T Cell/B Cell Interactions in the Establishment of Protective Immunity
Abstract
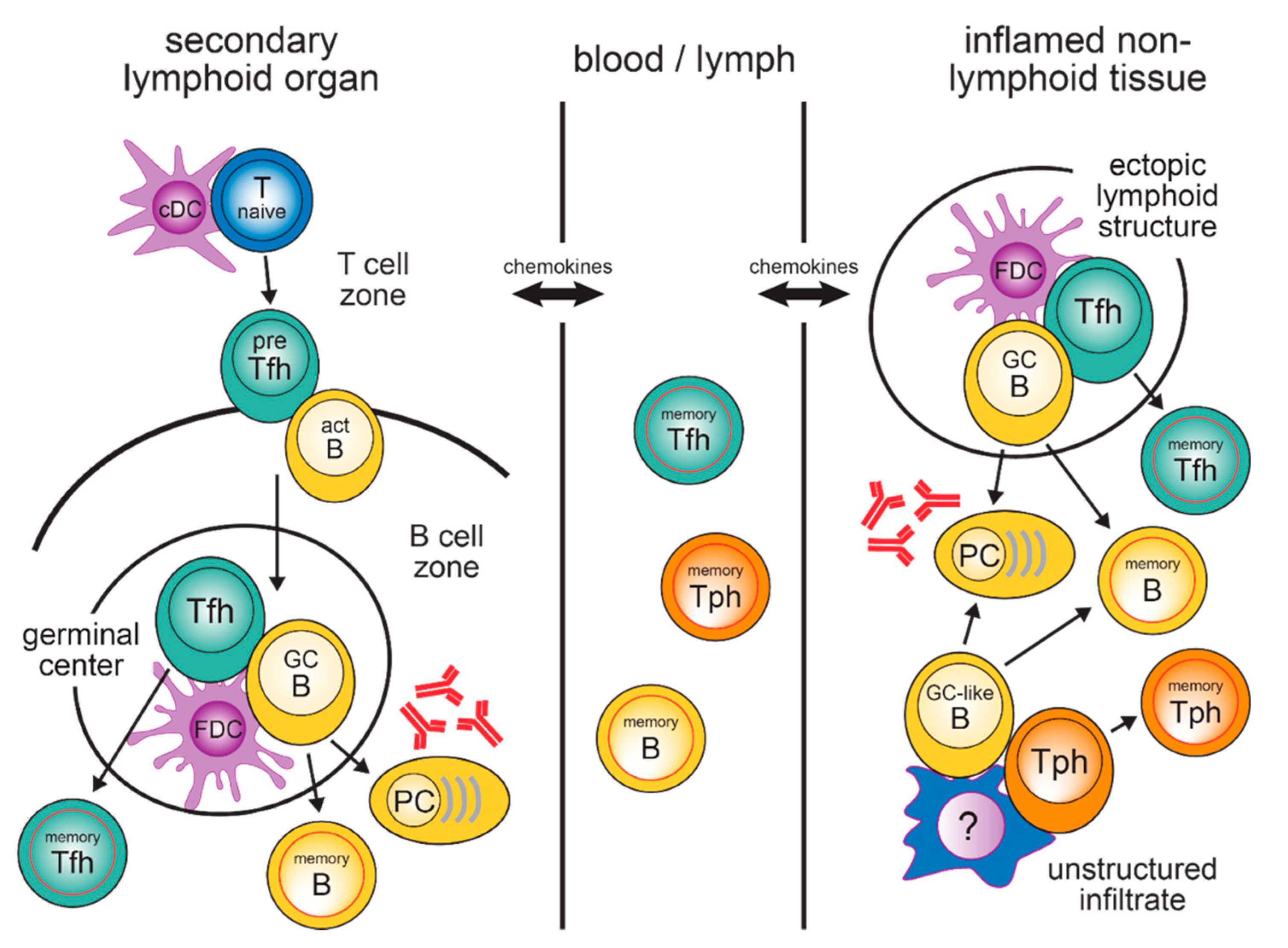
:1. Introduction
2. Generation of Tfh Cells in SLO
3. Circulating Tfh Memory Cells in the Blood
4. T and B Cells in Inflamed Tissues
5. Vaccination Strategies to Promote Tfh Cell Development
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of Humoral Immunity to Common Viral and Vaccine Antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef] [Green Version]
- Purtha, W.E.; Tedder, T.F.; Johnson, S.; Bhattacharya, D.; Diamond, M.S. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 2011, 208, 2599–2606. [Google Scholar] [CrossRef]
- Adachi, Y.; Onodera, T.; Yamada, Y.; Daio, R.; Tsuiji, M.; Inoue, T.; Kobayashi, K.; Kurosaki, T.; Ato, M.; Takahashi, Y. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J. Exp. Med. 2015, 212, 1709–1723. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2012, 12, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Dhenni, R.; Phan, T.G. The geography of memory B cell reactivation in vaccine-induced immunity and in autoimmune disease relapses. Immunol. Rev. 2020, 296, 62–86. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Kastenmüller, W.; Germain, R.N. Spatiotemporal Basis of Innate and Adaptive Immunity in Secondary Lymphoid Tissue. Annu Rev. Cell. Dev. Biol. 2014, 30, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, J.K.; Gowthaman, U.; Zhang, B.; Mattsson, J.; Szeponik, L.; Liu, D.; Wu, R.; White, T.; Calabro, S.; Xu, L.; et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci. Immunol. 2017, 2, eaam9169. [Google Scholar] [CrossRef] [PubMed]
- Goenka, R.; Barnett, L.G.; Silver, J.S.; O’Neill, P.J.; Hunter, C.A.; Cancro, M.P.; Laufer, T.M. Cutting Edge: Dendritic Cell-Restricted Antigen Presentation Initiates the Follicular Helper T Cell Program but Cannot Complete Ultimate Effector Differentiation. J. Immunol. 2011, 187, 1091–1095. [Google Scholar] [CrossRef] [Green Version]
- Shin, C.; Han, J.-A.; Koh, H.; Choi, B.; Cho, Y.; Jeong, H.; Ra, J.-S.; Sung, P.S.; Shin, E.-C.; Ryu, S.; et al. CD8α−Dendritic Cells Induce Antigen-Specific T Follicular Helper Cells Generating Efficient Humoral Immune Responses. Cell Rep. 2015, 11, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Eddahri, F.; Denanglaire, S.; Bureau, F.; Spolski, R.; Leonard, W.J.; Leo, O.; Andris, F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood 2009, 113, 2426–2433. [Google Scholar] [CrossRef]
- Ballesteros-Tato, A.; León, B.; Graf, B.A.; Moquin, A.; Adams, P.S.; Lund, F.E.; Randall, T.D. Interleukin-2 Inhibits Germinal Center Formation by Limiting T Follicular Helper Cell Differentiation. Immunity 2012, 36, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R.J.; Choi, Y.S.; Diamond, J.A.; Yang, J.A.; Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012, 209, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Afkarian, M.; Sedy, J.R.; Yang, J.; Jacobson, N.G.; Cereb, N.; Yang, S.Y.; Murphy, T.L.; Murphy, K.M. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat. Immunol. 2002, 3, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, S.A.; Schmidlin, H.; Nagasawa, M.; Blom, B.; Spits, H. IL-6 Triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol. Cell Biol. 2012, 90, 802–811. [Google Scholar] [CrossRef] [Green Version]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4 + T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef]
- Di Toro, D.; Winstead, C.J.; Pham, D.; Witte, S.; Andargachew, R.; Singer, J.R.; Wilson, C.G.; Zindl, C.L.; Luther, R.J.; Silberger, D.J.; et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 2018, 361, eaao2933. [Google Scholar] [CrossRef]
- Papillion, A.; Powell, M.D.; Chisolm, D.A.; Bachus, H.; Fuller, M.J.; Weinmann, A.S.; Villarino, A.; O’Shea, J.J.; León, B.; Oestreich, K.J.; et al. Inhibition of IL-2 responsiveness by IL-6 is required for the generation of GC-TFH cells. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Vogelzang, A.; McGuire, H.M.; Yu, D.; Sprent, J.; Mackay, C.R.; King, C. A Fundamental Role for Interleukin-21 in the Generation of T Follicular Helper Cells. Immunity 2008, 29, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.-H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A Novel Transcription Factor, T-bet, Directs Th1 Lineage Commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Qi, H. T follicular helper cells in space-time. Nat. Rev. Immunol. 2016, 16, 612–625. [Google Scholar] [CrossRef]
- Nakayamada, S.; Kanno, Y.; Takahashi, H.; Jankovic, D.; Lu, K.T.; Johnson, T.A.; Sun, H.; Vahedi, G.; Hakim, O.; Handon, R.; et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 2011, 35, 919–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oestreich, K.J.; Huang, A.C.; Weinmann, A.S. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 2011, 208, 1001–1013. [Google Scholar] [CrossRef]
- Oestreich, K.J.; Mohn, S.E.; Weinmann, A.S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 2012, 13, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Groom, J.R.; Richmond, J.; Murooka, T.T.; Sorensen, E.W.; Sung, J.H.; Bankert, K.; von Andrian, U.H.; Moon, J.J.; Mempel, T.R.; Luster, A.D. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 2012, 37, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Haynes, N.M.; Allen, C.D.C.; Lesley, R.; Ansel, K.M.; Killeen, N.; Cyster, J.G. Role of CXCR5 and CCR7 in Follicular Th Cell Positioning and Appearance of a Programmed Cell Death Gene-1 High Germinal Center-Associated Subpopulation. J. Immunol. 2007, 179, 5099–5108. [Google Scholar] [CrossRef] [Green Version]
- Hardtke, S.; Ohl, L.; Förster, R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 2005, 106, 1924–1931. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lu, E.; Yi, T.; Cyster, J.G. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature 2016, 533, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hercor, M.; Anciaux, M.; Denanglaire, S.; Debuisson, D.; Leo, O.; Andris, F. Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells. J. Leukoc. Biol. 2017, 101, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.; Ekland, E.H.; Ohl, L.; Nakano, H.; Lipp, M.; Förster, R.; Cyster, J.G. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 2002, 416, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Liu, D.; Li, J.; Zhang, X.; Chen, X.; Hou, S.; Peng, L.; Xu, C.; Liu, W.; et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013, 496, 523–527. [Google Scholar] [CrossRef]
- Avancena, P.; Song, T.; Yao, Y.; Fehlner-Peach, H.; Diamond, B.; Gu, H.; Rajewsky, K.; Zou, Y.-R. The magnitude of germinal center reactions is restricted by a fixed number of preexisting niches. Proc. Natl. Acad. Sci. USA 2021, 118, e2100576118. [Google Scholar] [CrossRef]
- Heesters, B.A.; Myers, R.C.; Carroll, M.C. Follicular dendritic cells: Dynamic antigen libraries. Nat. Rev. Immunol. 2014, 14, 495–504. [Google Scholar] [CrossRef]
- Weber, J.P.; Fuhrmann, F.; Hutloff, A. T-follicular helper cells survive as long-term memory cells. Eur. J. Immunol. 2012, 42, 1981–1988. [Google Scholar] [CrossRef]
- Suan, D.; Nguyen, A.; Moran, I.; Bourne, K.; Hermes, J.R.; Arshi, M.; Hampton, H.R.; Tomura, M.; Miwa, Y.; Kelleher, A.D.; et al. T Follicular Helper Cells Have Distinct Modes of Migration and Molecular Signatures in Naive and Memory Immune Responses. Immunity 2015, 42, 704–718. [Google Scholar] [CrossRef] [Green Version]
- Asrir, A.; Aloulou, M.; Gador, M.; Pérals, C.; Fazilleau, N. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat. Commun. 2017, 8, 847. [Google Scholar] [CrossRef] [Green Version]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef] [Green Version]
- Victora, G.D.; Schwickert, T.A.; Fooksman, D.R.; Kamphorst, A.O.; Meyer-Hermann, M.; Dustin, M.L.; Nussenzweig, M.C. Germinal Center Dynamics Revealed by Multiphoton Microscopy with a Photoactivatable Fluorescent Reporter. Cell 2010, 143, 592–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulman, Z.; Gitlin, A.D.; Weinstein, J.S.; Lainez, B.; Esplugues, E.; Flavell, R.A.; Craft, J.E.; Nussenzweig, M.C. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 2014, 345, 1058–1062. [Google Scholar] [CrossRef] [Green Version]
- Gitlin, A.D.; Shulman, Z.; Nussenzweig, M.C. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 2014, 509, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Merkenschlager, J.; Finkin, S.; Ramos, V.; Kraft, J.; Cipolla, M.; Nowosad, C.R.; Hartweger, H.; Zhang, W.; Olinares, P.D.B.; Gazumyan, A.; et al. Dynamic regulation of TFH selection during the germinal centre reaction. Nature 2021, 591, 458–463. [Google Scholar] [CrossRef]
- Fazilleau, N.; Eisenbraun, M.D.; Malherbe, L.; Ebright, J.N.; Pogue-Caley, R.R.; McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 2007, 8, 753–761. [Google Scholar] [CrossRef]
- Weber, J.P.; Fuhrmann, F.; Feist, R.K.; Lahmann, A.; Al Baz, M.S.; Gentz, L.-J.; Vu Van, D.; Mages, H.W.; Haftmann, C.; Riedel, R.; et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. J. Exp. Med. 2015, 212, 217–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linterman, M.A.; Denton, A.E.; Divekar, D.P.; Zvetkova, I.; Kane, L.; Ferreira, C.; Veldhoen, M.; Clare, S.; Dougan, G.; Espéli, M.; et al. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. Elife 2014, 3, e03180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatigny, S.; Höllbacher, B.; Motley, S.J.; Tan, C.; Hundhausen, C.; Buckner, J.H.; Smilek, D.; Khoury, S.J.; Ding, L.; Qin, T.; et al. Abatacept Targets T Follicular Helper and Regulatory T Cells, Disrupting Molecular Pathways That Regulate Their Proliferation and Maintenance. J. Immunol. 2019, 202, 1373–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiba, H.; Takeda, K.; Kojima, Y.; Usui, Y.; Harada, N.; Yamazaki, T.; Ma, J.; Tezuka, K.; Yagita, H.; Okumura, K. The Role of ICOS in the CXCR5+ Follicular B Helper T Cell Maintenance In Vivo. J. Immunol. 2005, 175, 2340–2348. [Google Scholar] [CrossRef] [Green Version]
- Pratama, A.; Srivastava, M.; Williams, N.J.; Papa, I.; Lee, S.K.; Dinh, X.T.; Hutloff, A.; Jordan, M.A.; Zhao, J.L.; Casellas, R.; et al. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat. Commun. 2015, 6, 6436. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Shih, C.; Wan, Z.; Ma, X.; Ma, W.; Luo, D.; Qi, H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 2015, 517, 214–218. [Google Scholar] [CrossRef]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274.e4. [Google Scholar] [CrossRef] [Green Version]
- Jacquemin, C.; Schmitt, N.; Contin-Bordes, C.; Liu, Y.; Narayanan, P.; Seneschal, J.; Maurouard, T.; Dougall, D.; Davizon, E.S.; Dumortier, H.; et al. OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response. Immunity 2015, 42, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Förster, R.; Emrich, T.; Kremmer, E.; Lipp, M. Expression of the G-Protein-Coupled Receptor BLR1 Defines Mature, Recirculating B Cells and a Subset of T-Helper Memory Cells. Blood 1994, 84, 830–840. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Tsai, L.M.; Leong, Y.A.; Hu, X.; Ma, C.S.; Chevalier, N.; Sun, X.; Vandenberg, K.; Rockman, S.; Ding, Y.; et al. Circulating Precursor CCR7loPD-1hi CXCR5+ CD4+ T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity 2013, 39, 770–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locci, M.; Havenar-Daughton, C.; Landais, E.; Wu, J.; Kroenke, M.A.; Arlehamn, C.L.; Su, L.F.; Cubas, R.; Davis, M.M.; Sette, A.; et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013, 39, 758–769. [Google Scholar] [CrossRef] [Green Version]
- Lüthje, K.; Kallies, A.; Shimohakamada, Y.; Belz, G.T.; Light, A.; Tarlinton, D.M.; Nutt, S.L. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 2012, 13, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bossaller, L.; Burger, J.; Draeger, R.; Grimbacher, B.; Knoth, R.; Plebani, A.; Durandy, A.; Baumann, U.; Schlesier, M.; Welcher, A.A.; et al. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+ CD4 Germinal Center Th Cells. J. Immunol. 2006, 177, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.S.; Avery, D.T.; Chan, A.; Batten, M.; Bustamante, J.; Boisson-Dupuis, S.; Arkwright, P.D.; Kreins, A.Y.; Averbuch, D.; Engelhard, D.; et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 2012, 119, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.P.; Gerosa, J.; Baronio, M.; Montin, D.; Licciardi, F.; Soresina, A.; Dellepiane, R.M.; Miano, M.; Baselli, L.A.; Volpi, S.; et al. Circulating follicular helper and follicular regulatory T cells are severely compromised in human CD40 deficiency: A case report. Front. Immunol. 2018, 9, 1761. [Google Scholar] [CrossRef] [PubMed]
- Martini, H.; Enright, V.; Perro, M.; Workman, S.; Birmelin, J.; Giorda, E.; Quinti, I.; Lougaris, V.; Baronio, M.; Warnatz, K.; et al. Importance of B cell co-stimulation in CD4(+) T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin. Exp. Immunol. 2011, 164, 381–387. [Google Scholar] [CrossRef]
- Brenna, E.; Davydov, A.N.; Ladell, K.; McLaren, J.E.; Bonaiuti, P.; Metsger, M.; Ramsden, J.D.; Gilbert, S.C.; Lambe, T.; Price, D.A.; et al. CD4+ T Follicular Helper Cells in Human Tonsils and Blood Are Clonally Convergent but Divergent from Non-Tfh CD4+ Cells. Cell Rep. 2020, 30, 137–152.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, L.A.; Buggert, M.; Manne, S.; Herati, R.S.; Sayin, I.; Kuri-Cervantes, L.; Bukh Brody, I.; O’Boyle, K.C.; Kaprielian, H.; Giles, J.R.; et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J. Clin. Investig. 2019, 129, 3185–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herati, R.S.; Muselman, A.; Vella, L.; Bengsch, B.; Parkhouse, K.; Del Alcazar, D.; Kotzin, J.; Doyle, S.A.; Tebas, P.; Hensley, S.E.; et al. Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci. Immunol. 2017, 2, eaag2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, R.; Schmitt, N.; Bentebibel, S.-E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeng-Adjei, N.; Portugal, S.; Tran, T.M.; Yazew, T.B.; Skinner, J.; Li, S.; Jain, A.; Felgner, P.L.; Doumbo, O.K.; Kayentao, K.; et al. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Rep. 2015, 13, 425–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentebibel, S.-E.; Lopez, S.; Obermoser, G.; Schmitt, N.; Mueller, C.; Harrod, C.; Flano, E.; Mejias, A.; Albrecht, R.A.; Blankenship, D.; et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 2013, 5, 176ra32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsakos, M.; Wheatley, A.K.; Loh, L.; Clemens, E.B.; Sant, S.; Nüssing, S.; Fox, A.; Chung, A.W.; Laurie, K.L.; Hurt, A.C.; et al. Circulating Tfh cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 2018, 10, eaan8405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, W.; Wen, B.; Xie, T.; Tang, P.; Hu, Y.; Huang, L.; Jin, K.; Zhang, P.; Liu, Z.; et al. Circulating CXCR3(+) Tfh cells positively correlate with neutralizing antibody responses in HCV-infected patients. Sci. Rep. 2019, 9, 10090. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Silva, D.G.; Martin, J.L.; Pratama, A.; Hu, X.; Chang, P.-P.; Walters, G.; Vinuesa, C.G. Interferon-y Excess Leads to Pathogenic Accumulation of Follicular Helper T Cells and Germinal Centers. Immunity 2012, 37, 880–892. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, R.L.; Liang, H.-E.; Locksley, R.M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 2009, 10, 385–393. [Google Scholar] [CrossRef]
- Fang, D.; Cui, K.; Mao, K.; Hu, G.; Li, R.; Zheng, M.; Riteau, N.; Reiner, S.L.; Sher, A.; Zhao, K.; et al. Transient T-bet expression functionally specifies a distinct T follicular helper subset. J. Exp. Med. 2018, 215, 2705–2714. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.S.; Laidlaw, B.J.; Lu, Y.; Wang, J.K.; Schulz, V.P.; Li, N.; Herman, E.I.; Kaech, S.M.; Gallagher, P.G.; Craft, J. STAT4 and T-bet control follicular helper T cell development in viral infections. J. Exp. Med. 2017, 215, 337–355. [Google Scholar] [CrossRef]
- Heit, A.; Schmitz, F.; Gerdts, S.; Flach, B.; Moore, M.S.; Perkins, J.A.; Robins, H.S.; Aderem, A.; Spearman, P.; Tomaras, G.D.; et al. Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J. Exp. Med. 2017, 214, 2139–2152. [Google Scholar] [CrossRef] [Green Version]
- Spensieri, F.; Borgogni, E.; Zedda, L.; Bardelli, M.; Buricchi, F.; Volpini, G.; Fragapane, E.; Tavarini, S.; Finco, O.; Rappuoli, R.; et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc. Natl. Acad. Sci. USA 2013, 110, 14330–14335. [Google Scholar] [CrossRef] [Green Version]
- Schultz, B.T.; Teigler, J.E.; Pissani, F.; Oster, A.F.; Kranias, G.; Alter, G.; Marovich, M.; Eller, M.A.; Dittmer, U.; Robb, M.L.; et al. Circulating HIV-Specific Interleukin-21+CD4+T Cells Represent Peripheral Tfh Cells with Antigen-Dependent Helper Functions. Immunity 2016, 44, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Boswell, K.L.; Paris, R.; Boritz, E.; Ambrozak, D.; Yamamoto, T.; Darko, S.; Wloka, K.; Wheatley, A.; Narpala, S.; McDermott, A.; et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014, 10, e1003853. [Google Scholar] [CrossRef]
- Simpson, N.; Gatenby, P.A.; Wilson, A.; Malik, S.; Fulcher, D.A.; Tangye, S.G.; Manku, H.; Vyse, T.J.; Roncador, G.; Huttley, G.A.; et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 234–244. [Google Scholar] [CrossRef]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Kusser, K.; Hartson, L.; Sprague, F.; Goodrich, S.; Woodland, D.L.; Lund, F.E.; Randall, T.D. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004, 10, 927–934. [Google Scholar] [CrossRef]
- Fleige, H.; Ravens, S.; Moschovakis, G.L.; Bölter, J.; Willenzon, S.; Sutter, G.; Häussler, S.; Kalinke, U.; Prinz, I.; Förster, R. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J. Exp. Med. 2014, 211, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef]
- Rendeiro, A.F.; Ravichandran, H.; Bram, Y.; Chandar, V.; Kim, J.; Meydan, C.; Park, J.; Foox, J.; Hether, T.; Warren, S.; et al. The spatial landscape of lung pathology during COVID-19 progression. Nature 2021, 593, 564–569. [Google Scholar] [CrossRef]
- Ramos da Silva, S.; Ju, E.; Meng, W.; Paniz Mondolfi, A.E.; Dacic, S.; Green, A.; Bryce, C.; Grimes, Z.; Fowkes, M.; Sordillo, E.M.; et al. Broad Severe Acute Respiratory Syndrome Coronavirus 2 Cell Tropism and Immunopathology in Lung Tissues From Fatal Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 1842–1854. [Google Scholar] [CrossRef]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.B.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care Med. 2020, 203, 192–201. [Google Scholar] [CrossRef]
- Hutloff, A. T Follicular Helper-Like Cells in In amed Non-Lymphoid Tissues. Front. Immunol. 2018, 9, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu Van, D.; Beier, K.C.; Pietzke, L.-J.; Al Baz, M.S.; Feist, R.K.; Gurka, S.; Hamelmann, E.; Kroczek, R.A.; Hutloff, A. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat. Commun. 2016, 7, 10875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu Van, D.; Bauer, L.; Kroczek, R.A.; Hutloff, A. ICOS Costimulation Differentially Affects T Cells in Secondary Lymphoid Organs and Inflamed Tissues. Am. J. Respir. Cell. Mol. Biol. 2018, 59, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bocharnikov, A.V.; Keegan, J.; Wacleche, V.S.; Cao, Y.; Fonseka, C.Y.; Wang, G.; Muise, E.S.; Zhang, K.X.; Arazi, A.; Keras, G.; et al. PD-1hiCXCR5– T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 2019, 4, e130062. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.K.; Mittereder, N.; Kuta, E.; Delaney, T.; Burwell, T.; Dacosta, K.; Zhao, W.; Cheng, L.I.; Brown, C.; Boutrin, A.; et al. T follicular helper–like cells contribute to skin fibrosis. Sci. Transl. Med. 2018, 10, eaaf5307. [Google Scholar] [CrossRef] [Green Version]
- Christophersen, A.; Lund, E.G.; Snir, O.; Solà, E.; Kanduri, C.; Dahal-Koirala, S.; Zühlke, S.; Molberg, Ø.; Utz, P.J.; Rohani-Pichavant, M.; et al. Distinct phenotype of CD4(+) T cells driving celiac disease identified in multiple autoimmune conditions. Nat. Med. 2019, 25, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, H.; Zhou, S.; Zhou, Y.; Zheng, J.; Zhu, H.; Pan, M. The Pathogenic Role of CD4+ Tissue-Resident Memory T Cells Bearing T Follicular Helper-Like Phenotype in Pemphigus Lesions. J. Investig. Dermatol. 2021, 141, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, D.-Q.; Xiao, Q.; Liu, Y.-B.; Song, J.; Liang, Y.; Ruan, J.-W.; Wang, Z.-Z.; Li, J.-X.; Pan, L.; et al. Defective STING expression potentiates IL-13 signaling in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Blokland, S.L.M.; Hillen, M.R.; Kruize, A.A.; Meller, S.; Homey, B.; Smithson, G.M.; Radstake, T.R.D.J.; van Roon, J.A.G. Increased CCL25 and T Helper Cells Expressing CCR9 in the Salivary Glands of Patients With Primary Sjögren’s Syndrome: Potential New Axis in Lymphoid Neogenesis. Arthritis Rheumatol. 2017, 69, 2038–2051. [Google Scholar] [CrossRef] [Green Version]
- Pontarini, E.; Murray-Brown, W.J.; Croia, C.; Lucchesi, D.; Conway, J.; Rivellese, F.; Fossati-Jimack, L.; Astorri, E.; Prediletto, E.; Corsiero, E.; et al. Unique expansion of IL-21+ Tfh and Tph cells under control of ICOS identifies Sjögren’s syndrome with ectopic germinal centres and MALT lymphoma. Ann. Rheum. Dis. 2020, 79, 1588–1599. [Google Scholar] [CrossRef]
- Bauer, L.; Müller, L.J.; Volkers, S.M.; Heinrich, F.; Mashreghi, M.F.; Ruppert, C.; Sander, L.E.; Hutloff, A. Follicular helper-like T cells in the lung highlight a novel role of B cells in sarcoidosis. Am. J. Respir. Crit. Care. Med. 2021. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Migliori, E.; Buisseret, L.; de Wind, A.; Brohée, S.; Garaud, S.; Noël, G.; Dang Chi, V.L.; Lodewyckx, J.-N.; Naveaux, C.; et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2017, 2, e91487. [Google Scholar] [CrossRef] [Green Version]
- Masopust, D.; Soerens, A.G. Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol. 2019, 37, 521–546. [Google Scholar] [CrossRef]
- Hogan, R.J.; Zhong, W.; Usherwood, E.J.; Cookenham, T.; Roberts, A.D.; Woodland, D.L. Protection from Respiratory Virus Infections Can Be Mediated by Antigen-Specific Cd4+ T Cells that Persist in the Lungs. J. Exp. Med. 2001, 193, 981–986. [Google Scholar] [CrossRef]
- Bingaman, A.W.; Patke, D.S.; Mane, V.R.; Ahmadzadeh, M.; Ndejembi, M.; Bartlett, S.T.; Farber, D.L. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur. J. Immunol. 2005, 35, 3173–3186. [Google Scholar] [CrossRef]
- Oja, A.E.; Piet, B.; Helbig, C.; Stark, R.; van der Zwan, D.; Blaauwgeers, H.; Remmerswaal, E.B.M.; Amsen, D.; Jonkers, R.E.; Moerland, P.D.; et al. Trigger-happy resident memory CD4+ T cells inhabit the human lungs. Mucosal Immunol. 2018, 11, 654–667. [Google Scholar] [CrossRef]
- Thome, J.J.C.; Yudanin, N.; Ohmura, Y.; Kubota, M.; Grinshpun, B.; Sathaliyawala, T.; Kato, T.; Lerner, H.; Shen, Y.; Farber, D.L. Spatial Map of Human T Cell Compartmentalization and Maintenance over Decades of Life. Cell 2014, 159, 814–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.T.; Ong, D.E.H.; Lim, F.S.H.; Teng, K.W.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GeurtsvanKessel, C.H.; Willart, M.A.M.; Bergen, I.M.; van Rijt, L.S.; Muskens, F.; Elewaut, D.; Osterhaus, A.D.M.E.; Hendriks, R.; Rimmelzwaan, G.F.; Lambrecht, B.N. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus–infected mice. J. Exp. Med. 2009, 206, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheon, I.; Wu, Y.; Li, C.; Wang, Z.; Gao, X.; Chen, Y.; Takahashi, Y.; Fu, Y.; Dent, A.; et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol. 2021, 6, eabb6852. [Google Scholar] [CrossRef]
- Swarnalekha, N.; Schreiner, D.; Litzler, L.C.; Iftikhar, S.; Kirchmeier, D.; Künzli, M.; Son, Y.M.; Sun, J.; Moreira, E.A.; King, C.G. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021, 6, eabb6808. [Google Scholar]
- Teijaro John, R.; Verhoeven, D.; Carly, P.A.; Turner, D.; Farber, D.L. Memory CD4 T Cells Direct Protective Responses to Influenza Virus in the Lungs through Helper-Independent Mechanisms. J. Virol. 2010, 84, 9217–9226. [Google Scholar] [CrossRef] [Green Version]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrançois, L.; Farber, D.L. Cutting Edge: Tissue-Retentive Lung Memory CD4 T Cells Mediate Optimal Protection to Respiratory Virus Infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef] [Green Version]
- Allie, S.R.; Bradley, J.E.; Mudunuru, U.; Schultz, M.D.; Graf, B.A.; Lund, F.E.; Randall, T.D. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 2019, 20, 97–108. [Google Scholar] [CrossRef]
- Joo, H.M.; He, Y.; Sangster, M.Y. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc. Natl. Acad. Sci. USA 2008, 105, 3485–3490. [Google Scholar] [CrossRef] [Green Version]
- Onodera, T.; Takahashi, Y.; Yokoi, Y.; Ato, M.; Kodama, Y.; Hachimura, S.; Kurosaki, T.; Kobayashi, K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA 2012, 109, 2485–2490. [Google Scholar] [CrossRef] [Green Version]
- Sakai, S.; Kauffman, K.D.; Schenkel, J.M.; McBerry, C.C.; Mayer-Barber, K.D.; Masopust, D.; Barber, D.L. Cutting Edge: Control of Mycobacterium tuberculosis Infection by a Subset of Lung Parenchyma–Homing CD4 T Cells. J. Immunol. 2014, 192, 2965–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Zhao, J.; Mangalam, A.K.; Channappanavar, R.; Fett, C.; Meyerholz, D.K.; Agnihothram, S.; Baric, R.S.; David, C.S.; Perlman, S. Airway Memory CD4+ T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity 2016, 44, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Barker, K.A.; Etesami, N.S.; Shenoy, A.T.; Arafa, E.I.; Lyon de Ana, C.; Smith, N.M.S.; Martin, I.M.C.; Goltry, W.N.; Barron, A.M.S.; Browning, J.L.; et al. Lung-resident memory B cells protect against bacterial pneumonia. J. Clin. Investig. 2021, 131, e141810. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Sugimoto-Ishige, A.; Harada, Y.; Adachi, Y.; Usami, Y.; Kaji, T.; Inoue, K.; Hasegawa, H.; Watanabe, T.; Hijikata, A.; et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 2016, 17, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Ho, N.I.; Huis In ’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef]
- Choi, Y.S.; Eto, D.; Yang, J.A.; Lao, C.; Crotty, S. Cutting Edge: STAT1 Is Required for IL-6–Mediated Bcl6 Induction for Early Follicular Helper Cell Differentiation. J. Immunol. 2013, 190, 3049–3053. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswamy, J.K.; Alsén, S.; Yrlid, U.; Eisenbarth, S.C.; Williams, A. Determination of T Follicular Helper Cell Fate by Dendritic Cells. Front. Immunol. 2018, 9, 2169. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent Antigen and Germinal Center B Cells Sustain T Follicular Helper Cell Responses and Phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Deenick, E.K.; Chan, A.; Ma, C.S.; Gatto, D.; Schwartzberg, P.L.; Brink, R.; Tangye, S.G. Follicular Helper T Cell Differentiation Requires Continuous Antigen Presentation that Is Independent of Unique B Cell Signaling. Immunity 2010, 33, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riteau, N.; Radtke, A.J.; Shenderov, K.; Mittereder, L.; Oland, S.D.; Hieny, S.; Jankovic, D.; Sher, A. Water-in-Oil-Only Adjuvants Selectively Promote T Follicular Helper Cell Polarization through a Type I IFN and IL-6-Dependent Pathway. J. Immunol. 2016, 197, 3884–3893. [Google Scholar] [CrossRef] [Green Version]
- Lahmann, A.; Bauer, L.; Hutloff, A. Identification of Follicular T-Cell Subsets in Murine and Human Tissues. Methods Mol. Biol. 2021, 2285, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.; Baldeon, A.D.; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccin. Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.L.; Pierson, W.; Bolland, D.J.; Mkindi, C.; Carr, E.J.; Wang, J.; Houard, S.; Wingett, S.W.; Audran, R.; Wallin, E.F.; et al. The adjuvant GLA-SE promotes human Tfh cell expansion and emergence of public TCRβ clonotypes. J. Exp. Med. 2019, 216, 1857–1873. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific B Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.; Fernández-Tejada, A.; Barjaktarović, Ž.; Bouzalas, I.; Brimnes, J.; Chernysh, S.; Gizurarson, S.; Gursel, I.; Jakopin, Ž.; Lawrenz, M.; et al. Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunol. Rev. 2020, 296, 169–190. [Google Scholar] [CrossRef]
- Glenny, A.T. Insoluble precipitates in diphtheria and tetanus immunization. Br. Med. J. 1930, 2, 244–245. [Google Scholar] [CrossRef] [Green Version]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springerplus 2015, 4, 181. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G.; et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef] [PubMed]
- Spensieri, F.; Siena, E.; Borgogni, E.; Zedda, L.; Cantisani, R.; Chiappini, N.; Schiavetti, F.; Rosa, D.; Castellino, F.; Montomoli, E.; et al. Early Rise of Blood T Follicular Helper Cell Subsets and Baseline Immunity as Predictors of Persisting Late Functional Antibody Responses to Vaccination in Humans. PLoS ONE 2016, 11, e0157066. [Google Scholar] [CrossRef] [PubMed]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.-H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.-A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Givord, C.; Welsby, I.; Detienne, S.; Thomas, S.; Assabban, A.; Lima Silva, V.; Molle, C.; Gineste, R.; Vermeersch, M.; Perez-Morga, D.; et al. Activation of the endoplasmic reticulum stress sensor IRE1α by the vaccine adjuvant AS03 contributes to its immunostimulatory properties. NPJ Vaccines 2018, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Budroni, S.; Buricchi, F.; Cavallone, A.; Bourguignon, P.; Caubet, M.; Dewar, V.; D’Oro, U.; Finco, O.; Garçon, N.; El Idrissi, M.; et al. Antibody avidity, persistence, and response to antigen recall: Comparison of vaccine adjuvants. NPJ Vaccines 2021, 6, 78. [Google Scholar] [CrossRef]
- Treanor, J.J.; Essink, B.; Hull, S.; Reed, S.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Kohberger, R.; Dunkle, L.M. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant. Vaccine 2013, 31, 5760–5765. [Google Scholar] [CrossRef]
- Seydoux, E.; Liang, H.; Dubois Cauwelaert, N.; Archer, M.; Rintala, N.D.; Kramer, R.; Carter, D.; Fox, C.B.; Orr, M.T. Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses. J. Immunol. 2018, 201, 98–112. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Roeffen, W.; Singh, S.K.; Tiendrebeogo, R.W.; Christiansen, M.; Beebe, E.; Carter, D.; Fox, C.B.; Howard, R.F.; Reed, S.G.; et al. Synthetic TLR4 agonists enhance functional antibodies and CD4+ T-cell responses against the Plasmodium falciparum GMZ2.6C multi-stage vaccine antigen. Vaccine 2016, 34, 2207–2215. [Google Scholar] [CrossRef] [Green Version]
- Van Hoeven, N.; Joshi, S.W.; Nana, G.I.; Bosco-Lauth, A.; Fox, C.; Bowen, R.A.; Clements, D.E.; Martyak, T.; Parks, D.E.; Baldwin, S.; et al. A Novel Synthetic TLR-4 Agonist Adjuvant Increases the Protective Response to a Clinical-Stage West Nile Virus Vaccine Antigen in Multiple Formulations. PLoS ONE 2016, 11, e0149610. [Google Scholar]
- Leroux-Roels, I.; Devaster, J.-M.; Leroux-Roels, G.; Verlant, V.; Henckaerts, I.; Moris, P.; Hermand, P.; Van Belle, P.; Poolman, J.T.; Vandepapelière, P.; et al. Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: Randomised, controlled trials. Vaccine 2015, 33, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G.; Marchant, A.; Levy, J.; Van Damme, P.; Schwarz, T.F.; Horsmans, Y.; Jilg, W.; Kremsner, P.G.; Haelterman, E.; Clément, F.; et al. Impact of adjuvants on CD4+ T cell and B cell responses to a protein antigen vaccine: Results from a phase II, randomized, multicenter trial. Clin. Immunol. 2016, 169, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.M.; Ogbe, A.; Pedroza-Pacheco, I.; Doeleman, S.E.; Chen, Y.; Silk, S.E.; Barrett, J.R.; Elias, S.C.; Miura, K.; Diouf, A.; et al. Protein/AS01B vaccination elicits stronger, more Th2-skewed antigen-specific human T follicular helper cell responses than heterologous viral vectors. Cell. Rep. Med. 2021, 2, 100207. [Google Scholar] [CrossRef]
- Aljurayyan, A.N.; Sharma, R.; Upile, N.; Beer, H.; Vaughan, C.; Xie, C.; Achar, P.; Ahmed, M.S.; McNamara, P.S.; Gordon, S.B.; et al. A critical role of T follicular helper cells in human mucosal anti-influenza response that can be enhanced by immunological adjuvant CpG-DNA. Antivir. Res. 2016, 132, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claassen, E.; de Leeuw, W.; de Greeve, P.; Hendriksen, C.; Boersma, W. Freund’s complete adjuvant: An effective but disagreeable formula. Res. Immunol. 1992, 143, 478–483. [Google Scholar] [CrossRef]
- Chakarov, S.; Fazilleau, N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Mol. Med. 2014, 6, 590–603. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, E.; Liu, H.; Huckriede, A.; Hak, E. Safety and tolerability evaluation of the use of Montanide ISATM51 as vaccine adjuvant: A systematic review. Hum. Vaccines Immunother. 2016, 12, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-B.; Xu, J. Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant-Antigen Codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Fong, C.H.-Y.; Zhang, A.J.; Wu, W.-L.; Li, I.C.; Lee, A.C.-Y.; Dissanayake, T.K.; Chen, L.; Hung, I.F.-N.; Chan, K.-H.; et al. Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine. Vaccines 2020, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Gupta, G.; Adhikari, A.; Majumder, S.; Kar Mahapatra, S.; Bhattacharyya Majumdar, S.; Majumdar, S. Miltefosine triggers a strong proinflammatory cytokine response during visceral leishmaniasis: Role of TLR4 and TLR9. Int. Immunopharmacol. 2012, 12, 565–572. [Google Scholar] [CrossRef]
- Gibson, S.J.; Lindh, J.M.; Riter, T.R.; Gleason, R.M.; Rogers, L.M.; Fuller, A.E.; Oesterich, J.L.; Gorden, K.B.; Qiu, X.; McKane, S.W.; et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 2002, 218, 74–86. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Rasheed, M.A.U.; Havenar-Daughton, C.; Pham, M.; Legere, T.; Sher, Z.J.; Kovalenkov, Y.; Gumber, S.; Huang, J.Y.; Gottardo, R.; et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020, 5, eabb1025. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-Y.; Lin, M.-Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.-J.; Liu, L.T.-C.; Cheng, J.; Wu, Y.-C.; Wu, C.-C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Vono, M.; Eberhardt, C.S.; Mohr, E.; Auderset, F.; Christensen, D.; Schmolke, M.; Coler, R.; Meinke, A.; Andersen, P.; Lambert, P.-H.; et al. Overcoming the Neonatal Limitations of Inducing Germinal Centers through Liposome-Based Adjuvants Including C-Type Lectin Agonists Trehalose Dibehenate or Curdlan. Front. Immunol. 2018, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- van Dissel, J.T.; Soonawala, D.; Joosten, S.A.; Prins, C.; Arend, S.M.; Bang, P.; Tingskov, P.N.; Lingnau, K.; Nouta, J.; Hoff, S.T.; et al. Ag85B–ESAT-6 adjuvanted with IC31® promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine 2011, 29, 2100–2109. [Google Scholar] [CrossRef]
- Knudsen, N.P.H.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef]
- Lirussi, D.; Weissmann, S.F.; Ebensen, T.; Nitsche-Gloy, U.; Franz, H.B.G.; Guzmán, C.A. Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines. Pharmaceutics 2021, 13, 188. [Google Scholar] [CrossRef]
- Chauveau, L.; Bridgeman, A.; Tan, T.K.; Beveridge, R.; Frost, J.N.; Rijal, P.; Pedroza-Pacheco, I.; Partridge, T.; Gilbert-Jaramillo, J.; Knight, M.L.; et al. Inclusion of cGAMP within virus-like particle vaccines enhances their immunogenicity. EMBO Rep. 2021, 22, e52447. [Google Scholar] [CrossRef] [PubMed]
- Carnathan, D.G.; Kaushik, K.; Ellebedy, A.H.; Enemuo, C.A.; Gebru, E.H.; Dhadvai, P.; Rasheed, M.A.U.; Pauthner, M.G.; Ozorowski, G.; Ahmed, R.; et al. Harnessing Activin A Adjuvanticity to Promote Antibody Responses to BG505 HIV Envelope Trimers. Front. Immunol. 2020, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Agren, L.C.; Ekman, L.; Löwenadler, B.; Lycke, N.Y. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 1997, 158, 3936–3946. [Google Scholar] [PubMed]
- Eriksson, A.M.; Schön, K.M.; Lycke, N.Y. The Cholera Toxin-Derived CTA1-DD Vaccine Adjuvant Administered Intranasally Does Not Cause Inflammation or Accumulate in the Nervous Tissues. J. Immunol. 2004, 173, 3310–3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bemark, M.; Bergqvist, P.; Stensson, A.; Holmberg, A.; Mattsson, J.; Lycke, N.Y. A Unique Role of the Cholera Toxin A1-DD Adjuvant for Long-Term Plasma and Memory B Cell Development. J. Immunol. 2011, 186, 1399–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schussek, S.; Bernasconi, V.; Mattsson, J.; Wenzel, U.A.; Strömberg, A.; Gribonika, I.; Schön, K.; Lycke, N.Y. The CTA1-DD adjuvant strongly potentiates follicular dendritic cell function and germinal center formation, which results in improved neonatal immunization. Mucosal Immunol. 2020, 13, 545–557. [Google Scholar] [CrossRef]
- Gary, E.; O’Connor, M.; Chakhtoura, M.; Tardif, V.; Kumova, O.K.; Malherbe, D.C.; Sutton, W.F.; Haigwood, N.L.; Kutzler, M.A.; Haddad, E.K. Adenosine deaminase-1 enhances germinal center formation and functional antibody responses to HIV-1 Envelope DNA and protein vaccines. Vaccine 2020, 38, 3821–3831. [Google Scholar] [CrossRef]
- Robinson, C.; Baehr, C.; Schmiel, S.E.; Accetturo, C.; Mueller, D.L.; Pravetoni, M. Alum adjuvant is more effective than MF59 at prompting early germinal center formation in response to peptide-protein conjugates and enhancing efficacy of a vaccine against opioid use disorders. Hum. Vaccin. Immunother. 2019, 15, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, S.; Benson, R.A.; Gibson, V.B.; Pollock, A.H.; Garside, P.; Brewer, J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012, 26, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef] [PubMed]
- De Donato, S.; Granoff, D.; Minutello, M.; Lecchi, G.; Faccini, M.; Agnello, M.; Senatore, F.; Verweij, P.; Fritzell, B.; Podda, A. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine 1999, 17, 3094–3101. [Google Scholar] [CrossRef]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De Gregorio, E.; Seubert, A.; Wack, A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Moris, P.; van der Most, R.; Leroux-Roels, I.; Clement, F.; Dramé, M.; Hanon, E.; Leroux-Roels, G.G.; Van Mechelen, M. H5N1 Influenza Vaccine Formulated with AS03A Induces Strong Cross-Reactive and Polyfunctional CD4 T-Cell Responses. J. Clin. Immunol. 2011, 31, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Sparwasser, T.; Vabulas, R.M.; Villmow, B.; Lipford, G.B.; Wagner, H. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur. J. Immunol. 2000, 30, 3591–3597. [Google Scholar] [CrossRef]
- Klinman, D.M. Therapeutic Applications of CpG-Containing Oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998, 8, 181–184. [Google Scholar] [CrossRef]
- Pichyangkul, S.; Kum-Arb, U.; Yongvanitchit, K.; Limsalakpetch, A.; Gettayacamin, M.; Lanar, D.E.; Ware, L.A.; Stewart, V.A.; Heppner, D.G.; Mettens, P.; et al. Preclinical evaluation of the safety and immunogenicity of a vaccine consisting of Plasmodium falciparum liver-stage antigen 1 with adjuvant AS01B administered alone or concurrently with the RTS,S/AS01B vaccine in rhesus primates. Infect. Immun. 2008, 76, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Vandepapelière, P.; Horsmans, Y.; Moris, P.; Van Mechelen, M.; Janssens, M.; Koutsoukos, M.; Van Belle, P.; Clement, F.; Hanon, E.; Wettendorff, M.; et al. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 2008, 26, 1375–1386. [Google Scholar] [CrossRef]
- Bok, S.Y.; Jin, I.S.; Hong, N.; Won, K.S.; Woo, C.Y.; Cheol, K.M.; Seong, L.H.; Tak, J.H.; Hwan, Y.S.; La, C.M.; et al. Crucial Roles of Interleukin-7 in the Development of T Follicular Helper Cells and in the Induction of Humoral Immunity. J. Virol. 2014, 88, 8998–9009. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, L.; Sui, B.; Luo, Z.; Zhang, Y.; Wang, Y. Recombinant Rabies Virus Overexpressing OX40-Ligand Enhances Humoral Immune Responses by Increasing T Follicular Helper Cells and Germinal Center B Cells. Vaccines 2020, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Sulczewski, F.B.; Martino, L.A.; Almeida, B.d.S.; Zaneti, A.B.; Ferreira, N.S.; Amorim, K.N.d.S.; Yamamoto, M.M.; Apostolico, J.d.S.; Rosa, D.S.; Boscardin, S.B. Conventional type 1 dendritic cells induce TH1, TH1-like follicular helper T cells and regulatory T cells after antigen boost via DEC205 receptor. Eur. J. Immunol. 2020, 50, 1895–1911. [Google Scholar] [CrossRef]
- Godot, V.; Tcherakian, C.; Gil, L.; Cervera-Marzal, I.; Li, G.; Cheng, L.; Ortonne, N.; Lelièvre, J.-D.; Pantaleo, G.; Fenwick, C.; et al. TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice. PLoS Pathog. 2020, 16, e1009025. [Google Scholar] [CrossRef]
- Andersen, T.K.; Huszthy, P.C.; Gopalakrishnan, R.P.; Jacobsen, J.T.; Fauskanger, M.; Tveita, A.A.; Grødeland, G.; Bogen, B. Enhanced germinal center reaction by targeting vaccine antigen to major histocompatibility complex class II molecules. NPJ Vaccines 2019, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.A.; Ols, S.; Miura, K.; Rausch, K.; Narum, D.L.; Spångberg, M.; Juraska, M.; Wille-Reece, U.; Weiner, A.; Howard, R.F.; et al. TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 2018, 3, e120692. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Suh, H.; Li, A.V.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 2012, 109, 1080–1085. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin 3rd, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Pauthner, M.; Havenar-Daughton, C.; Sok, D.; Nkolola, J.P.; Bastidas, R.; Boopathy, A.V.; Carnathan, D.G.; Chandrashekar, A.; Cirelli, K.M.; Cottrell, C.A.; et al. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 2017, 46, 1073–1088.e6. [Google Scholar] [CrossRef] [Green Version]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019, 177, 1153–1171.e28. [Google Scholar] [CrossRef] [PubMed]
- Regules, J.A.; Cicatelli, S.B.; Bennett, J.W.; Paolino, K.M.; Twomey, P.S.; Moon, J.E.; Kathcart, A.K.; Hauns, K.D.; Komisar, J.L.; Qabar, A.N.; et al. Fractional Third and Fourth Dose of RTS,S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J. Infect. Dis. 2016, 214, 762–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H.; Hughes, T.K.; Pokkali, S.; Swanson, P.A.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.N.T.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral vaccination protects against transcervical infection with Chlamydia trachomatis and generate tissue-resident T cells post-challenge. NPJ Vaccines 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, H.M.; He, Y.; Sundararajan, A.; Huan, L.; Sangster, M.Y. Quantitative analysis of influenza virus-specific B cell memory generated by different routes of inactivated virus vaccination. Vaccine 2010, 28, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Rioux, G.; Mathieu, C.; Russell, A.; Bolduc, M.; Laliberté-Gagné, M.-E.; Savard, P.; Leclerc, D. PapMV nanoparticles improve mucosal immune responses to the trivalent inactivated flu vaccine. J. Nanobiotechnol. 2014, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Counoupas, C.; Ferrell, K.C.; Ashhurst, A.; Bhattacharyya, N.D.; Nagalingam, G.; Stewart, E.L.; Feng, C.G.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. NPJ Vaccines 2020, 5, 105. [Google Scholar] [CrossRef]
- Flórido, M.; Muflihah, H.; Lin, L.C.W.; Xia, Y.; Sierro, F.; Palendira, M.; Feng, C.G.; Bertolino, P.; Stambas, J.; Triccas, J.A.; et al. Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4+ memory T cells that are associated with protection against tuberculosis. Mucosal Immunol. 2018, 11, 1743–1752. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-X.; Wheatley, A.K.; Esterbauer, R.; Jegaskanda, S.; Glass, J.J.; Masopust, D.; De Rose, R.; Kent, S.J. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime–boost immunization. Mucosal Immunol. 2018, 11, 994–1007. [Google Scholar] [CrossRef] [Green Version]
- Çuburu, N.; Graham, B.S.; Buck, C.B.; Kines, R.C.; Pang, Y.-Y.S.; Day, P.M.; Lowy, D.R.; Schiller, J.T. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Investig. 2012, 122, 4606–4620. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Magnusson, M.K.; Quiding-Järbrink, M.; Lundgren, A. Activated T follicular helper-like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B-cell memory. Sci. Rep. 2018, 8, 2729. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef]
- Knight, F.C.; Gilchuk, P.; Kumar, A.; Becker, K.W.; Sevimli, S.; Jacobson, M.E.; Suryadevara, N.; Wang-Bishop, L.; Boyd, K.L.; Crowe Jr, J.E.; et al. Mucosal Immunization with a pH-Responsive Nanoparticle Vaccine Induces Protective CD8(+) Lung-Resident Memory T Cells. ACS Nano 2019, 13, 10939–10960. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef]
- Zacharias, Z.R.; Ross, K.A.; Hornick, E.E.; Goodman, J.T.; Narasimhan, B.; Waldschmidt, T.J.; Legge, K.L. Polyanhydride Nanovaccine Induces Robust Pulmonary B and T Cell Immunity and Confers Protection Against Homologous and Heterologous Influenza A Virus Infections. Front. Immunol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Rakhra, K.; Abraham, W.; Wang, C. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci. Immunol. 2021, 6, eabd8003. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Kuang, Y.; Liang, J.; Jones, M.; Swain, S.L. Influenza Vaccine–Induced CD4 Effectors Require Antigen Recognition at an Effector Checkpoint to Generate CD4 Lung Memory and Antibody Production. J. Immunol. 2020, 205, 2077–2090. [Google Scholar] [CrossRef]
- Lapuente, D.; Storcksdieck genannt Bonsmann, M.; Maaske, A.; Stab, V.; Heinecke, V.; Watzstedt, K.; Heß, R.; Westendorf, A.M.; Bayer, W.; Ehrhardt, C.; et al. IL-1β as mucosal vaccine adjuvant: The specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal Immunol. 2018, 11, 1265–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, E.A.; Darrah, P.A.; Foulds, K.E.; Hoffer, E.; Caffrey-Carr, A.; Norenstedt, S.; Perbeck, L.; Seder, R.A.; Kedl, R.M.; Loré, K. Monocytes Acquire the Ability to Prime Tissue-Resident T Cells via IL-10-Mediated TGF-β Release. Cell. Rep. 2019, 28, 1127–1135.e4. [Google Scholar] [CrossRef] [Green Version]
- Kingstad-Bakke, B.; Toy, R.; Lee, W.; Pradhan, P.; Vogel, G.; Marinaik, C.B.; Larsen, A.; Gates, D.; Luu, T.; Pandey, B.; et al. Polymeric Pathogen-Like Particles-Based Combination Adjuvants Elicit Potent Mucosal T Cell Immunity to Influenza A Virus. Front. Immunol. 2021, 11, 559382. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition and immunity: Lessons for COVID-19. Eur. J. Clin. Nutr. 2021, 75, 1309–1318. [Google Scholar] [CrossRef]
- Sudfeld, C.R.; Navar, A.M.; Halsey, N.A. Effectiveness of measles vaccination and vitamin A treatment. Int. J. Epidemiol. 2010, 39 (Suppl. 1), i48–i55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Measles vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2009, 84, 349–360.
- Scholz, J.; Kuhrau, J.; Heinrich, F.; Heinz, G.A.; Hutloff, A.; Worm, M.; Heine, G. Vitamin A controls the allergic response through T follicular helper cell as well as plasmablast differentiation. Allergy. Eur. J. Allergy Clin. Immunol. 2021, 76, 1109–1122. [Google Scholar] [CrossRef]
- Yang, C.; Yao, H.; Wu, Y.; Sun, G.; Yang, W.; Li, Z.; Shang, L. Status and risks of selenium deficiency in a traditional selenium-deficient area in Northeast China. Sci. Total Environ. 2021, 762, 144103. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, Q.; Chen, Z.; Liang, K.; Gao, X.; Wang, X.; Makota, F.V.; Ong, H.S.; Wan, Y.; Luo, K.; et al. The metabolic hormone leptin promotes the function of T(FH) cells and supports vaccine responses. Nat. Commun. 2021, 12, 3073. [Google Scholar] [CrossRef]

| Adjuvant | Influence on Humoral Immune Response | Licensed in Humans | Literature | ||||
|---|---|---|---|---|---|---|---|
| alum | alum | Tfh cell formation but lower numbers compared to combinations with TLR agonists | licensed | [128,132,133] | |||
| AS04 (MPL + alum) | in combination with MPL strong activation of T and B cells, persistent antibody, and cellular responses, induces biased Th1 immune responses → targeting of viral infections | licensed | [127,134,135,136] | ||||
| oil-in-water emulsions | squalene-based | MF59 | promotion of potent immune responses in mice and humans | licensed | [137,138] | ||
| AS03 (resembles MF59, combined with vitamin E) | potent Tfh cell activation in mice, high proportion of high-avidity antibodies after antigen recall in humans, persistence of cellular and humoral responses, induces marked antibody response, used in vaccines where antibody mediated protection is important | licensed | [127,139,140] | ||||
| GLA-SE (GLA + squalene) | promotes Tfh cell expansion in mice and men and effective, long-lived antibody production in humans, stable emulsion superior in enhancing adjuvanticity in GLA | clinical trials | [128,141,142] | ||||
| SLA-SE (SLA + squalene) | strong antibody and CD4+ T cell responses in mice | [143,144] | |||||
| saponin-based | AS02 (MPL + QS-21) | induces strong humoral and T cell mediated immune responses, used for pathogens that require strong T cell response, enhances humoral immune response in elderly people | clinical trials | [127,145] | |||
| Liposomes | AS01 (MPL + Saponin) | persistence of cellular and humoral responses in humans, robust innate stimulation, highly potent stimulation of CD4+ T cells and specific antibody responses in humans, designed to strengthen CD8+ T cell response | licensed | [127,140,146,147] | |||
| AS015 (AS01 combined with CpG) | CpG promotes Tfh cell and antibody responses to influenza vaccination | not licensed | [148] | ||||
| Water-in-oil emulsions | IFA | strong Tfh cell polarization in mice, strong side effects in humans and therefore not used, addition of CpG improved Tfh cell differentiation | not licensed | [124,149,150] | |||
| CFA | triggers Tfh cells and additionally Th1, Th17 and Th2 responses in mice | not licensed | [124] | ||||
| Montanide | strong Tfh cell polarization in mice, side effects in humans | not licensed | [124,151] | ||||
| immunostimulators | TLR4 agonists | LPS | not usable for humans due to toxicity | [152] | |||
| synthetic TLR4 agonists | miltefosine (TLR4 and TLR9 agonist) | enhances Tfh cell responses and GC reaction in mice, induces both Th1 and Th2 antigen-specific cytokine responses, MTF improves efficacy of influenza vaccine against homologous and heterologous viruses by improving Tfh and antibody response, might be used for other than influenza vaccines as well | [153,154] | ||||
| MPL | included in AS01 and AS04 | ||||||
| GLA | GLA-SE | ||||||
| SLA | SLA-SE | ||||||
| TLR7 agonists | 3M052 | promotes DC maturation and cellular response, enhanced Tfh cell generation compared to alum in NHP | [152,155,156] | ||||
| TLR9 agonists | CpG | included in AS015, combination of SARS-CoV-2 spike protein with CpG 1018 and alum elicited Th1-dominant immune responses with high neutralizing antibodies in mice | [157] | ||||
| IC31 | induction of strong Th1 response in mice and humans, increase in Tfh cells in mice | clinical trials | [158,159] | ||||
| C-type lectin agonist | mincle agonist | CAF01 | promotes GC responses and prolonged humoral responses in murine neonates, strong Th1/Th17 responses in mice | clinical trials | [158,160] | ||
| STING agonist | chemically modified cyclic dinucleotide | induces Tfh and Th1 cell responses in neonatal cord blood, three-dose vaccination schedule is beneficial in mice leading to higher antibody titres; presence of cGAMP within HIV-derived virus-like particles enhanced adaptive immune responses, increased Tfh cell numbers in draining lymphnode | [161,162] | ||||
| Activin A | Study in NHP, HIV model: Activin A administration shows no differences in Tfh cell numbers, but decreased number of Tfr cells → either by promotion of Tfh cells or inhibitory role on Tfr development, improved antibody response and PC development | [163] | |||||
| CTA1-DD | CTA1-DD adjuvant well documented in mice, long term plasma cell and memory B cell development Effective mucosal and systemic adjuvant Can bind complement on FDCs, thereby directly affects DC function, directly influences gene transcription in FDCs, greatly upregulates Cxcl13 gene expression → strongly promotes GC B cell and Tfh cell development in neonate mice | [164,165,166,167] | |||||
| Adenosine deaminase-1 | HIV-1 envelope (env) DNA vaccine: co-immunization with plasmid-encoded adenosine deaminase-1 in the context of an HIV-1 env DNA vaccine increases draining lymphnode Tfh cell frequencies and increases env-binding antibody in the serum of vaccinated mice → no increase in Tfh cell numbers compared to other groups but enhanced Tfh effector functions (increased serum antibody levels) | [168] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritzau-Jost, J.; Hutloff, A. T Cell/B Cell Interactions in the Establishment of Protective Immunity. Vaccines 2021, 9, 1074. https://doi.org/10.3390/vaccines9101074
Ritzau-Jost J, Hutloff A. T Cell/B Cell Interactions in the Establishment of Protective Immunity. Vaccines. 2021; 9(10):1074. https://doi.org/10.3390/vaccines9101074
Chicago/Turabian StyleRitzau-Jost, Julia, and Andreas Hutloff. 2021. "T Cell/B Cell Interactions in the Establishment of Protective Immunity" Vaccines 9, no. 10: 1074. https://doi.org/10.3390/vaccines9101074
APA StyleRitzau-Jost, J., & Hutloff, A. (2021). T Cell/B Cell Interactions in the Establishment of Protective Immunity. Vaccines, 9(10), 1074. https://doi.org/10.3390/vaccines9101074






