Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Antibody ELISA
2.3. ELISpot Assay
2.4. Statistical Analysis
3. Results
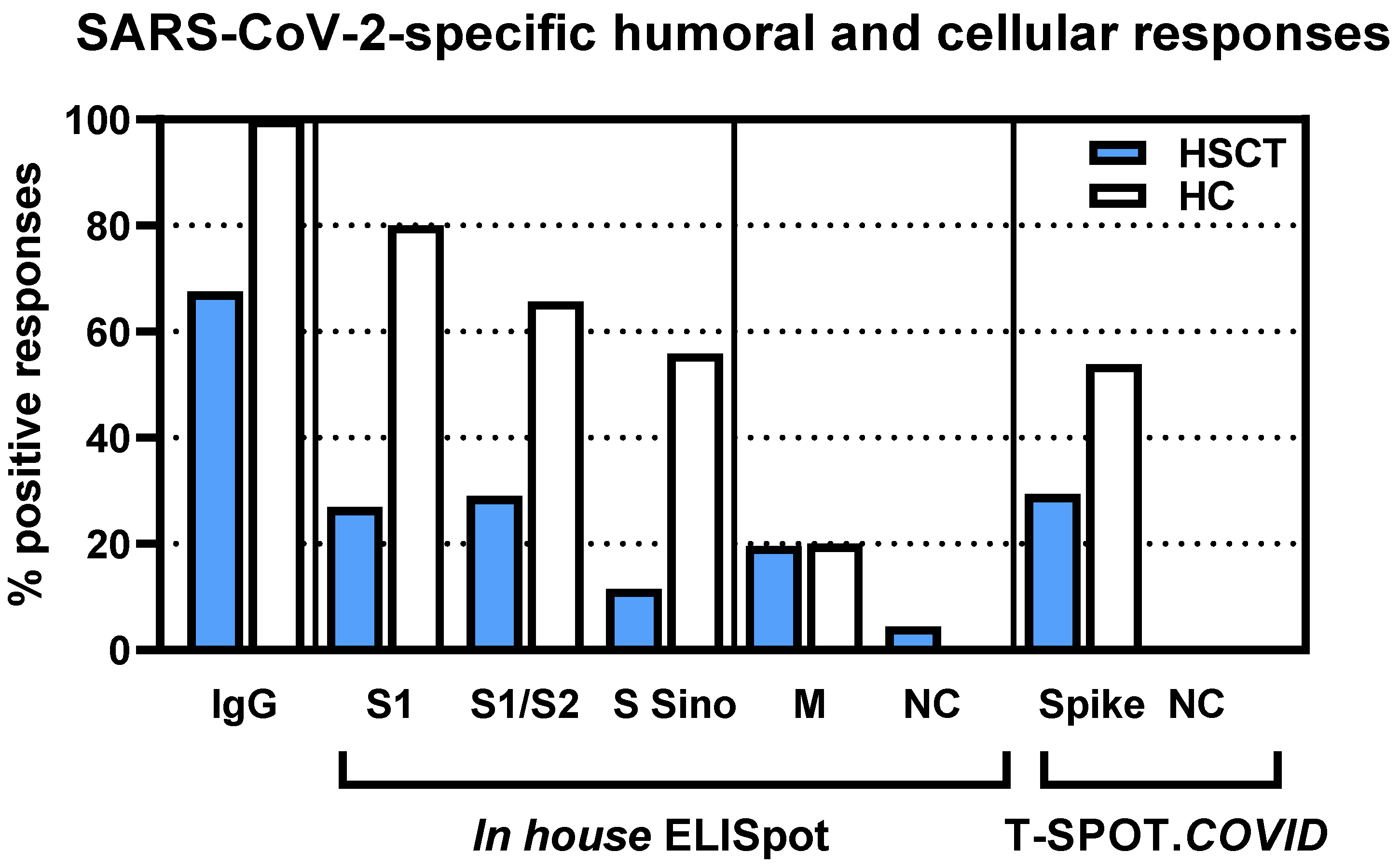
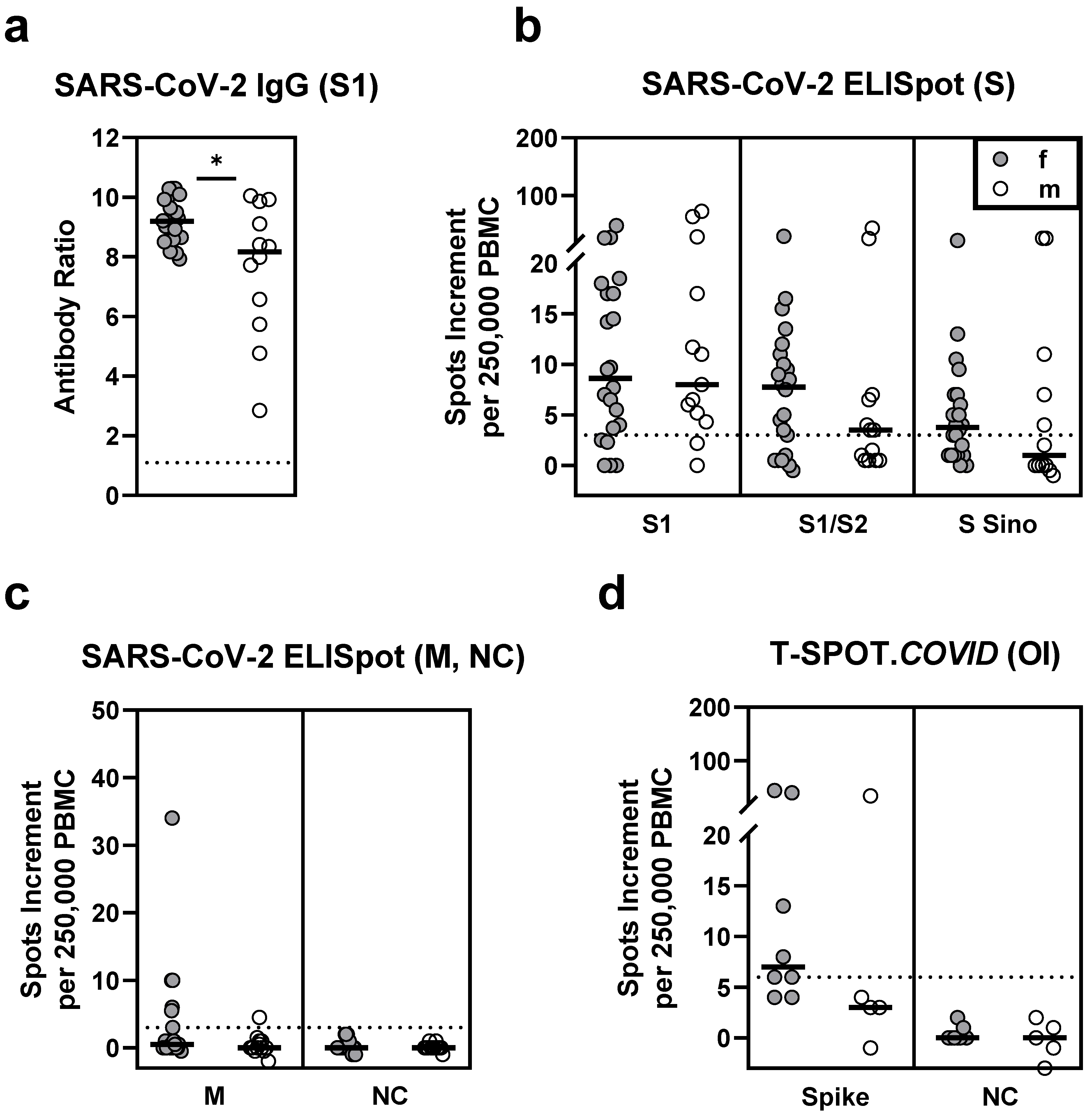
3.1. Comparison of Vaccination Responses in Patients after Hematopoietic Stem Cell Transplantation and Healthy Controls
3.2. Correlation of SARS-CoV-2-Specific Immunity Measured by Various Assays
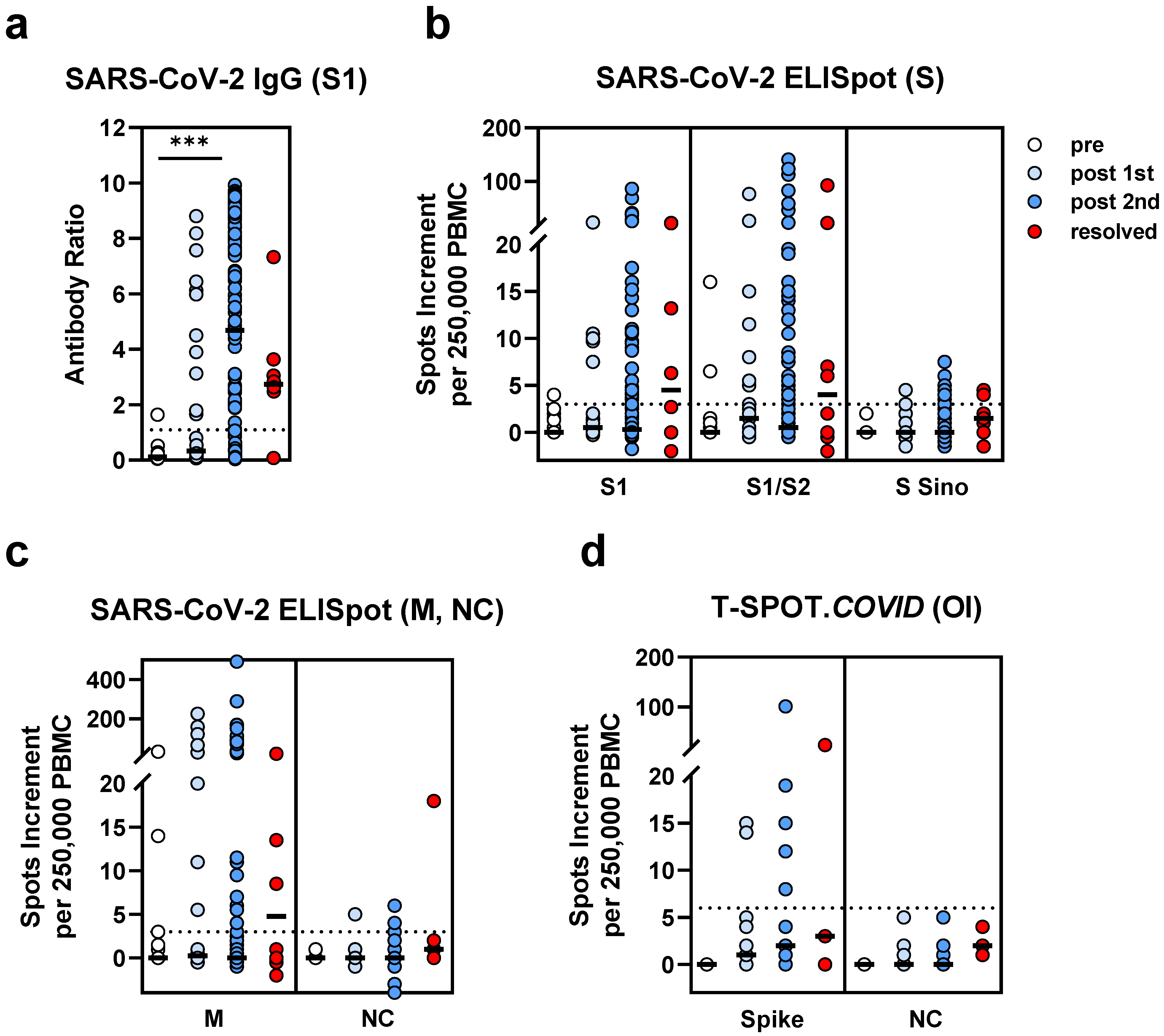
3.3. Comparison of SARS-CoV-2-Specific Immunity in Stem Cell Transplant Recipients Prior to and Post Vaccination and after Infection
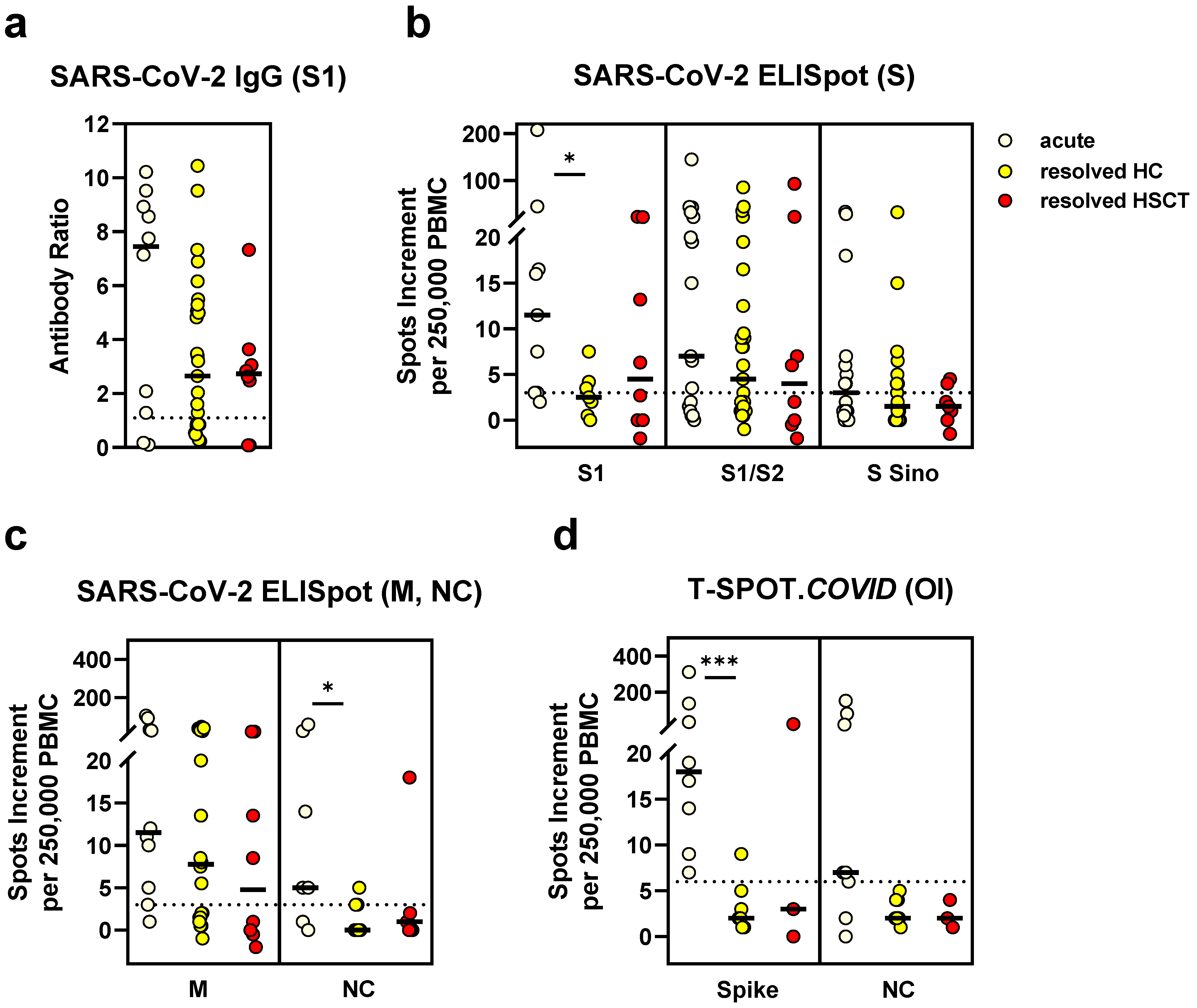
3.4. Comparison of SARS-CoV-2-Specific Immunity in Stem Cell Transplant Recipients and Controls after SARS-CoV-2 Infection
3.5. Correlation of SARS-CoV-2 Immunity and Clinical Parameters
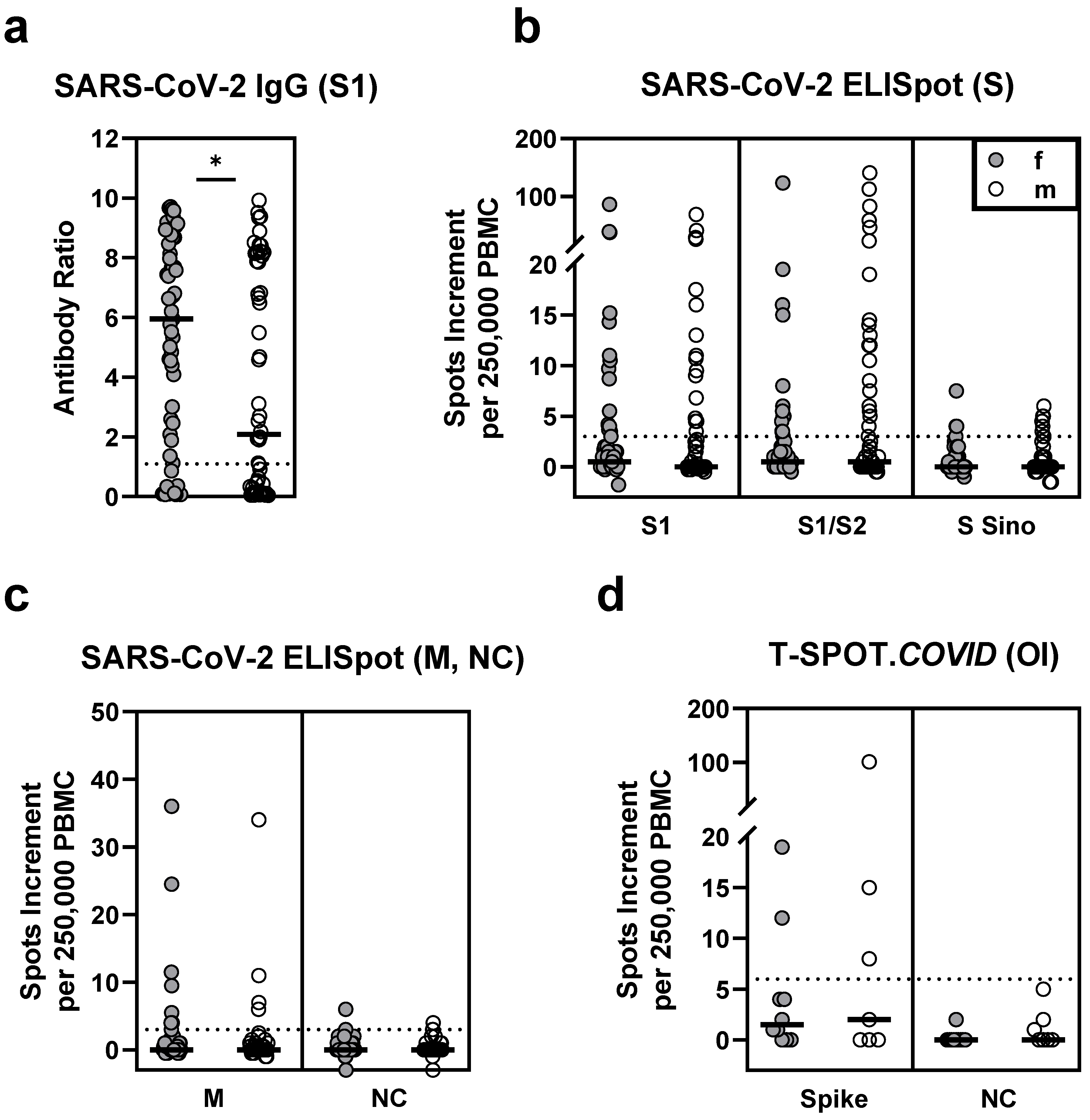
3.5.1. Sex
3.5.2. Age
3.5.3. Interval between Transplantation and Testing
3.5.4. Interval between Vaccination and Testing
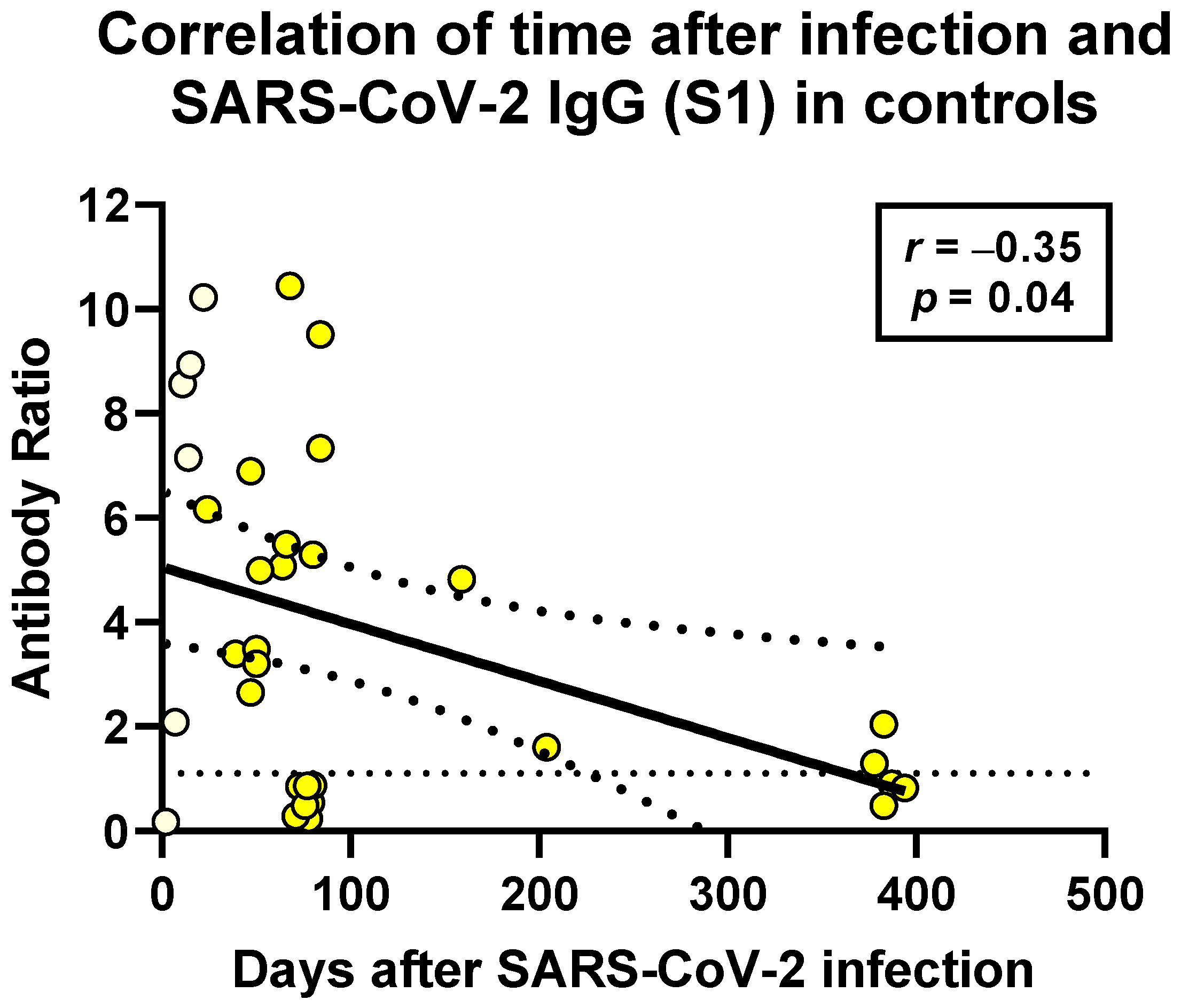
3.5.5. Interval between SARS-CoV-2 Infection and Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ljungman, P.; de la Camara, R.; Mikulska, M.; Tridello, G.; Aguado, B.; Zahrani, M.A.; Apperley, J.; Berceanu, A.; Bofarull, R.M.; Calbacho, M.; et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia 2021. [Google Scholar] [CrossRef]
- Easdale, S.; Shea, R.; Ellis, L.; Bazin, J.; Davis, K.; Dallas, F.; Thistlethwayte, E.; Ethell, M.; Potter, M.; Arias, C.; et al. Serological responses following a single dose of SARS-CoV-2 vaccination in allogeneic stem cell transplant recipients. Transplant. Cell Ther. 2021. [Google Scholar] [CrossRef]
- Chevallier, P.; Coste-Burel, M.; Le Bourgeois, A.; Peterlin, P.; Garnier, A.; Bene, M.C.; Imbert, B.M.; Drumel, T.; Le Gouill, S.; Moreau, P.; et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. eJHaem 2021. [Google Scholar] [CrossRef] [PubMed]
- Maneikis, K.; Sablauskas, K.; Ringeleviciute, U.; Vaitekenaite, V.; Cekauskiene, R.; Kryzauskaite, L.; Naumovas, D.; Banys, V.; Peceliunas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thummler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg. Infect. Dis. 2021, 27. [Google Scholar] [CrossRef]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, E200–E212. [Google Scholar] [CrossRef]
- Polewska, K.; Tylicki, P.; Biedunkiewicz, B.; Rucinska, A.; Szydlowska, A.; Kubanek, A.; Rosenberg, I.; Rodak, S.; Slizien, W.; Renke, M.; et al. Safety and Tolerability of the BNT162b2 mRNA COVID-19 Vaccine in Dialyzed Patients. COViNEPH Project. Medicina 2021, 57, 732. [Google Scholar] [CrossRef]
- Ali, H.; Ngo, D.; Aribi, A.; Arslan, S.; Dadwal, S.; Marcucci, G.; Nakamura, R.; Forman, S.J.; Chen, J.; Al Malki, M.M. Safety and Tolerability of SARS-CoV2 Emergency-Use Authorized Vaccines for Allogeneic Hematopoietic Stem Cell Transplant Recipients. Transpl. Cell Ther. 2021. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Korth, J.; Jahn, M.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hadicke, B.; Eisenberger, U.; Gackler, A.; Dittmer, U.; Witzke, O.; Wilde, B.; et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021, 13, 756. [Google Scholar] [CrossRef]
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulie, C.; Barrou, B.; Marcelin, A.G.; et al. Poor Antibody Response after Two Doses of SARS-CoV-2 vaccine in Transplant Recipients. Clin. Infect. Dis. 2021, 10. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transpl. 2021, 21, 2727–2739. [Google Scholar] [CrossRef]
- Kolb, T.; Fischer, S.; Müller, L.; Lübke, N.; Hillebrandt, J.; Andrée, M.; Schmitz, M.; Schmidt, C.; Kücükköylü, S.; Koster, L.; et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360 2021. [Google Scholar] [CrossRef]
- Miele, M.; Busa, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am. J. Transpl. 2021, 21, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Rashidi-Alavijeh, J.; Frey, A.; Passenberg, M.; Korth, J.; Zmudzinski, J.; Anastasiou, O.E.; Saner, F.H.; Jahn, M.; Lange, C.M.; Willuweit, K. Humoral Response to SARS-Cov-2 Vaccination in Liver Transplant Recipients–A Single-Center Experience. Vaccines 2021, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwobel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 100178. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.E.; Li, A.; Gentili, M.; Perugino, C.A.; Weiss, Z.F.; Bowman, K.; Ankomah, P.; Liu, H.; Lewis, G.D.; Bitar, N.; et al. Vaccine serologic responses among transplant patients associate with COVID-19 infection and T peripheral helper cells. medRxiv 2021. [Google Scholar] [CrossRef]
- Rimar, D.; Slobodin, G.; Paz, A.; Henig, I.; Zuckerman, T. SARS-COV-2 vaccination after stem cell transplantation for scleroderma. Ann. Rheum. Dis. 2021, 80, 1354–1355. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Anft, M.; Paniskaki, K.; Blazquez-Navarro, A.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Konik, M.J.; Meister, T.L.; Pfaender, S.; et al. The Magnitude and Functionality of SARS-CoV-2 Reactive Cellular and Humoral Immunity in Transplant Population Is Similar to the General Population Despite Immunosuppression. Transplantation 2021. [Google Scholar] [CrossRef]
- Herrera, S.; Colmenero, J.; Pascal, M.; Escobedo, M.; Castel, M.A.; Sole-Gonzalez, E.; Palou, E.; Egri, N.; Ruiz, P.; Mosquera, M.; et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transpl. 2021. [Google Scholar] [CrossRef]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transpl. 2021, 40, 754–758. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.N.; et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021, 397, 1178–1181. [Google Scholar] [CrossRef]
- Parry, H.; Tut, G.; Faustini, S.; Stephens, C.; Saunders, P.; Bentley, C.; Hilyard, K.; Brown, K.; Amirthalingam, G.; Charlton, S.; et al. BNT162b2 Vaccination in People over 80 Years of Age Induces Strong Humoral Immune Responses with Cross Neutralisation of P.1 Brazilian Variant. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3816840 (accessed on 30 July 2021).
- Rahimi, G.; Rahimi, B.; Panahi, M.; Abkhiz, S.; Saraygord-Afshari, N.; Milani, M.; Alizadeh, E. An overview of Betacoronaviruses-associated severe respiratory syndromes, focusing on sex-type-specific immune responses. Int. Immunopharmacol. 2021, 92, 107365. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Ward, J.W.; Shouval, D.; Zanetti, A. Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Offit, P.A., Orenstein, W.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 342–374.e317. [Google Scholar]
- Bienzle, U.; Gunther, M.; Neuhaus, R.; Vandepapeliere, P.; Vollmar, J.; Lun, A.; Neuhaus, P. Immunization with an adjuvant hepatitis B vaccine after liver transplantation for hepatitis B-related disease. Hepatology 2003, 38, 811–819. [Google Scholar] [CrossRef] [PubMed]

| Variable | Group | Absolute Number or Median (Range) |
|---|---|---|
| Sex | Female | 61 |
| Male | 56 | |
| Age (years) | 59 (21–77) | |
| Underlying disease | Acute leukemia Myelodysplastic syndromes Myeloproliferative neoplasia Lymphoma Other/not specified | 70 18 14 12 3 |
| Vaccine | Comirnaty® (Biontech/Pfizer) | 111 |
| Spikevax® (Moderna Biotech) | 3 | |
| Vaxzevria® (AstraZeneca) | 2 | |
| Vaxzevria® followed by Comirnaty® | 1 | |
| Interval transplantation- 2nd vaccination | 30 months (5–391) | |
| Interval transplantation- testing | 31 months (6–392) | |
| Interval vaccination-testing 2nd | 31 days (11–137) | |
| 1st | 71 days (33–158) |
| Cohort | Antigen for ELISpot | r | p |
|---|---|---|---|
| HSCT | S1 | 0.45 | <0.0001 |
| S1/S2 | 0.22 | 0.02 | |
| S Sino | 0.16 | 0.09 | |
| Spike (T-SPOT.COVID) 1 | 0.52 | 0.04 | |
| Healthy controls | S1 | 0.22 | 0.22 |
| S1/S2 | 0.20 | 0.25 | |
| S Sino | 0.40 | 0.02 | |
| Spike (T-SPOT.COVID) 1 | 0.14 | 0.64 |
| Antigen for ELISpot 1 | Antigen for ELISpot 2 | r | p |
|---|---|---|---|
| S1 | S1/S2 | 0.48 | <0.0001 |
| S1 | S Sino | 0.34 | 0.0003 |
| S1/S2 | S Sino | 0.11 | 0.24 |
| S1 | Spike (T-SPOT.COVID) 1 | 0.53 | 0.03 |
| S1/S2 | Spike (T-SPOT.COVID) 1 | 0.42 | 0.09 |
| Test | Female (n = 61) | Male (n = 56) | p |
|---|---|---|---|
| IgG | 6.0 (0.07–9.7) | 2.1 (0.04–9.9) | 0.03 |
| S1 ELISpot | 0.5 (−2–87) | 0 (−0.5–69) | 0.49 |
| S1/S2 ELISpot | 0.5 (−0.5–124) | 0.5 (−0.5–141) | 0.52 |
| S Sino ELISpot | 0 (−1–7.5) | 0 (−1.5–6) | 0.76 |
| Spike (T-SPOT.COVID) 2 | 1.5 (0–19) | 2.0 (0–101) | 0.86 |
| Parameter | Test | r | p |
|---|---|---|---|
| Age | IgG | −0.22 | 0.02 |
| S1 ELISpot | −0.05 | 0.59 | |
| S1/S2 ELISpot | 0.06 | 0.55 | |
| S Sino ELISpot | −0.10 | 0.28 | |
| Spike (T-SPOT.COVID) 1 | −0.31 | 0.23 | |
| Interval HSCT-testing | IgG | 0.31 | 0.0006 |
| S1 ELISpot | 0.30 | 0.001 | |
| S1/S2 ELISpot | 0.32 | 0.0004 | |
| S Sino ELISpot | 0.06 | 0.52 | |
| Spike (T-SPOT.COVID) 1 | 0.67 | 0.004 | |
| Interval 2nd vaccination-testing | IgG | −0.05 | 0.59 |
| S1 ELISpot | −0.28 | 0.003 | |
| S1/S2 ELISpot | −0.21 | 0.02 | |
| S Sino ELISpot | −0.14 | 0.14 | |
| Spike (T-SPOT.COVID) 1 | −0.08 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. https://doi.org/10.3390/vaccines9101075
Lindemann M, Klisanin V, Thümmler L, Fisenkci N, Tsachakis-Mück N, Ditschkowski M, Schwarzkopf S, Klump H, Reinhardt HC, Horn PA, et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines. 2021; 9(10):1075. https://doi.org/10.3390/vaccines9101075
Chicago/Turabian StyleLindemann, Monika, Vesna Klisanin, Laura Thümmler, Neslinur Fisenkci, Nikolaos Tsachakis-Mück, Markus Ditschkowski, Sina Schwarzkopf, Hannes Klump, Hans Christian Reinhardt, Peter A. Horn, and et al. 2021. "Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients" Vaccines 9, no. 10: 1075. https://doi.org/10.3390/vaccines9101075
APA StyleLindemann, M., Klisanin, V., Thümmler, L., Fisenkci, N., Tsachakis-Mück, N., Ditschkowski, M., Schwarzkopf, S., Klump, H., Reinhardt, H. C., Horn, P. A., & Koldehoff, M. (2021). Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines, 9(10), 1075. https://doi.org/10.3390/vaccines9101075






