Durvalumab as Consolidation Therapy in Post-Concurrent Chemoradiation (CCRT) in Unresectable Stage III Non-Small Cell Lung Cancer Patients: A Multicenter Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluation of the CRT Response, Survival, and Treatment-Induced AEs of Durvalumab
2.3. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics and CRT Treatment Information of the Study Patients
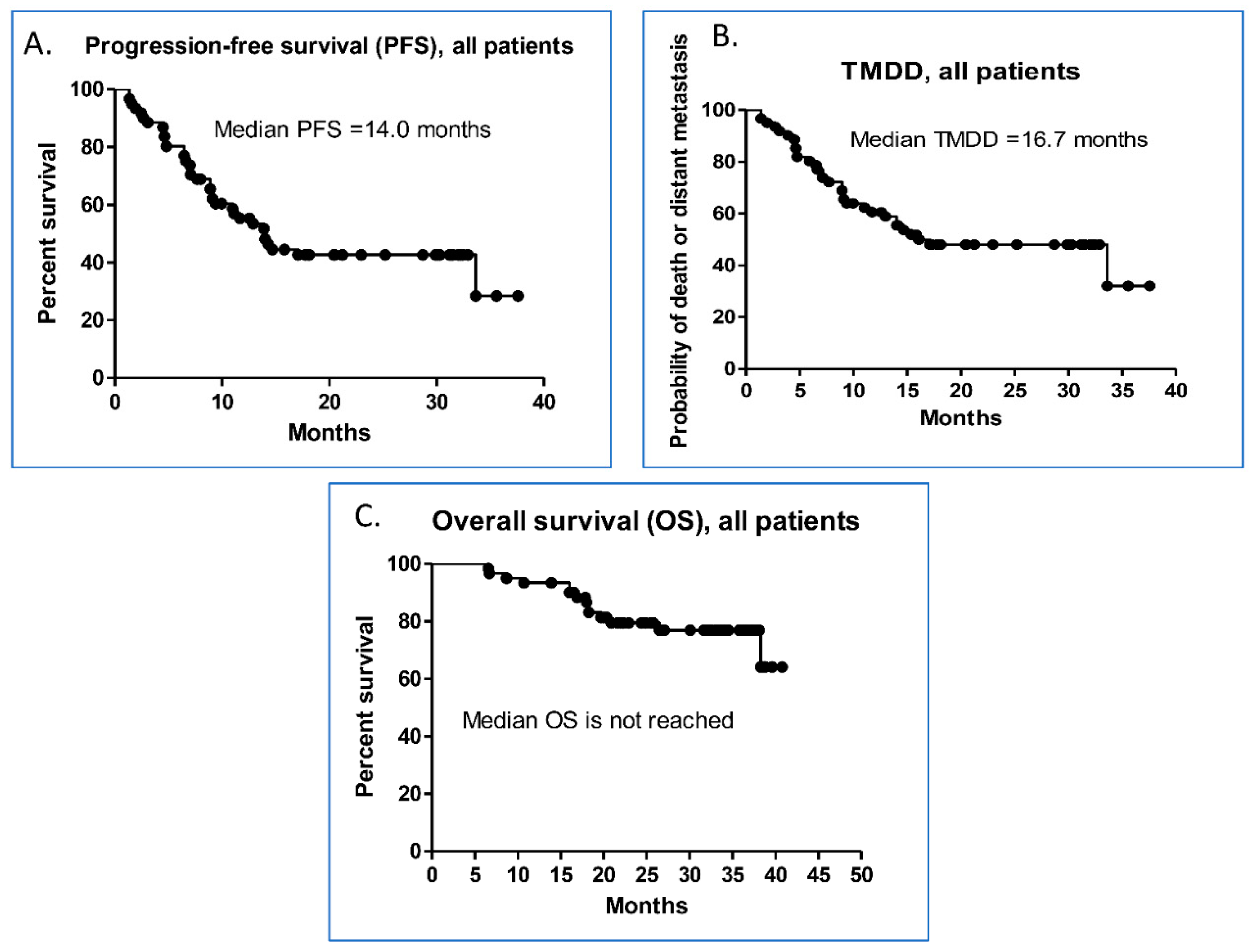
3.2. Efficacy of Durvalumab and the Predictive Factors Associated with PFS
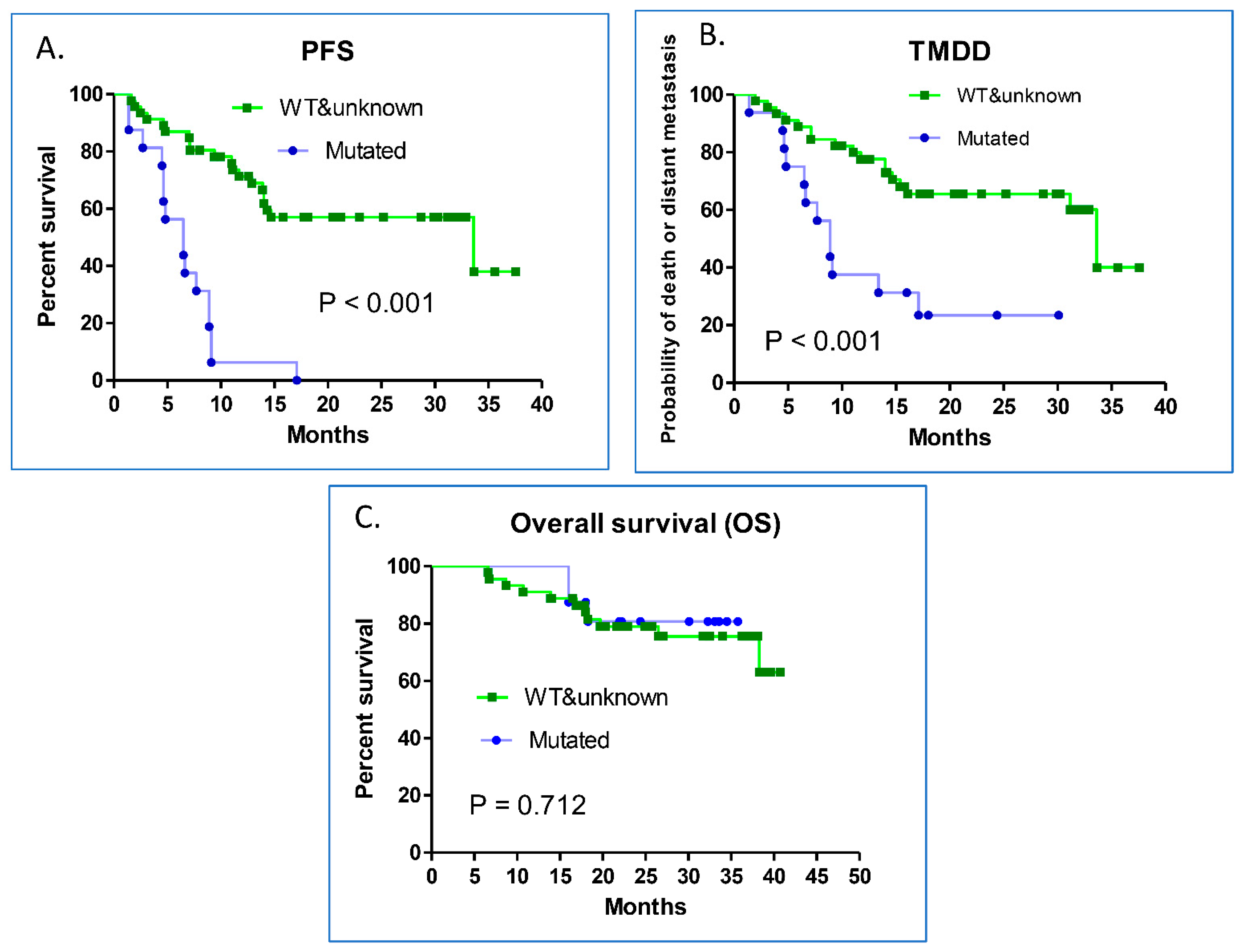
3.3. Comparisons of the Post-CCRT PFS, and TMDD Based on EGFR Mutation Status
3.4. Durvalumab-Induced AEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non–Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, T.; Sharma, N.; Machtay, M. Controversies in the management of stage III non-small-cell lung cancer. Expert Rev. Anticancer. Ther. 2014, 14, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Chang, J.W.; Wang, C.C.; Wu, C.T.; Lin, Y.C.; Wang, C.L.; Lin, T.Y.; Li, S.H.; Wu, Y.C.; Kuo, S.C.; et al. Oral vi-norelbine plus cisplatin with concomitant radiotherapy as induction therapy for stage III non-small cell lung cancer: Results of a single-arm prospective cohort study. Thorac. Cancer 2019, 10, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Tabchi, S.; Kassouf, E.; El Rassy, E.; Kourie, H.R.; Martin, J.; Campeau, M.-P.; Tehfe, M.; Blais, N. Management of stage III non–small cell lung cancer. Semin. Oncol. 2017, 44, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Rosenman, J.G.; Schell, M.J.; Halle, J.; Russo, S.; Rivera, M.P.; Clark, J.; Limentani, S.; Fraser, R.; Mitchell, W.; et al. Induction carboplatin/paclitaxel followed by concurrent carboplatin/paclitaxel and dose-escalating conformal thoracic ra-diation therapy in unresectable stage IIIA/B nonsmall cell lung carcinoma: A modified Phase I trial. Cancer 2000, 89, 534–542. [Google Scholar] [CrossRef]
- Zatloukal, P.; Petruzelka, L.; Zemanova, M.; Havel, L.; Janku, F.; Judas, L.; Kubik, A.; Krepela, E.; Fiala, P.; Pecen, L. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: A ran-domized study. Lung Cancer 2004, 46, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., 3rd; Shepherd, F.A.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.-L.; Chang, Y.-C.; Ko, H.-L.; Chi, M.-S.; Wang, H.-E.; Hsu, P.-S.; Lin, C.-C.; Yeh, D.Y.-W.; Kao, S.-J.; Jiang, J.-S.; et al. Optimizing Survival of Patients With Marginally Operable Stage IIIA Non–Small-Cell Lung Cancer Receiving Chemoradiotherapy With or Without Surgery. Clin. Lung Cancer 2016, 17, 550–557. [Google Scholar] [CrossRef]
- Tsujino, K.; Kurata, T.; Yamamoto, S.; Kawaguchi, T.; Kubo, A.; Isa, S.; Hasegawa, Y.; Ou, S.-H.I.; Takada, M.; Ando, M. Is Consolidation Chemotherapy after Concurrent Chemo-Radiotherapy Beneficial for Patients with Locally Advanced Non–Small-Cell Lung Cancer? A Pooled Analysis of the Literature. J. Thorac. Oncol. 2013, 8, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ding, X.; Kong, D.; Zhang, L.; Guo, Y.; Ren, J.; Hu, X.; Yang, J.; Gao, S. The effect of consolidation chemotherapy after concurrent chemoradiotherapy on the survival of patients with locally advanced non-small cell lung cancer: A meta-analysis. Int. J. Clin. Oncol. 2016, 22, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Dutta, P.; Liu, J.; Sabri, N.; Song, Y.; Li, W.; Li, J. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J. Cell. Mol. Med. 2018, 23, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, N.; Gorenberg, M.; Agbarya, A. First Line Immunotherapy for Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Cobo, M.; Rodríguez-Abreu, D.; Parente, D.P.; Gracia, P.R.; González, J.G. Practical Issues in the Use of Atezolizumab for Patients with Non-Small Cell Lung Cancer: Case Reports and Literature Review. Oncol. Ther. 2021, 9, 41–53. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival with Durvalumab After Chemoradiotherapy in Stage III NSCLC—An Update from the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, S.C.A.C.B.H.S.Q.L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef]
- Okita, R.; Maeda, A.; Shimizu, K.; Nojima, Y.; Saisho, S.; Nakata, M. Effect of platinum-based chemotherapy on the expression of natural killer group 2 member D ligands, programmed cell death-1 ligand 1 and HLA class I in non-small cell lung cancer. Oncol. Rep. 2019, 42, 839–848. [Google Scholar] [CrossRef]
- Belluomini, L.; Fiorica, F.; Frassoldati, A. Immune Checkpoint Inhibitors and Radiotherapy in NSCLC Patients: Not Just a Fluke. Oncol. Ther. 2019, 7, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Check-point Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Ku langara, K. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non–Small-cell Lung Cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, O.; Cardona, A.F.; Martín, C.; Más-López, L.; Corrales-Rodríguez, L.; Bramuglia, G.; Castillo-Fernandez, O.; Meyerson, M.; Amieva-Rivera, E.; Campos-Parra, A.D.; et al. Updated Frequency of EGFR and KRAS Mutations in NonSmall-Cell Lung Cancer in Latin America: The Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J. Thorac. Oncol. 2015, 10, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.C.; Wang, C.W.; Kuo, S.C.H.; Lin, S.M.; Lo, Y.L.; Huang, A.C.C.; Chiu, L.C.; Yang, C.T. The Co-Expression of Pro-grammed Death-Ligand 1 (PD-L1) in Untreated EGFR-Mutated Metastatic Lung Adenocarcinoma. Biomedicines 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, J.S.; Yang, T.Y.; Chen, K.C.; Hsu, K.H.; Chen, H.Y.; Chang, G.C. Retrospective study of erlotinib in patients with ad-vanced squamous lung cancer. Lung Cancer 2012, 77, 128–133. [Google Scholar] [CrossRef]
- Cheung, A.H.-K.; Tong, J.H.-M.; Chung, L.-Y.; Chau, S.-L.; Ng, C.S.-H.; Wan, I.Y.; To, K.-F. EGFR mutation exists in squamous cell lung carcinoma. Pathology 2020, 52, 323–328. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.J.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C.-H. Checkpoint Inhibitors in Metastatic EGFR- Mutated Non–Small Cell Lung Cancer—A Meta-Analysis. J. Thorac. Oncol. 2017, 12, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.; Mezquita, L.; Thai, A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Aredo, J.V.; Mambetsariev, I.; Hellyer, J.A.; Amini, A.; Neal, J.W.; Padda, S.K.; McCoach, C.E.; Riess, J.W.; Cabebe, E.C.; Naidoo, J.; et al. Durvalumab for Stage III EGFR-Mutated NSCLC After Definitive Chemoradiotherapy. J. Thorac. Oncol. 2021, 16, 1030–1041. [Google Scholar] [CrossRef]
- Yang, J.C.; Shepherd, F.A.; Kim, D.W.; Lee, G.W.; Lee, J.S.; Chang, G.C.; Lee, S.S.; Wei, Y.F.; Lee, Y.G.; Laus, G.; et al. Osi-mertinib Plus Durvalumab versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC following Previous EGFR TKI Therapy: CAURAL Brief Report. J. Thorac. Oncol. 2019, 14, 933–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef]
- Metro, G.; Baglivo, S.; Moretti, R.; Bellezza, G.; Sidoni, A.; Roila, F. Is There a Role for Multiple Lines of Anti-HER2 Therapies Administered Beyond Progression in HER2-Mutated Non-Small Cell Lung Cancer? A Case Report and Literature Review. Oncol. Ther. 2020, 8, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osi-mertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Lu, S.; Casarini, I.; Kato, T.; Cobo, M.; Özgüroğlu, M.; Hodge, R.; van der Gronde, T.; Saggese, M.; Ramalingam, S.S. Osimertinib Maintenance After Definitive Chemoradiation in Patients with Unresectable EGFR Mutation Positive Stage III Non–small-cell Lung Cancer: LAURA Trial in Progress. Clin. Lung Cancer 2021, 22, 371–375. [Google Scholar] [CrossRef]

| Total | N = 61 |
|---|---|
| Gender | |
| Male | 49 |
| Female | 12 |
| Age year (median/range) | 63 (32–86) |
| ECOG PS | |
| 0–1 | 59 |
| 2 | 2 |
| Smoking status | |
| Non-smoker | 18 |
| Former/current smoker | 43 |
| Histology | |
| Adenocarcinoma | 42 |
| Squamous cell carcinoma | 15 |
| NSCLC * | 4 |
| Stage | |
| IIIA | 19 |
| IIIB | 35 |
| IIIC | 7 |
| EGFR mutation | |
| Mutated EGFR | 16 |
| Wild-type | 33 |
| Unknown | 12 |
| PD-L1 expression (TPS) | |
| Positive (≥1%) | 27 |
| Negative (<1%) | 16 |
| Unknown | 18 |
| Chemotherapy regimens Platinum-based doublet with | |
| Docetaxel | 24 |
| Vinorelbine | 31 |
| Etoposide | 1 |
| Pemetrexed | 5 |
| Dose of radiation therapy | |
| 5000–6000 cGy | 3 |
| 6000–6600 cGy | 51 |
| 6600–7000 cGy | 7 |
| Response to neoadjuvant CCRT | |
| PR | 34 |
| SD | 27 |
| Neutrophil-to-lymphocyte ratio (NLR) | |
| Low (<3.0) | 23 |
| High (≥3.0) | 38 |
| Timing of first dose of durvalumab administrated post-CCRT, months (median/range) | 1.8 (0.2–3.9) |
| Median follow-up time, months | 27.0 (6.7–40.7) |
| Variables | Patients (N) | Median PFS (months) | Univariate Analysis p-Value HR (95% CI) | Multivariate Analysis | |
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | ||||
| Age | |||||
| <60 years | 25 | 14 | 0.572 | ||
| ≥60 years | 36 | 17.6 | 0.817 (0.406–1.646) | ||
| Gender | |||||
| Male | 49 | 13.9 | 0.535 | ||
| Female | 12 | 15.8 | 0.688 (0.210–2.247) | ||
| Smoking status | |||||
| Non-smoker | 21 | 15.8 | 0.111 | ||
| Former/current smoker | 40 | 13.9 | 2.162 (0.838–5.576) | ||
| Histology | |||||
| Adenocarcinoma | 42 | 11.7 | 0.787 | ||
| Non-adenocarcinoma | 19 | 18.1 | 0.876 (0.334–2.298) | ||
| Stage | |||||
| IIIA | 19 | 15.8 | |||
| IIIB | 35 | 13.9 | 0.902 | ||
| IIIC | 7 | 7.7 | 1.041 (0.547–1.981) | ||
| EGFR mutation | |||||
| Mutated EGFR | 16 | 6.5 | <0.001 | 10.47 (4.55–24.07) | <0.001 |
| Wild-type and unknown | 45 | 33.63 | 12.22 (4.296–34.765) | ||
| PD-L1 expression (TPS) | |||||
| Positive (≥1%) | 27 | 14 | |||
| Negative (<1%) | 16 | 12.9 | 0.855 | ||
| Unknown | 18 | 14.3 | 0.956 (0.590–1.549) | ||
| NLR | |||||
| Low NLR (<3.0) | 23 | 17.1 | 0.400 | ||
| High NLR (≥3.0) | 38 | 12.9 | 1.375 (0.654–2.891) | ||
| Response to neoadjuvant CCRT | |||||
| PR | 34 | 14.7 | 0.377 | ||
| SD | 27 | 14 | 0.704 (0.324–1.532) | ||
| Variables | Mutated EGFR | Wild Type and Unknown | p-Value |
|---|---|---|---|
| Gender | |||
| Male | 12 | 37 | |
| Female | 4 | 8 | 0.715 |
| Age year (mean ± SD) | 60.3 ± 7.1 | 63.7 ± 12.9 | 0.587 |
| ECOG PS | |||
| 0–1 | 15 | 44 | |
| 2 | 1 | 1 | 0.459 |
| Smoking status | |||
| Non-smoker | 10 | 33 | |
| Former/current smoker | 6 | 12 | 0.526 |
| Histology | |||
| Adenocarcinoma | 15 | 27 | |
| Squamous cell carcinoma | 0 | 15 | |
| NSCLC | 1 | 3 | 0.026 |
| Stage | |||
| IIIA | 5 | 14 | |
| IIIB | 9 | 26 | |
| IIIC | 2 | 5 | 0.988 |
| PD-L1 expression (TPS) | |||
| Positive (≥1%) | 7 | 20 | |
| Negative (<1%) | 5 | 11 | |
| Unknown | 4 | 14 | 0.836 |
| Response to neoadjuvant CCRT | |||
| PR | 8 | 26 | |
| SD | 8 | 19 | 0.77 |
| NLR | |||
| Low NLR (<3.0) | 7 | 16 | |
| High NLR (≥3.0) | 9 | 29 | 0.565 |
| Timing of the first dose of durvalumab administrated post-CCRT (median, months) | 1.6 | 1.9 | 0.252 |
| Mutated EGFR Total Patients n = 12 | Wild type and Unknown Total Patients n = 17 | |
|---|---|---|
| Metastatic sites | ||
| Lung to lung | 4 | 4 |
| Pleura | 3 | 1 |
| Pericardium | 0 | 1 |
| Brain | 4 | 5 |
| Bone | 5 | 4 |
| Liver | 2 | 2 |
| Adrenal gland | 1 | 2 |
| Subsequent systemic therapy following durvalumab | Number of patients (n) | Number of patients (n) |
| Afatinib | 4 | 0 |
| Erlotinib | 2 | 1 |
| Gefitinib | 1 | 0 |
| Osimertinib | 2 | 0 |
| Erlotinib + bevacizumab | 2 | 0 |
| AZD3759 | 1 | 0 |
| Platinum-based chemotherapy | 0 | 7 |
| Single agent chemotherapy | 0 | 5 |
| Supportive care | 0 | 4 |
| Adverse Events (AEs) | All, N = 61 (%) | Grade 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|
| Skin rash/pruritis | 32 (52.5%) | 32 (52.5%) | 0 | 0 |
| Nausea or anorexia | 3 (4.9%) | 3 (4.9%) | 0 | 0 |
| Diarrhea | 6 (9.8%) | 6 (9.8%) | 0 | 0 |
| Amylase or lipase increased | 4 (6.6%) | 4 (6.6%) | 0 | 0 |
| Liver-transaminases increased | 6 (9.8%) | 5 (8.2%) | 1 (1.6%) | 0 |
| Pneumonitis | 11 (18%) | 8 (13.1%) | 3 (4.9%) | 0 |
| Headache or dizziness | 4 (6.6%) | 4 (6.6%) | 0 | 0 |
| Hypothyroidism | 4 (6.6%) | 4 (6.6%) | 0 | 0 |
| Adrenal insufficiency | 6 (9.8%) | 6 (9.8%) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Chiu, L.-C.; Ju, J.-S.; Lin, Y.-C.; Fang, Y.-F.; Yang, C.-T.; Hsu, P.-C. Durvalumab as Consolidation Therapy in Post-Concurrent Chemoradiation (CCRT) in Unresectable Stage III Non-Small Cell Lung Cancer Patients: A Multicenter Observational Study. Vaccines 2021, 9, 1122. https://doi.org/10.3390/vaccines9101122
Wang C-C, Chiu L-C, Ju J-S, Lin Y-C, Fang Y-F, Yang C-T, Hsu P-C. Durvalumab as Consolidation Therapy in Post-Concurrent Chemoradiation (CCRT) in Unresectable Stage III Non-Small Cell Lung Cancer Patients: A Multicenter Observational Study. Vaccines. 2021; 9(10):1122. https://doi.org/10.3390/vaccines9101122
Chicago/Turabian StyleWang, Chin-Chou, Li-Chung Chiu, Jia-Shiuan Ju, Yu-Ching Lin, Yueh-Fu Fang, Cheng-Ta Yang, and Ping-Chih Hsu. 2021. "Durvalumab as Consolidation Therapy in Post-Concurrent Chemoradiation (CCRT) in Unresectable Stage III Non-Small Cell Lung Cancer Patients: A Multicenter Observational Study" Vaccines 9, no. 10: 1122. https://doi.org/10.3390/vaccines9101122
APA StyleWang, C.-C., Chiu, L.-C., Ju, J.-S., Lin, Y.-C., Fang, Y.-F., Yang, C.-T., & Hsu, P.-C. (2021). Durvalumab as Consolidation Therapy in Post-Concurrent Chemoradiation (CCRT) in Unresectable Stage III Non-Small Cell Lung Cancer Patients: A Multicenter Observational Study. Vaccines, 9(10), 1122. https://doi.org/10.3390/vaccines9101122






