Direct and Indirect Proof of SARS-CoV-2 Infections in Indigenous Wiwa Communities in North-Eastern Colombia—A Cross-Sectional Assessment Providing Preliminary Surveillance Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. SARS-CoV-2 Diagnostic Tests
2.3. COVID-19 Case Definitions
2.4. Statistics
2.5. Ethics
3. Results
3.1. Study Population and Basic Demographic Data
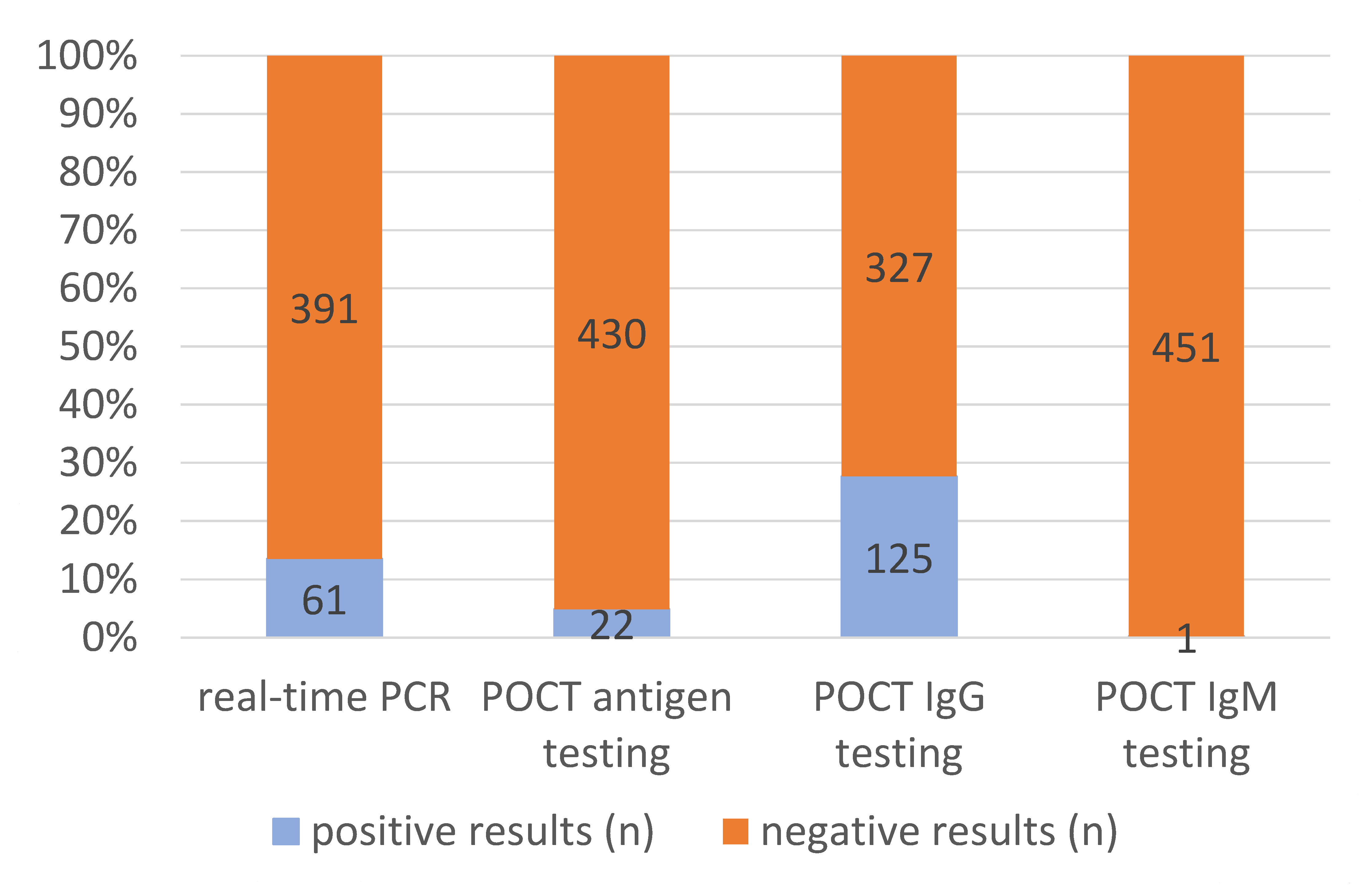
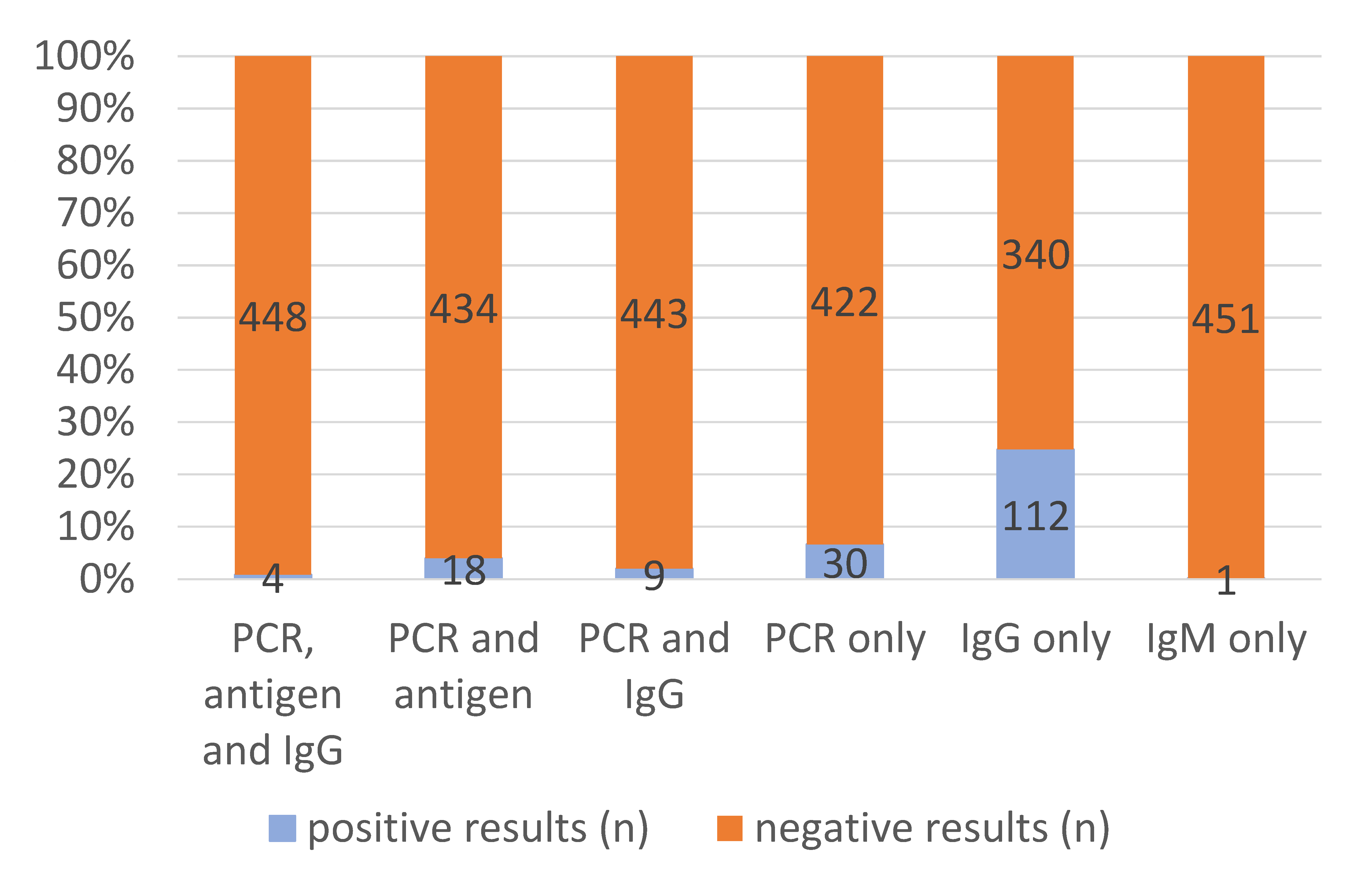
3.2. Molecular and Serological SARS-CoV-2 Diagnostic Test Results
3.3. Association of SARS-CoV-2 Test Results with Clinical and Epidemiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Amigo, I. Indigenous communities in Brazil fear pandemic’s impact. Science 2020, 368, 352. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, C. COVID-19: Review Indigenous peoples’ data. Nature 2020, 580, 185. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, L.; Fearnside, P.M. Protect Indigenous peoples from COVID-19. Science 2020, 368, 251. [Google Scholar] [PubMed] [Green Version]
- Kaplan, H.S.; Trumble, B.C.; Stieglitz, J.; Mamany, R.M.; Cayuba, M.G.; Moye, L.M.; Alami, S.; Kraft, T.; Gutierrez, R.Q.; Adrian, J.C.; et al. Voluntary collective isolation as a best response to COVID-19 for indigenous populations? A case study and protocol from the Bolivian Amazon. Lancet 2020, 395, 1727–1734. [Google Scholar] [CrossRef]
- Curtice, K.; Choo, E. Indigenous populations: Left behind in the COVID-19 response. Lancet 2020, 395, 1753. [Google Scholar] [CrossRef]
- Power, T.; Wilson, D.; Best, O.; Brockie, T.; Bourque Bearskin, L.; Millender, E.; Lowe, J. COVID-1 9 and Indigenous Peoples: An imperative for action. J. Clin. Nurs. 2020, 29, 2737–2741. [Google Scholar] [CrossRef]
- Banning, J. How Indigenous people are coping with COVID-19. CMAJ 2020, 192, E787–E788. [Google Scholar] [CrossRef]
- Banning, J. Why are Indigenous communities seeing so few cases of COVID-19? CMAJ 2020, 192, E993–E994. [Google Scholar] [CrossRef]
- Júnior, J.G.; Moreira, M.M.; Pinheiro, W.R.; de Amorim, L.M.; Lima, C.K.T.; da Silva, C.G.L.; Neto, M.L.R. The mental health of those whose rights have been taken away: An essay on the mental health of indigenous peoples in the face of the 2019 Coronavirus (2019-nCoV) outbreak. Psychiatry Res. 2020, 289, 113094. [Google Scholar] [CrossRef]
- Reinders, S.; Alva, A.; Huicho, L.; Blas, M.M. Indigenous communities’ responses to the COVID-19 pandemic and consequences for maternal and neonatal health in remote Peruvian Amazon: A qualitative study based on routine programme supervision. BMJ Open 2020, 10, e044197. [Google Scholar] [CrossRef]
- Santos, V.S.; Souza Araújo, A.A.; de Oliveira, J.R.; Quintans-Júnior, L.J.; Martins-Filho, P.R. COVID-19 mortality among Indigenous people in Brazil: A nationwide register-based study. J. Public Health 2021, 43, e250–e251. [Google Scholar] [CrossRef] [PubMed]
- Baqui, P.; Bica, I.; Marra, V.; Ercole, A.; van der Schaar, M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: A cross-sectional observational study. Lancet Glob. Health 2020, 8, e1018–e1026. [Google Scholar] [CrossRef]
- Argoty-Pantoja, A.D.; Robles-Rivera, K.; Rivera-Paredez, B.; Salmerón, J. COVID-19 fatality in Mexico’s indigenous populations. Public Health 2021, 193, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Charlier, P.; Varison, L. Is COVID-19 being used as a weapon against Indigenous Peoples in Brazil? Lancet 2020, 396, 1069–1070. [Google Scholar] [CrossRef]
- Cupertino, G.A.; Cupertino, M.D.C.; Gomes, A.P.; Braga, L.M.; Siqueira-Batista, R. COVID-19 and Brazilian Indigenous Populations. Am. J. Trop. Med. Hyg. 2020, 103, 609–612. [Google Scholar] [CrossRef]
- Hillier, S.A.; Chaccour, E.; Al-Shammaa, H.; Vorstermans, J. Canada’s response to COVID-19 for Indigenous Peoples: A way forward? Can. J. Public Health 2020, 111, 1000–1001. [Google Scholar] [CrossRef]
- Goha, A.; Mezue, K.; Edwards, P.; Madu, K.; Baugh, D.; Tulloch-Reid, E.E.; Nunura, F.; Doubeni, C.A.; Madu, E. Indigenous people and the COVID-19 pandemic: The tip of an iceberg of social and economic inequities. J. Epidemiol. Community Health 2021, 75, 207–208. [Google Scholar]
- Teixeira, S.C. Circumnavigating the challenges of COVID-19 for Indigenous people: Perspectives for public health. Public Health 2020, 186, 127–128. [Google Scholar] [CrossRef]
- Montag, D.; Barboza, M.; Cauper, L.; Brehaut, I.; Alva, I.; Bennett, A.; Sanchez-Choy, J.; Barletti, J.P.S.; Valenzuela, P.; Manuyama, J. Healthcare of Indigenous Amazonian Peoples in response to COVID-19: Marginality, discrimination and revaluation of ancestral knowledge in Ucayali, Peru. BMJ Glob. Health 2021, 6, e004479. [Google Scholar] [CrossRef]
- Finlay, S.; Wenitong, M. Aboriginal Community Controlled Health Organisations are taking a leading role in COVID-19 health communication. Aust. N. Z. J. Public Health 2020, 44, 251–252. [Google Scholar] [CrossRef]
- Mesa Vieira, C.; Franco, O.H.; Gómez Restrepo, C.; Abel, T. COVID-19: The forgotten priorities of the pandemic. Maturitas 2020, 136, 38–41. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz-Restrepo, F.; Alvis-Zakzuk, N.J.; De la Hoz-Gomez, J.F.; De la Hoz, A.; Gómez Del Corral, L.; Alvis-Guzmán, N. Is Colombia an example of successful containment of the 2020 COVID-19 pandemic? A critical analysis of the epidemiological data, March to July 2020. Int. J. Infect. Dis. 2020, 99, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.; Muñoz, M.; Patiño, L.H.; Hernández, C.; González-Casabianca, F.; Carroll, I.; Santos-Vega, M.; Cascante, J.; Angel, A.; Feged-Rivadeneira, A. Deciphering the introduction and transmission of SARS-CoV-2 in the Colombian Amazon Basin. PLoS Negl. Trop. Dis. 2021, 15, e0009327. [Google Scholar] [CrossRef] [PubMed]
- Kann, S.; Kunz, M.; Hansen, J.; Sievertsen, J.; Crespo, J.J.; Loperena, A.; Arriens, S.; Dandekar, T. Chagas Disease: Detection of Trypanosoma cruzi by a New, High-Specific Real Time PCR. J. Clin. Med. 2020, 9, 1517. [Google Scholar] [CrossRef]
- Hur, K.H.; Park, K.; Lim, Y.; Jeong, Y.S.; Sung, H.; Kim, M.N. Evaluation of Four Commercial Kits for SARS-CoV-2 Real-Time Reverse-Transcription Polymerase Chain Reaction Approved by Emergency-Use-Authorization in Korea. Front. Med. 2020, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Jeong, H.; Chang, E.; Choe, P.G.; Kang, C.K.; Park, W.B.; Kim, T.S.; Kwon, W.Y.; Oh, M.D.; Kim, N.J. Clinical Application of the Standard Q COVID-19 Ag Test for the Detection of SARS-CoV-2 Infection. J. Korean Med. Sci. 2021, 36, e101. [Google Scholar] [CrossRef]
- Emmerich, P.; Murawski, C.; Ehmen, C.; von Possel, R.; Pekarek, N.; Oestereich, L.; Duraffour, S.; Pahlmann, M.; Struck, N.; Eibach, D.; et al. Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin. Trop. Med. Int. Health 2021, 26, 621–631. [Google Scholar] [CrossRef]
- Kann, S.; Bruennert, D.; Hansen, J.; Mendoza, G.A.C.; Gonzalez, J.J.C.; Quintero, C.L.A.; Duraffour, S.; Pahlmann, M.; Struck, N.; Eibach, D.; et al. High Prevalence of Intestinal Pathogens in Indigenous in Colombia. J. Clin. Med. 2020, 9, 2786. [Google Scholar] [CrossRef]
- Frickmann, H.; Alker, J.; Hansen, J.; Dib, J.C.; Aristizabal, A.; Concha, G.; Schotte, U.; Kann, S. Seasonal Differences in Cyclospora cayetanensis Prevalence in Colombian Indigenous People. Microorganisms 2021, 9, 627. [Google Scholar] [CrossRef]
- Cifuentes, M.P.; Rodriguez-Villamizar, L.A.; Rojas-Botero, M.L.; Alvarez-Moreno, C.A.; Fernández-Niño, J.A. Socioeconomic inequalities associated with mortality for COVID-19 in Colombia: A cohort nationwide study. J. Epidemiol. Community Health 2021, 75, 610–615. [Google Scholar] [CrossRef] [PubMed]
| Origin of Study Participants | Number of Tested Individuals (n) | Estimated Number of Inhabitants (n) | Calculated Proportion of Screened Inhabitants (%) |
|---|---|---|---|
| San Juan | 189 | 40,069 | 0.5% |
| Sabana de Higuieron | 88 | 176 | 50.0% |
| Piñoncito | 74 | 486 | 15.2% |
| Ahuyamal | 44 | 109 | 40.4% |
| Surimena | 33 | 106 | 31.1% |
| Others | 24 | 534,697 | <0.1% |
| Total | 452 | 575,643 | <0.1% |
| Risk Categories | Negative | Active | Previous | Active + Previous | ||||||||||
| n | % | n | % | OR | p | n | % | OR | p | n | % | OR | p | |
| Overall | ||||||||||||||
| Total | 278 | 61.5 | 61 | 13.5 | x | x | 113 | 25.0 | x | x | 174 | 38.5 | x | x |
| Sex | ||||||||||||||
| Female (ref) | 156 | 56.1 | 40 | 14.4 | 1 | x | 82 | 29.5 | 1 | x | 122 | 43.9 | 1 | x |
| Male | 122 | 70.1 | 21 | 12.1 | 0.671 | 0.1773 | 31 | 17.8 | 0.483 | 0.0028 | 52 | 29.9 | 0.545 | 0.0031 |
| Ethnicity | ||||||||||||||
| Indigenous (ref) | 243 | 64.0 | 47 | 12.4 | 1 | x | 90 | 23.7 | 1 | x | 137 | 36.1 | 1 | x |
| Columbian | 35 | 48.6 | 14 | 19.4 | 2.068 | 0.0402 | 23 | 31.9 | 1.775 | 0.0522 | 37 | 51,3 | 1.875 | 0.0152 |
| Village | ||||||||||||||
| Ahuyamal | 31 | 70.5 | 0 | 0 | x | 0.9613 | 13 | 29.5 | 0.606 | 0.1811 | 13 | 29.5 | 0.295 | 0.0007 |
| Piñoncito | 47 | 63.5 | 4 | 5.4 | 0.116 | <0.0001 | 23 | 31.1 | 0.707 | 0.2631 | 27 | 36.5 | 0.404 | 0.0014 |
| Sabana de Higuieron | 78 | 88.6 | 0 | 0 | x | 0.9386 | 10 | 11.4 | 0.185 | <0.0001 | 10 | 11.4 | 0.090 | <0.0001 |
| San Juan (ref) | 78 | 41.3 | 57 | 30.2 | 1 | x | 54 | 28.6 | 1 | x | 111 | 58.8 | 1 | x |
| Surimena | 32 | 97.0 | 0 | 0 | x | 0.9607 | 1 | 3.0 | 0.045 | 0.0027 | 1 | 3.0 | 0.022 | 0.0002 |
| Other | 12 | 50.0 | 0 | 0 | x | 0.9759 | 12 | 50.0 | 1.444 | 0.4086 | 12 | 50.0 | 0.703 | 0.4164 |
| Age | ||||||||||||||
| 0−<15 | 62 | 65.3 | 14 | 14.7 | 0.912 | 0.8022 | 19 | 20.0 | 0.748 | 0.3630 | 33 | 34.7 | 0.810 | 0.4271 |
| 15−<25 | 48 | 56.5 | 9 | 10.6 | 0.757 | 0.5121 | 28 | 32.9 | 1.425 | 0.2365 | 37 | 43.5 | 1.173 | 0.5517 |
| 25−<45 (ref) | 105 | 60.3 | 26 | 14.9 | 1 | x | 43 | 24.7 | 1 | x | 69 | 39.6 | 1 | x |
| 45−<60 | 38 | 67.9 | 7 | 12.5 | 0.744 | 0.5256 | 11 | 19.6 | 0.707 | 0.3707 | 18 | 31.1 | 0.721 | 0.3144 |
| ≥60 | 20 | 54.1 | 5 | 13.5 | 1.010 | 0.9860 | 12 | 32.4 | 1.465 | 0.3486 | 17 | 45.9 | 1.293 | 0.4802 |
| Systolic blood pressure | ||||||||||||||
| Systolic blood pressure per 1 mmHg | 255 | x | 50 | x | 1.016 | 0.1299 | 106 | x | 1.007 | 0.3892 | 156 | x | 1.009 | 0.1693 |
| Diastolic blood pressure | ||||||||||||||
| Diastolic blood pressure per 1 mmHg | 255 | x | 50 | x | 1.026 | 0.1232 | 105 | x | 0.977 | 0.0788 | 155 | x | 0.994 | 0.5900 |
| Heart rate | ||||||||||||||
| Heart rate per 1 beat per minute | 278 | x | 61 | x | 1.032 | 0.0044 | 113 | x | 0.984 | 0.0937 | 174 | x | 1.002 | 0.7950 |
| Body temperature | ||||||||||||||
| Temperature per 1 °C | 278 | x | 61 | x | 0.664 | 0.1289 | 113 | x | 0.748 | 0.1894 | 174 | x | 0.725 | 0.0836 |
| O2 saturation | ||||||||||||||
| O2 per 1% | 277 | x | 61 | x | 1.058 | 0.5312 | 113 | x | 0.957 | 0.5239 | 174 | x | 0.990 | 0.8728 |
| Risk Categories | Negative | Active | Previous | Active + Previous | ||||||||||
| n | % | n | % | OR | p | n | % | OR | p | n | % | OR | p | |
| Overall | ||||||||||||||
| Total | 278 | 61.5 | 61 | 13.5 | x | x | 113 | 25.0 | x | x | 174 | 38.5 | x | x |
| Sex | ||||||||||||||
| Female (ref) | 156 | 56.1 | 40 | 14.4 | 1 | x | 82 | 29.5 | 1 | x | 122 | 43.9 | 1 | x |
| Male | 122 | 70.1 | 21 | 12.1 | 1.341 | 0.4050 | 31 | 17.8 | 2.033 | 0.0065 | 52 | 29.9 | 1.700 | 0.0203 |
| Ethnicity | ||||||||||||||
| Indigene (ref) | 243 | 64.0 | 47 | 12.4 | 1 | x | 90 | 23.7 | 1 | x | 137 | 36.1 | 1 | x |
| Columbian | 35 | 48.6 | 14 | 19.4 | 2.394 | 0.0258 | 23 | 31.9 | 1.184 | 0.6240 | 37 | 51.3 | 1.476 | 0.1888 |
| Village | ||||||||||||||
| Ahuyamal | 31 | 70.5 | 0 | 0 | x | 0.9608 | 13 | 29.5 | 1.932 | 0.1078 | 13 | 29.5 | 3.935 | 0.0004 |
| Piñoncito | 47 | 63.5 | 4 | 5.4 | 11.108 | <0.0001 | 23 | 31.1 | 1.471 | 0.2557 | 27 | 36.5 | 2.884 | 0.0005 |
| Sabana de Higuieron | 78 | 88.6 | 0 | 0 | x | 0.9371 | 10 | 11.4 | 5.585 | <0.0001 | 10 | 11.4 | 12.448 | <0.0001 |
| San Juan (ref) | 78 | 41.3 | 57 | 30.2 | 1 | x | 54 | 28.6 | 1 | x | 111 | 58.8 | 1 | x |
| Surimena | 32 | 97.0 | 0 | 0 | x | 0.9599 | 1 | 3.0 | 23.873 | 0.0023 | 1 | 3.0 | 52.148 | 0.0001 |
| Other | 12 | 50.0 | 0 | 0 | x | 0.9752 | 12 | 50.0 | 0.872 | 0.7663 | 12 | 50.0 | 1.610 | 0.2873 |
| Heart rate | ||||||||||||||
| Heart rate per 1 beat per minute | 278 | x | 61 | x | 0.984 | 0.2218 | 113 | x | 1.022 | 0.0459 | 174 | x | 1.007 | 0.4630 |
| Recorded Symptom | Individuals with Active SARS-CoV-2 Infection (n = 61) | Individuals with Previous SARS-CoV-2 Infection (n = 113) | Individuals without Active or Previous SARS-CoV-2 Infection (n = 278) |
|---|---|---|---|
| Headache | 3 | 2 | 2 |
| Joint and muscular pain | 1 | - | 2 |
| Flu-like symptoms | 1 | 2 | - |
| Sore throat | - | 2 | 2 |
| Recent history of fever/chills | 4 | - | 3 |
| Loss of smell/taste | 2 | - | 1 |
| Fatigue | 1 | - | - |
| Rash | - | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha, G.; Frickmann, H.; Oey, A.; Strengert, M.; Kreienbrock, L.; Kann, S. Direct and Indirect Proof of SARS-CoV-2 Infections in Indigenous Wiwa Communities in North-Eastern Colombia—A Cross-Sectional Assessment Providing Preliminary Surveillance Data. Vaccines 2021, 9, 1120. https://doi.org/10.3390/vaccines9101120
Concha G, Frickmann H, Oey A, Strengert M, Kreienbrock L, Kann S. Direct and Indirect Proof of SARS-CoV-2 Infections in Indigenous Wiwa Communities in North-Eastern Colombia—A Cross-Sectional Assessment Providing Preliminary Surveillance Data. Vaccines. 2021; 9(10):1120. https://doi.org/10.3390/vaccines9101120
Chicago/Turabian StyleConcha, Gustavo, Hagen Frickmann, Anke Oey, Monika Strengert, Lothar Kreienbrock, and Simone Kann. 2021. "Direct and Indirect Proof of SARS-CoV-2 Infections in Indigenous Wiwa Communities in North-Eastern Colombia—A Cross-Sectional Assessment Providing Preliminary Surveillance Data" Vaccines 9, no. 10: 1120. https://doi.org/10.3390/vaccines9101120
APA StyleConcha, G., Frickmann, H., Oey, A., Strengert, M., Kreienbrock, L., & Kann, S. (2021). Direct and Indirect Proof of SARS-CoV-2 Infections in Indigenous Wiwa Communities in North-Eastern Colombia—A Cross-Sectional Assessment Providing Preliminary Surveillance Data. Vaccines, 9(10), 1120. https://doi.org/10.3390/vaccines9101120






