Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks
Abstract
:1. Introduction
2. Materials and Methods
3. Results
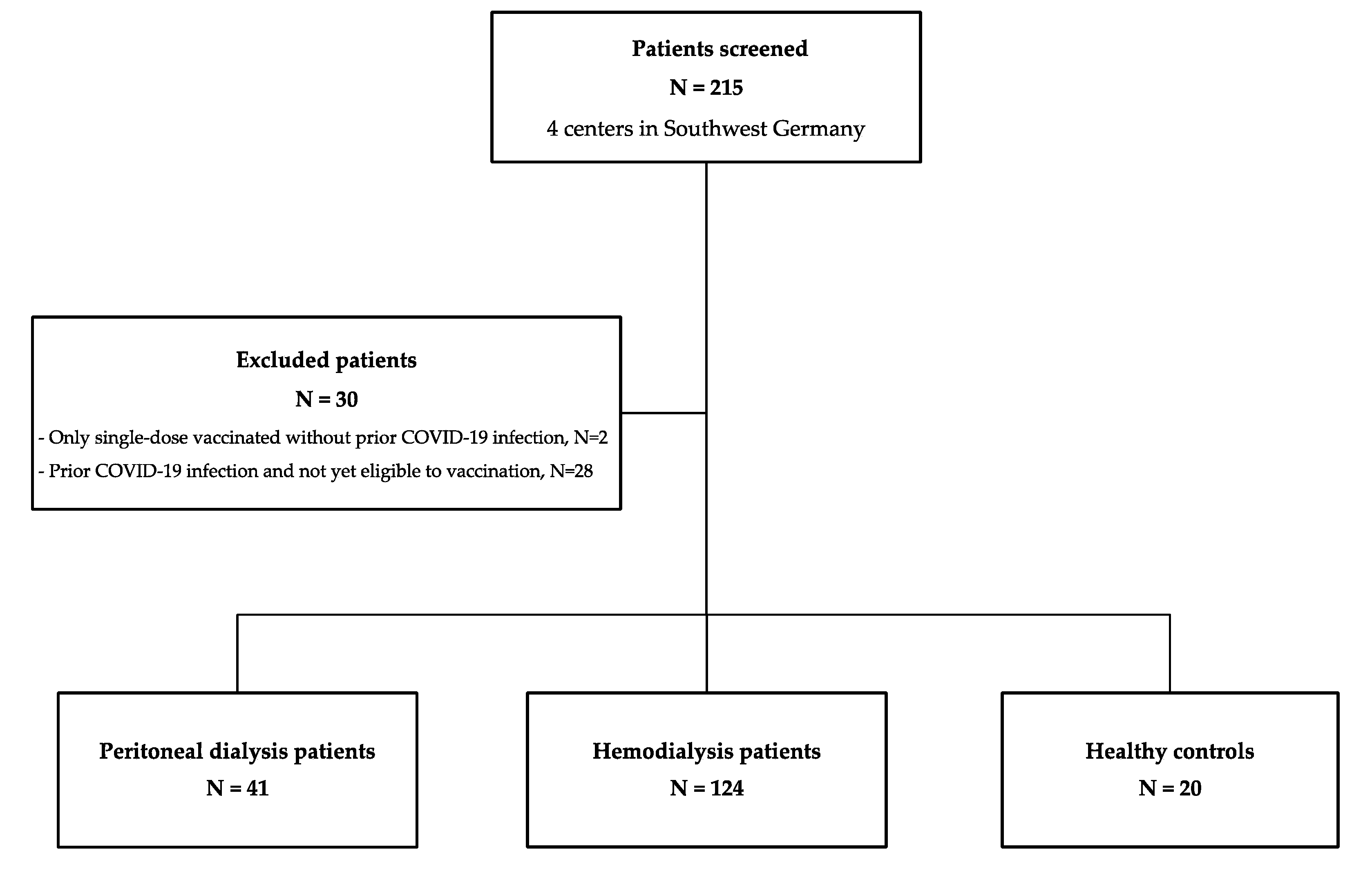
3.1. Baseline Characteristics
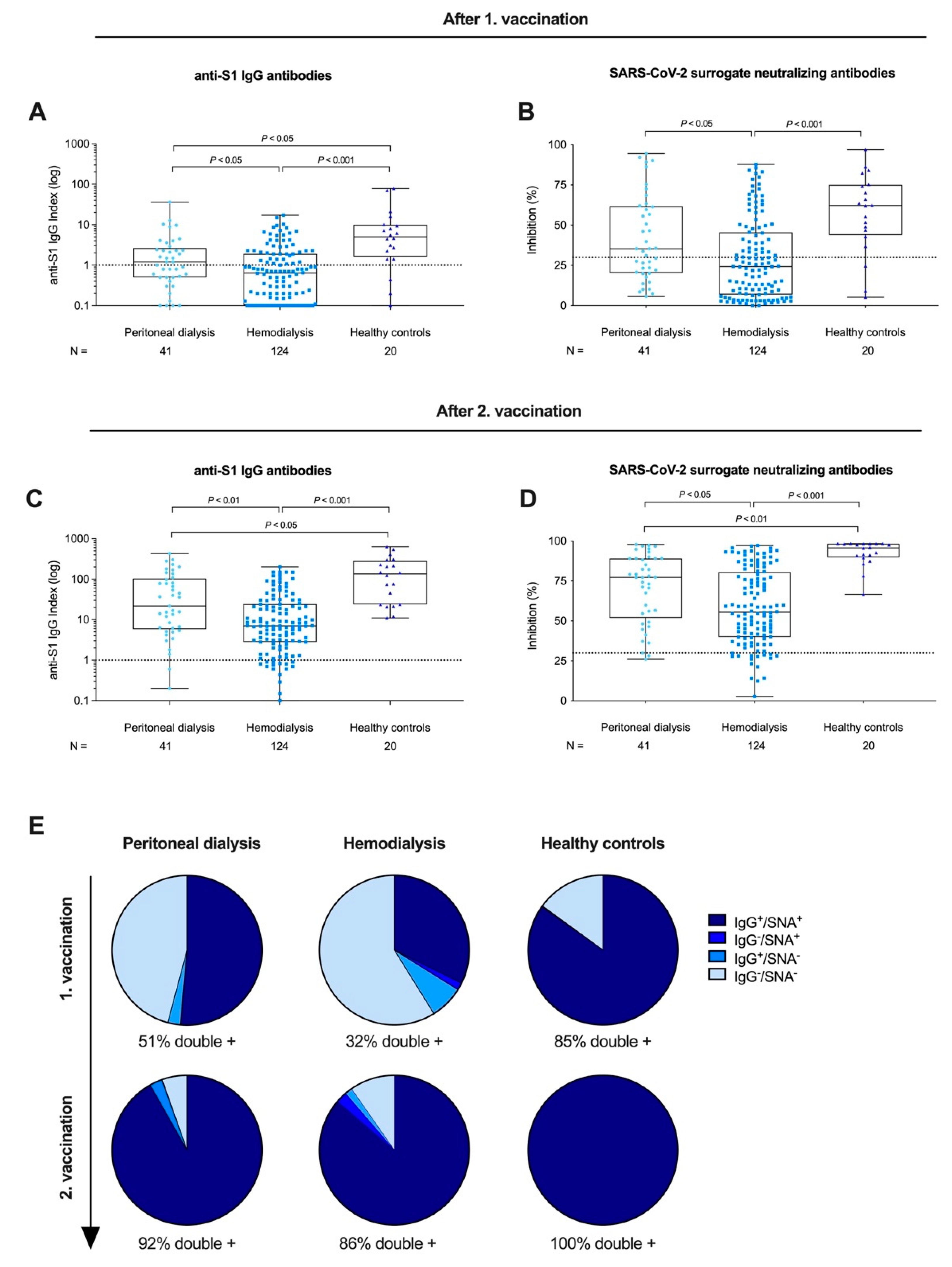
3.2. Anti-S1 IgG and Surrogate Neutralizing Antibodies after First and Second BNT162b2 Vaccination in Peritoneal Dialysis Patients, Hemodialysis Patients, and Healthy Controls
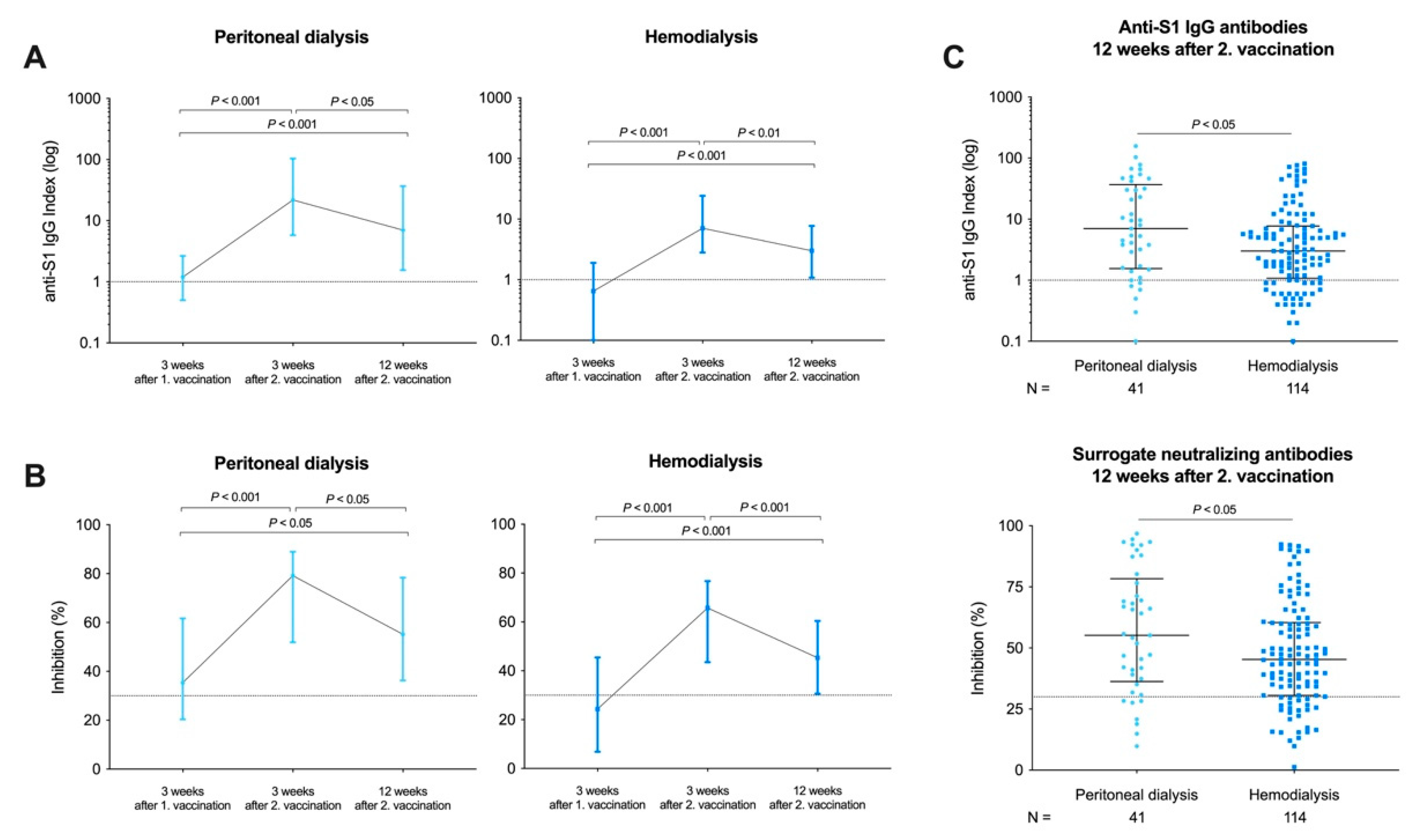
3.3. Longevity of Humoral Responses in Peritoneal Dialysis and Hemodialysis Patients after a Time Course of 12 Weeks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaier, M.; Leick, A.; Uhlmann, L.; Kälble, F.; Morath, C.; Eckstein, V.; Ho, A.; Mueller-Tidow, C.; Meuer, S.; Mahnke, K.; et al. End-Stage Renal Disease, Dialysis, Kidney Transplantation and Their Impact on CD4 +T-Cell Differentiation. Immunology 2018, 7, 3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Abate, M.; Andrade, H.P.; et al. Outcomes of Patients with End-Stage Kidney Disease Hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef]
- Speer, C.; Göth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kälble, F.; Kreysing, M.; Reichel, P.; et al. Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, CJN.03500321. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Meyer, B. Waning Antibodies to SARS-CoV-2—Don’t Panic. Lancet Reg. Health Eur. 2021, 4, 100115. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.-L.; Mullier, F.; Elsen, M.; Eucher, C.; Eeckhoudt, S.V.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.-M.; et al. Antibody Titres Decline 3-Month Post-Vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xia, H.; Zou, J.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Cutler, M.; Cooper, D.; Muik, A.; et al. BNT162b2-Elicited Neutralization of B.1.617 and Other SARS-CoV-2 Variants. Nature 2021, 596, 273–275. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 Spike 69/70 Deletion, E484K and N501Y Variants by BNT162b2 Vaccine-Elicited Sera. Nat. Med. 2021, 27, 620–621. [Google Scholar] [CrossRef]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; Liu, J.; Errico, J.M.; Xie, X.; Suryadevara, N.; et al. Resistance of SARS-CoV-2 Variants to Neutralization by Monoclonal and Serum-Derived Polyclonal Antibodies. Nat. Med. 2021, 27, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, S.M.; Park, S.J.; Kim, E.H.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Casel, M.A.B.; Rollon, R.; Jang, S.G.; et al. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg. Microbes Infect. 2021, 10, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Benning, L.; Töllner, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Schaier, M.; Bartenschlager, M.; Schnitzler, P.; Zeier, M.; et al. Neutralizing Antibody Response against Variants of Concern after Vaccination of Dialysis Patients with BNT162b2. Kidney Int. 2021, 100, 700–702. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 Surrogate Virus Neutralization Test Based on Antibody-Mediated Blockage of ACE2-Spike Protein-Protein Interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Benning, L.; Töllner, M.; Hidmark, A.; Schaier, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Buylaert, M.; Grenz, J.; Ponath, G.; et al. Heterologous ChAdOx1 NCoV-19/BNT162b2 Prime-Boost Vaccination Induces Strong Humoral Responses among Health Care Workers. Vaccines 2021, 9, 857. [Google Scholar] [CrossRef]
- Frantzen, L.; Cavaillé, G.; Thibeaut, S.; El-Haik, Y. Efficacy of the BNT162b2 MRNA COVID-19 Vaccine in a Haemodialysis Cohort. Nephrol. Dial. Transplant. 2021, 36, gfab165. [Google Scholar] [CrossRef]
- Speer, C.; Morath, C.; Töllner, M.; Buylaert, M.; Göth, D.; Nusshag, C.; Kälble, F.; Schaier, M.; Grenz, J.; Kreysing, M.; et al. Humoral Responses to Single-Dose BNT162b2 MRNA Vaccination in Dialysis Patients Previously Infected With SARS-CoV-2. Front. Med. 2021, 8, 721286. [Google Scholar] [CrossRef]
- Jiang, H.-J.; Tang, H.; Xiong, F.; Chen, W.-L.; Tian, J.-B.; Sun, J.; Dong, J.-W.; Wang, X.-H.; Jin, X.-F.; Ding, Y.-Q.; et al. COVID-19 in Peritoneal Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2020, 16, CJN.07200520. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, D.; Broseta, J.J.; Maduell, F.; Bedini, J.L.; Vera, M. Humoral Response of MRNA-1273 SARS-CoV-2 Vaccine in Peritoneal Dialysis Patients. Kidney Int. 2021, 100, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Kao, M.-T.; Huang, C.-C. A Comparison of Responsiveness to Hepatitis B Vaccination in Patients on Hemodialysis and Peritoneal Dialysis. Vaccine 2005, 23, 3957–3960. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef]
- Blachere, N.E.; Hacisuleyman, E.; Darnell, R.B. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 385, e7. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising Antibody Activity against SARS-CoV-2 VOCs, B.1.617.2 and B.1.351 by BNT162b2 Vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and Cellular Immunity to SARS-CoV-2 Vaccination in Renal Transplant versus Dialysis Patients: A Prospective, Multicenter Observational Study Using MRNA-1273 or BNT162b2 MRNA Vaccine. Lancet Reg. Health Eur. 2021, 100178, in press. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral Response after Three Doses of BNT162b2 MRNA COVID-19 Vaccine in Patients on Hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, NEJMoa2109072, in press. [Google Scholar] [CrossRef]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and Stable Mobilization of CD8+ T Cells by SARS-CoV-2 MRNA Vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef] [PubMed]
| Group 1 Peritoneal Dialysis | Group 2 Hemodialysis | Group 3 Healthy Controls | p Value | |
|---|---|---|---|---|
| Number of patients, N | 41 | 124 | 20 | |
| Age at enrollment (years), median (IQR) | 65 (56–78) | 70 (57–80) | 60 (56–77) | 0.52 |
| Sex (female), N (%) | 15 (37) | 52 (42) | 12 (60) | 0.21 |
| BMI, median (IQR) Missing data, N (%) | 27 (23–30) 3 (7) | 25 (22–29) 11 (9) | - - | 0.69 |
| Dialysis vintage (months), median (IQR) | 31 (20–46) | 46 (34–59) | - | <0.001 a |
| Cause of nephropathy Diabetes, N (%) Vascular, N (%) PKD, N (%) Glomerulonephritis, N (%) Chronic pyelonephritis, N (%) Other, N (%) Missing data, N (%) | 7 (17) 10 (24) 2 (5) 9 (22) 2 (5) 9 (22) 2 (5) | 29 (23) 31 (27) 3 (2) 20 (16) 2 (2) 31 (27) 8 (6) | - - - - - - - | 0.40 0.94 0.43 0.40 0.24 0.69 - |
| Comorbidities Arterial hypertension, N (%) Diabetes, N (%) Cancer, N (%) Smoker (active/former), N (%) CAD, N (%) PAD, N (%) Chronic lung disease, N (%) Chronic liver disease, N (%) Missing data, N (%) | 36 (88) 15 (37) 4 (10) 13 (32) 21 (51) 10 (24) 8 (20) 7 (17) 2 (5) | 118 (95) 55 (44) 9 (7) 26 (21) 72 (58) 43 (35) 20 (16) 10 (8) 8 (6) | - - - - - - - - - - | 0.10 0.38 0.61 0.16 0.44 0.22 0.62 0.10 - |
| Characteristics | B | 95% CI | SE | p Value |
|---|---|---|---|---|
| Age (years) | −1.9 | −2.6, −1.2 | 0.4 | <0.001 |
| Sex (female versus male) | 10.5 | −5.4, 26.4 | 8.0 | 0.20 |
| Dialysis modality (PD versus HD) | 49.1 | 29.1, 69.1 | 10.1 | <0.001 |
| BMI > 30 (yes versus no) | 9.3 | −10.2, 28.8 | 9.3 | 0.35 |
| Dialysis vintage (months) | −0.1 | −0.7, 0.6 | 0.4 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speer, C.; Schaier, M.; Nusshag, C.; Töllner, M.; Buylaert, M.; Kälble, F.; Reichel, P.; Grenz, J.; Süsal, C.; Zeier, M.; et al. Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks. Vaccines 2021, 9, 1130. https://doi.org/10.3390/vaccines9101130
Speer C, Schaier M, Nusshag C, Töllner M, Buylaert M, Kälble F, Reichel P, Grenz J, Süsal C, Zeier M, et al. Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks. Vaccines. 2021; 9(10):1130. https://doi.org/10.3390/vaccines9101130
Chicago/Turabian StyleSpeer, Claudius, Matthias Schaier, Christian Nusshag, Maximilian Töllner, Mirabel Buylaert, Florian Kälble, Paula Reichel, Julia Grenz, Caner Süsal, Martin Zeier, and et al. 2021. "Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks" Vaccines 9, no. 10: 1130. https://doi.org/10.3390/vaccines9101130
APA StyleSpeer, C., Schaier, M., Nusshag, C., Töllner, M., Buylaert, M., Kälble, F., Reichel, P., Grenz, J., Süsal, C., Zeier, M., Schnitzler, P., Morath, C., Klein, K., & Benning, L. (2021). Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks. Vaccines, 9(10), 1130. https://doi.org/10.3390/vaccines9101130







