M.; Wood, K. Montgomery county’s public health service uses operations research to plan emergency mass dispensing and vaccination clinics. Interfaces 2006, 36, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Arinaminpathy, N.; Ratmann, O.; Koelle, K.; Epstein, S.L.; Price, G.E.; Viboud, C.; Miller, M.A.; Grenfell, B.T. Impact of cross-protective vaccines on epidemiological and evolutionary dynamics of influenza. Proc. Natl. Acad. Sci. USA 2012, 109, 3173–3177. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Cho, S.H.; Zhang, F. Contracting for on-time delivery in the US influenza vaccine supply chain. Manuf. Serv. Oper. Manag. 2016, 18, 332–346. [Google Scholar] [CrossRef] [Green Version]
- Bertsimas, D.; Digalakis Jr, V.; Jacquillat, A.; Li, M.L.; Previero, A. Where to locate COVID-19 mass vaccination facilities? Nav. Res. Logist. (NRL) 2021. [Google Scholar] [CrossRef]
- Chowdhury, P.; Paul, S.K.; Kaisar, S.; Moktadir, M.A. COVID-19 pandemic related supply chain studies: A systematic review. Transp. Res. Part Logist. Transp. Rev. 2021, 148, 102271. [Google Scholar] [CrossRef]
- Adeleke, O.J.; Olukanni, D.O. Facility location problems: Models, techniques, and applications in waste management. Recycling 2020, 5, 10. [Google Scholar] [CrossRef]
- Daskin, M.S. Network and Discrete Location: Models, Algorithms, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Lee, E.K.; Chen, C.H.; Pietz, F.; Benecke, B. Modeling and optimizing the public-health infrastructure for emergency response. Interfaces 2009, 39, 476–490. [Google Scholar] [CrossRef]
- Cormen, T.H.; Leiserson, C.E.; Rivest, R.L.; Stein, C. Introduction to Algorithms; MIT Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Stork, J.; Eiben, A.E.; Bartz-Beielstein, T. A new taxonomy of global optimization algorithms. Nat. Comput. 2020, 1–24. [Google Scholar] [CrossRef]
- Talbi, E.G. Metaheuristics: From Design to Implementation; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 74. [Google Scholar]
- Nesmachnow, S. An overview of metaheuristics: Accurate and efficient methods for optimisation. Int. J. Metaheuristics 2014, 3, 320–347. [Google Scholar] [CrossRef]
- Stegherr, H.; Heider, M.; Hähner, J. Classifying Metaheuristics: Towards a unified multi-level classification system. Nat. Comput. 2020, 1–17. [Google Scholar] [CrossRef]
- Ezugwu, A.E.; Shukla, A.K.; Nath, R.; Akinyelu, A.A.; Agushaka, J.O.; Chiroma, H.; Muhuri, P.K. Metaheuristics: A comprehensive overview and classification along with bibliometric analysis. Artif. Intell. Rev. 2021, 1–80. [Google Scholar] [CrossRef]
- Feo, T.A.; Resende, M.G. Greedy randomized adaptive search procedures. J. Glob. Optim. 1995, 6, 109–133. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markhorst, B.; Zver, T.; Malbasic, N.; Dijkstra, R.; Otto, D.; van der Mei, R.; Moeke, D. A Data-Driven Digital Application to Enhance the Capacity Planning of the COVID-19 Vaccination Process. Vaccines 2021, 9, 1181. https://doi.org/10.3390/vaccines9101181
Markhorst B, Zver T, Malbasic N, Dijkstra R, Otto D, van der Mei R, Moeke D. A Data-Driven Digital Application to Enhance the Capacity Planning of the COVID-19 Vaccination Process. Vaccines. 2021; 9(10):1181. https://doi.org/10.3390/vaccines9101181
Chicago/Turabian StyleMarkhorst, Berend, Tara Zver, Nina Malbasic, Renze Dijkstra, Daan Otto, Rob van der Mei, and Dennis Moeke. 2021. "A Data-Driven Digital Application to Enhance the Capacity Planning of the COVID-19 Vaccination Process" Vaccines 9, no. 10: 1181. https://doi.org/10.3390/vaccines9101181
APA StyleMarkhorst, B., Zver, T., Malbasic, N., Dijkstra, R., Otto, D., van der Mei, R., & Moeke, D. (2021). A Data-Driven Digital Application to Enhance the Capacity Planning of the COVID-19 Vaccination Process. Vaccines, 9(10), 1181. https://doi.org/10.3390/vaccines9101181





