Course and Lethality of SARS-CoV-2 Epidemic in Nursing Homes after Vaccination in Florence, Italy
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
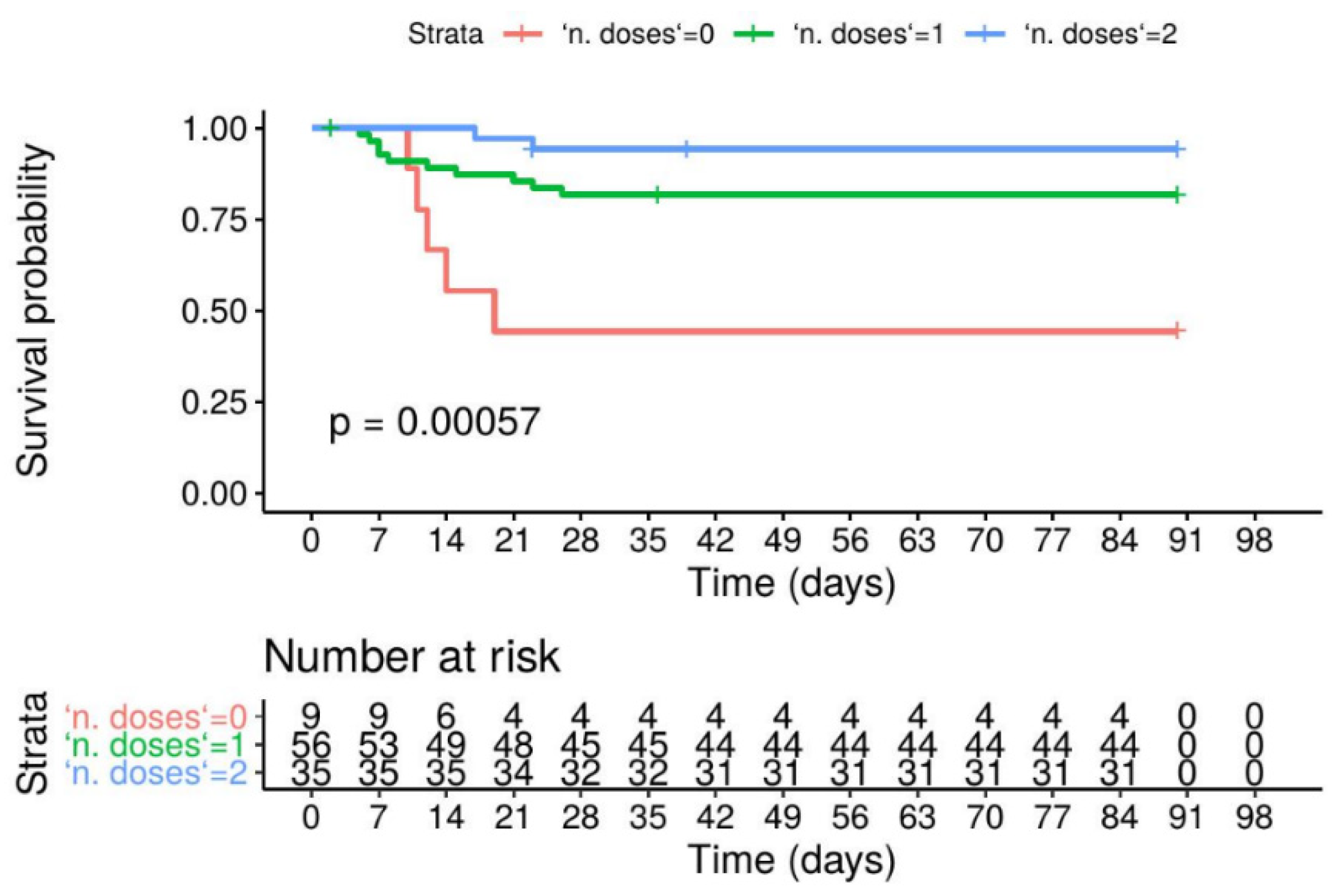
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, J.K.; Bayne, G.; Evans, C.; Garbe, F.; Gorman, D.; Honhold, N.; McCormick, D.; Othieno, R.; Stevenson, J.E.; Swietlik, S.; et al. Evolution and effects of COVID-19 outbreaks in care homes: A population analysis in 189 care homes in one geographical region of the UK. Lancet Health Longev. 2020, 1, e21–e31. [Google Scholar] [CrossRef]
- Rutten, J.J.S.; van Loon, A.M.; van Kooten, J.; van Buul, L.W.; Joling, K.J.; Smalbrugge, M.; Hertogh, C.M.P.M. Clinical Suspicion of COVID-19 in Nursing Home Residents: Symptoms and Mortality Risk Factors. J. Am. Med. Dir. Assoc. 2020, 21, 1791–1797.e1. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.N.; Junghans, C.; Downes, R.; Sendall, C.; Lai, H.; McKirdy, A.; Elliott, P.; Howard, R.; Wingfield, D.; Priestman, M.; et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J. Infect. 2020, 81, 411–419. [Google Scholar] [CrossRef]
- Tang, O.; Bigelow, B.F.; Sheikh, F.; Peters, M.; Zenilman, J.M.; Bennett, R.; Katz, M.J. Outcomes of Nursing Home COVID-19 Patients by Initial Symptoms and Comorbidity: Results of Universal Testing of 1970 Residents. J. Am. Med. Dir. Assoc. 2020, 21, 1767–1773.e1. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Pawelec, G.; Castle, S.; Loeb, M. Immunosenescence and vaccination in nursing home residents. Clin. Infect. Dis. 2009, 48, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M. The effect of aging of the immune system on vaccination responses. Hum. Vaccines Immunother. 2013, 9, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Saito, R.; Tanabe, N.; Nishikawa, M.; Sasaki, A.; Gejyo, F.; Suzuki, H. Antibody Response to Influenza Vaccination in Nursing Home Residents and Healthcare Workers During Four Successive Seasons in Niigata, Japan. Infect. Control Hosp. Epidemiol. 2005, 26, 859–866. [Google Scholar] [CrossRef]
- Bellei, N.C.J.; Carraro, E.; Castelo, A.; Granato, C.F.H. Risk factors for poor immune response to influenza vaccination in elderly people. Braz. J. Infect. Dis. 2006, 10, 269–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Helfand, B.K.I.; Webb, M.; Gartaganis, S.L.; Fuller, L.; Kwon, C.S.; Inouye, S.K. The Exclusion of Older Persons from Vaccine and Treatment Trials for Coronavirus Disease 2019—Missing the Target. JAMA Intern. Med. 2020, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Antonelli Incalzi, R.; Trevisan, C.; Del Signore, S.; Volpato, S.; Fumagalli, S.; Monzani, F.; Bellelli, G.; Gareri, P.; Mossello, E.; Malara, A.; et al. Are vaccines against COVID-19 tailored to the most vulnerable people? Vaccine 2021, 39, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Canaday, D.H.; Carias, L.; Oyebanji, O.A.; Keresztesy, D.; Wilk, D.; Payne, M.; Aung, H.; St Denis, K.; Lam, E.C.; Wilson, B.; et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naive nursing home residents. medRxiv Prepr. Serv. Health Sci. 2021, 61–64. [Google Scholar] [CrossRef]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; De Vriese, A.S.; Reynders, M. Humoral and cellular immunogenicity of the BNT162b2 mRNA Covid-19 Vaccine in nursing home residents. Clin. Infect Dis. 2021, ciab300. [Google Scholar] [CrossRef]
- Benvenuti, E.; Rivasi, G.; Bulgaresi, M.; Barucci, R.; Lorini, C.; Balzi, D.; Faraone, A.; Fortini, G.; Vaccaro, G.; Del Lungo, I.; et al. Caring for nursing home residents with COVID-19: A “hospital-at-nursing home” intermediate care intervention. Aging Clin Exp Res. 2021, 1–8, Epub ahead of print. [Google Scholar] [CrossRef]
- Chidambaram, P.; Garfield, R.; Neuman, T.; McDermott, D.; Rice, C.; Anderson, E. New COVID-19 Cases and Deaths among Nursing Home Residents Have Dropped Since Vaccinations Began. Available online: https://www.kff.org/coronavirus-covid-19/slide/new-covid-19-cases-and-deaths-among-nursing-home-residents-have-dropped-since-vaccinations-began (accessed on 30 April 2021).
- Menéndez Colino, R.; Merello de Miguel, A.; Argentina, F.; Barcons Marqués, M.; Chaparro Jiménez, B.; López Hernández, P.; Jiménez Bueno, S.; Montero Vega, M.D.; García Rodríguez, J.; Ferrer Simo, B.; et al. Evolution of COVID-19 at nursing homes from the second wave to vaccination. Description of a coordination program between Primary Care, Geriatrics and Public Health. Rev. Esp. Salud Publica 2021, 95, 1–11. [Google Scholar]
- Mor, V.; Gutman, R.; Yang, X.; White, E.M.; McConeghy, K.W.; Feifer, R.A.; Blackman, C.R.; Kosar, C.M.; Bardenheier, B.H.; Gravenstein, S.A. Short-term impact of nursing home SARS-CoV-2 vaccinations on new infections, hospitalizations, and deaths. J. Am. Geriatr. Soc. 2021, 69, 2063–2069. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Hartronft, S.; McConeghy, K.; Kennedy, M.; Intrator, O.; Minor, L.; Hubert, T.L.; Goldstein, M.K. Proportion of SARS-CoV-2 positive tests and vaccination in Veterans Affairs Community Living Centers. J. Am. Geriatr. Soc. 2021, 69, 2090–2095. [Google Scholar] [CrossRef]
- Domi, M.; Leitson, M.; Gifford, D.; Nicolaou, A.; Sreenivas, K.; Bishnoi, C. The BNT162b2 vaccine is associated with lower new COVID-19 cases in nursing home residents and staff. J. Am. Geriatr. Soc. 2021, 69, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- McEllistrem, M.C.; Clancy, C.J.; Buehrle, D.J.; Lucas, A.; Decker, B.K. Single dose of a mRNA SARS-CoV-2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID-19. Clin. Infect. Dis. 2021, 73, e1365–e1367. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, M.C.; Pergam, S.A.; Shah, P.D. Cocooning against COVID-19: The argument for vaccinating caregivers of patients with cancer. Cancer 2021, 127, cncr.33598. [Google Scholar] [CrossRef] [PubMed]
- Teran, R.A.; Walblay, K.A.; Shane, E.L.; Xydis, S.; Gretsch, S.; Gagner, A.; Samala, U.; Choi, H.; Zelinski, C.; Black, S.R. Postvaccination SARS-CoV-2 Infections Among Skilled Nursing Facility Residents and Staff Members—Chicago, Illinois, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 632–638. [Google Scholar] [CrossRef] [PubMed]
- CDC. When You’ve Been Fully Vaccinated. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html (accessed on 30 April 2021).

| All | Unvaccinated | Partially Vaccinated | Fully Vaccinated | p | |
|---|---|---|---|---|---|
| (N = 100) | (N = 9) | (N = 56) | (N = 35) | ||
| Sex | 0.170 | ||||
| Female | 67 (67%) | 5 (56%) | 35 (63%) | 28 (80%) | |
| Male | 33 (33%) | 4 (44%) | 21 (38%) | 7 (20%) | |
| Median age (IQR) | 86.0 (79.0–91.0) | 74.5 (71.7–82.5) | 85.0 (80.2–90.0) | 89.0 (80.0–92.5) | <0.001 |
| Symptoms | <0.001 | ||||
| Yes | 51 (51%) | 7 (78%) | 39 (70%) | 5 (14%) | |
| No | 49 (49%) | 2 (22%) | 17 (30%) | 30 (86%) | |
| Symptoms severity | <0.001 | ||||
| Absent/minimal | 57 (57%) | 2 (22%) | 25 (45%) | 30 (86%) | |
| Mild | 30 (30%) | 3 (33%) | 22 (39%) | 5 (14%) | |
| Severe | 13 (13%) | 4 (44%) | 9 (16%) | 0 (0%) | |
| COVID-19 hospitalization | 0.040 | ||||
| Yes | 12 (12%) | 3 (33%) | 8 (14%) | 1 (3%) | |
| No | 88 (88%) | 6 (67%) | 48 (86%) | 34 (97%) | |
| COVID-19 deaths | <0.001 | ||||
| Yes | 17 (17%) | 5 (56%) | 10 (18%) | 2 (6%) | |
| No | 83 (83%) | 4 (44%) | 46 (82%) | 33 (94%) |
| Variables | Unadjusted Model | Adjusted Model | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p | Hazard Ratio (95%CI) | p | |
| Sex | 0.93 | 0.43 | ||
| Female | 0.65 (0.25–1.7) | 0.67 (0.25–1.81) | ||
| Male | - | - | ||
| Age (years) | 1.0 (0.98–1.1) | 0.16 | 1.09 (1.02–1.17) | 0.013 |
| Number of doses | ||||
| 0 | - | - | ||
| 1 | 0.26 (0.09–0.76) | 0.014 | 0.16 (0.05–0.49) | 0.002 |
| 2 | 0.07 (0.01–0.38) | 0.002 | 0.04 (0.006–0.22) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivasi, G.; Bulgaresi, M.; Mossello, E.; Buscemi, P.; Lorini, C.; Balzi, D.; Barucci, R.; Del Lungo, I.; Gangemi, S.; Giardini, S.; et al. Course and Lethality of SARS-CoV-2 Epidemic in Nursing Homes after Vaccination in Florence, Italy. Vaccines 2021, 9, 1174. https://doi.org/10.3390/vaccines9101174
Rivasi G, Bulgaresi M, Mossello E, Buscemi P, Lorini C, Balzi D, Barucci R, Del Lungo I, Gangemi S, Giardini S, et al. Course and Lethality of SARS-CoV-2 Epidemic in Nursing Homes after Vaccination in Florence, Italy. Vaccines. 2021; 9(10):1174. https://doi.org/10.3390/vaccines9101174
Chicago/Turabian StyleRivasi, Giulia, Matteo Bulgaresi, Enrico Mossello, Primo Buscemi, Chiara Lorini, Daniela Balzi, Riccardo Barucci, Ilaria Del Lungo, Salvatore Gangemi, Sante Giardini, and et al. 2021. "Course and Lethality of SARS-CoV-2 Epidemic in Nursing Homes after Vaccination in Florence, Italy" Vaccines 9, no. 10: 1174. https://doi.org/10.3390/vaccines9101174
APA StyleRivasi, G., Bulgaresi, M., Mossello, E., Buscemi, P., Lorini, C., Balzi, D., Barucci, R., Del Lungo, I., Gangemi, S., Giardini, S., Piga, C., Barghini, E., Boni, S., Bulli, G., Carrai, P., Crociani, A., Faraone, A., Lo Forte, A., Martella, L., ... Benvenuti, E. (2021). Course and Lethality of SARS-CoV-2 Epidemic in Nursing Homes after Vaccination in Florence, Italy. Vaccines, 9(10), 1174. https://doi.org/10.3390/vaccines9101174










