Establishment of an ELISpot Assay to Detect Cellular Immunity against S. pneumoniae in Vaccinated Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Vaccines
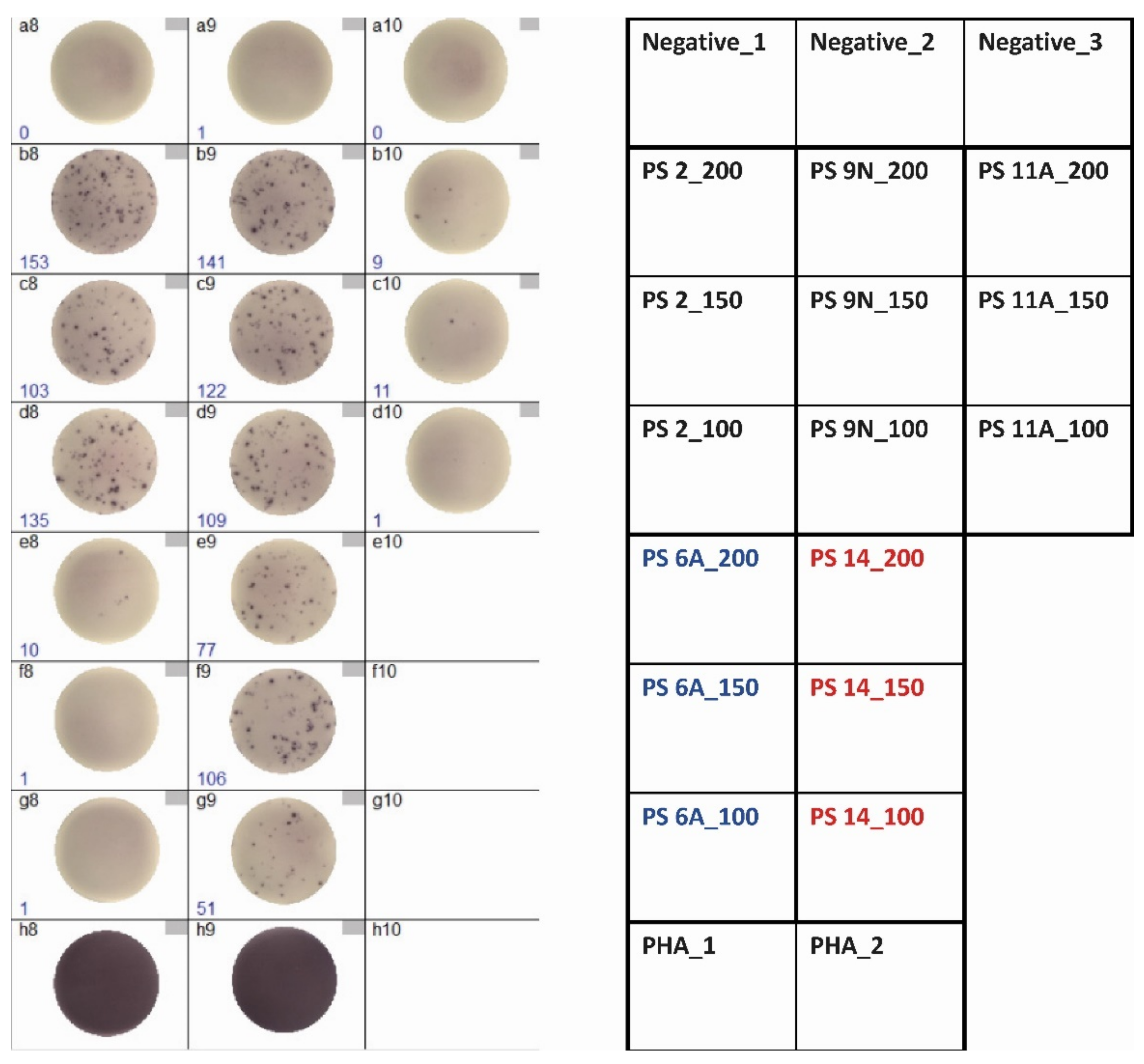
2.3. Determination of Cellular Immunity against Pneumococci by ELISpot
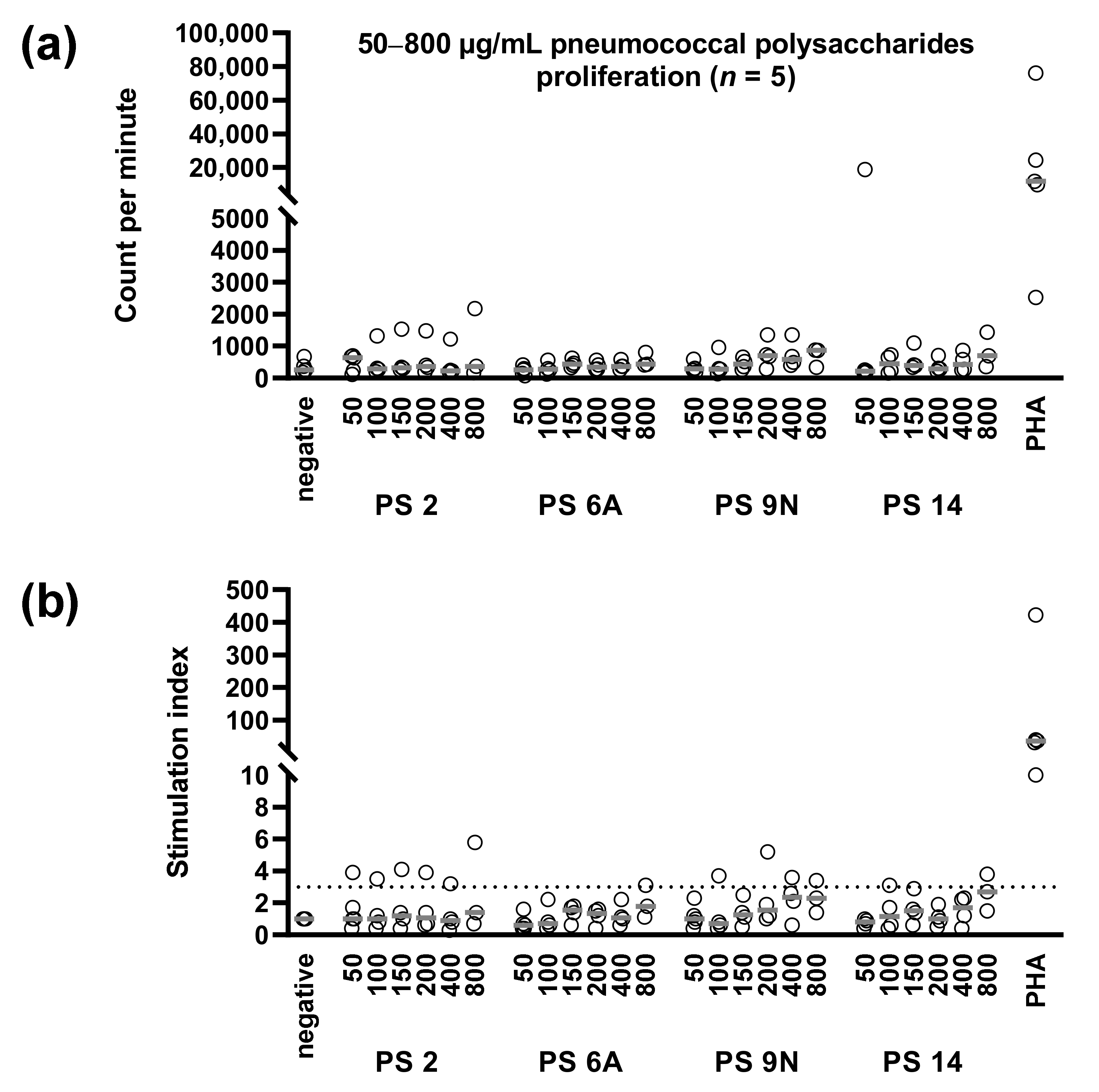
2.4. Determination of Cellular Immunity against Pneumococci by Proliferation Assay
2.5. Determination of Antibodies against Pneumococci
2.6. Statistical Analysis
3. Results
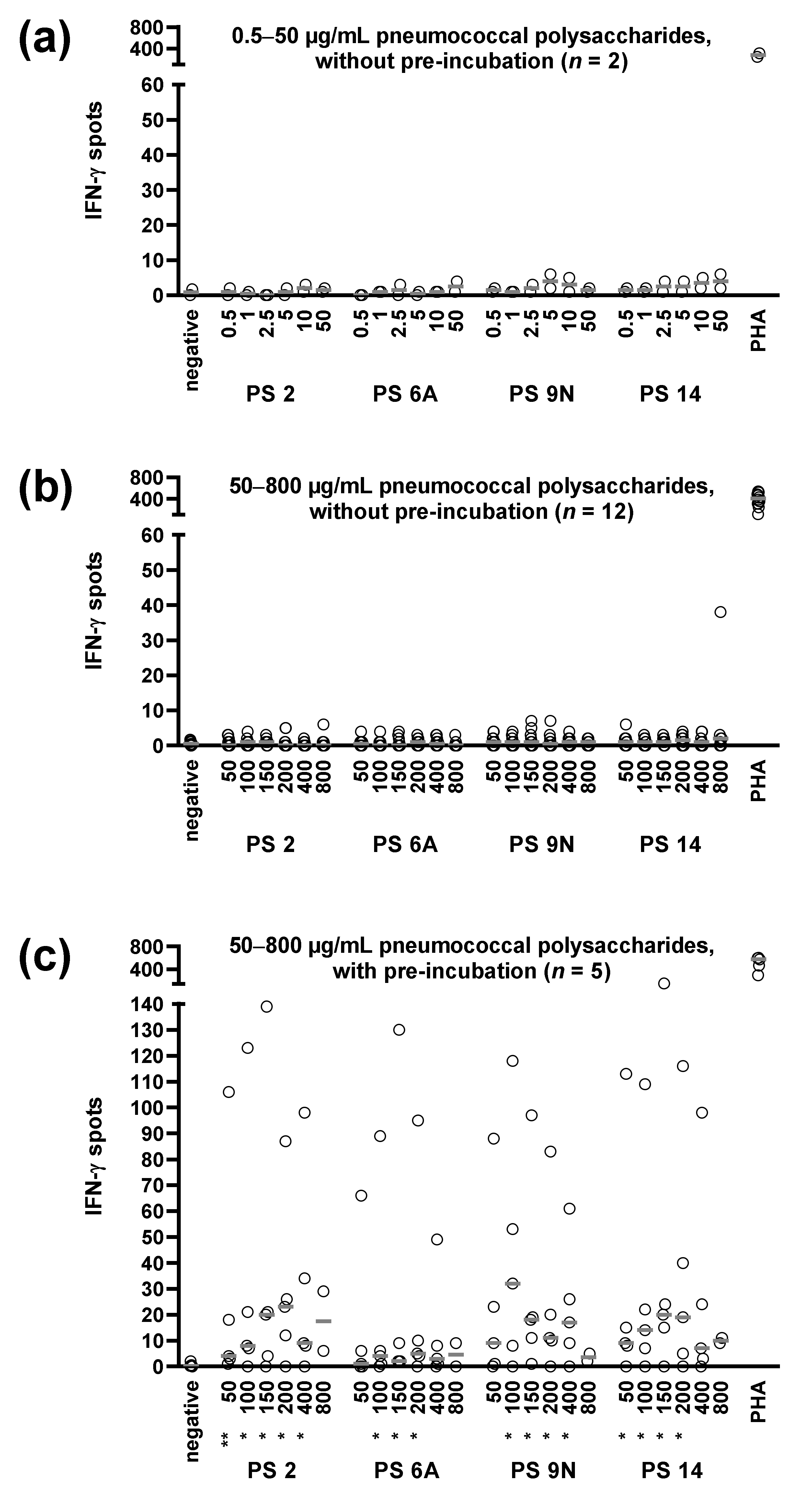
3.1. Optimization of ELISpot Conditions
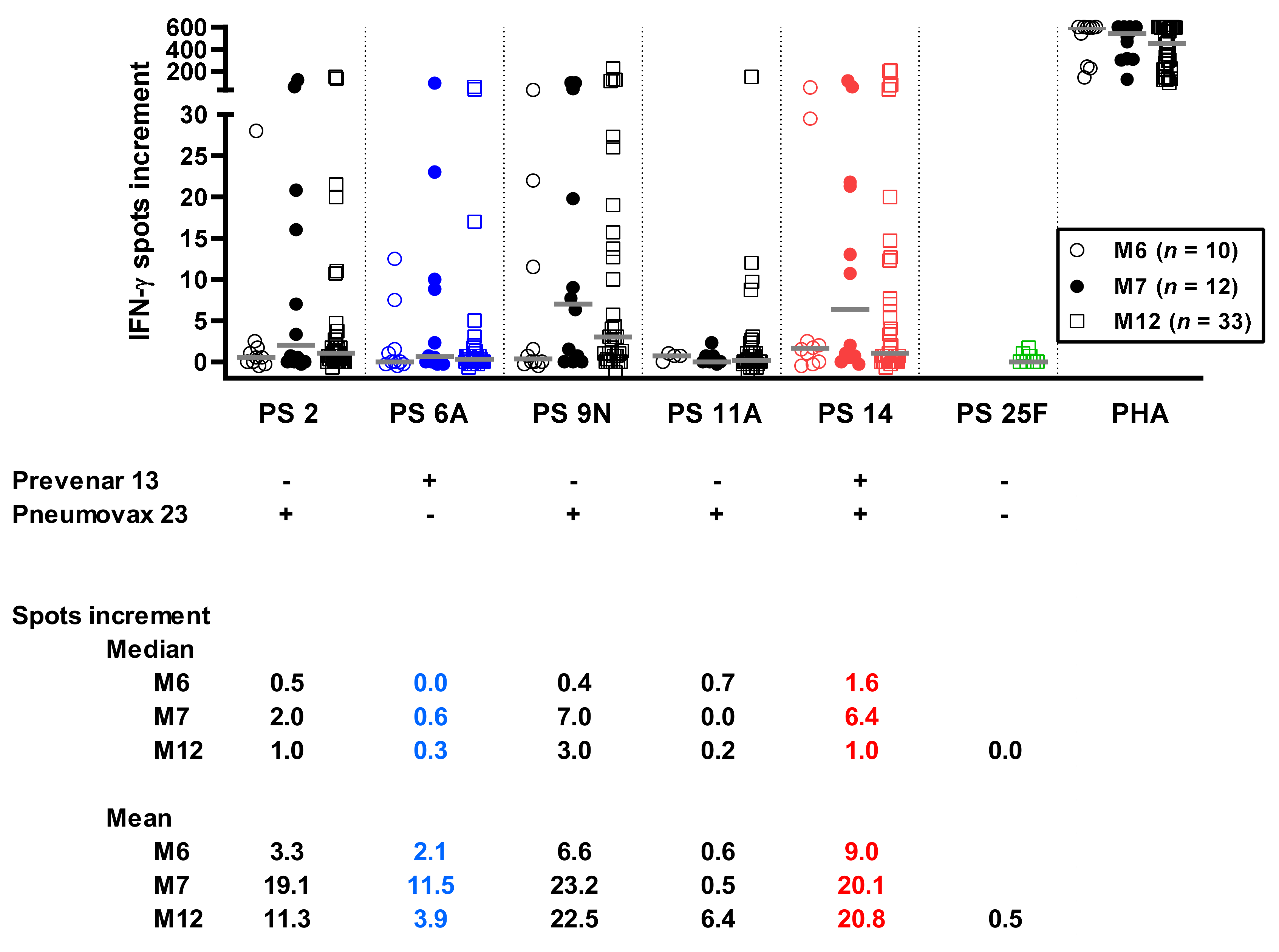
3.2. Time Course of Pneumococcus-Specific ELISpot Responses
3.3. Concentration Dependency of Pneumococcus-Specific Proliferative Responses
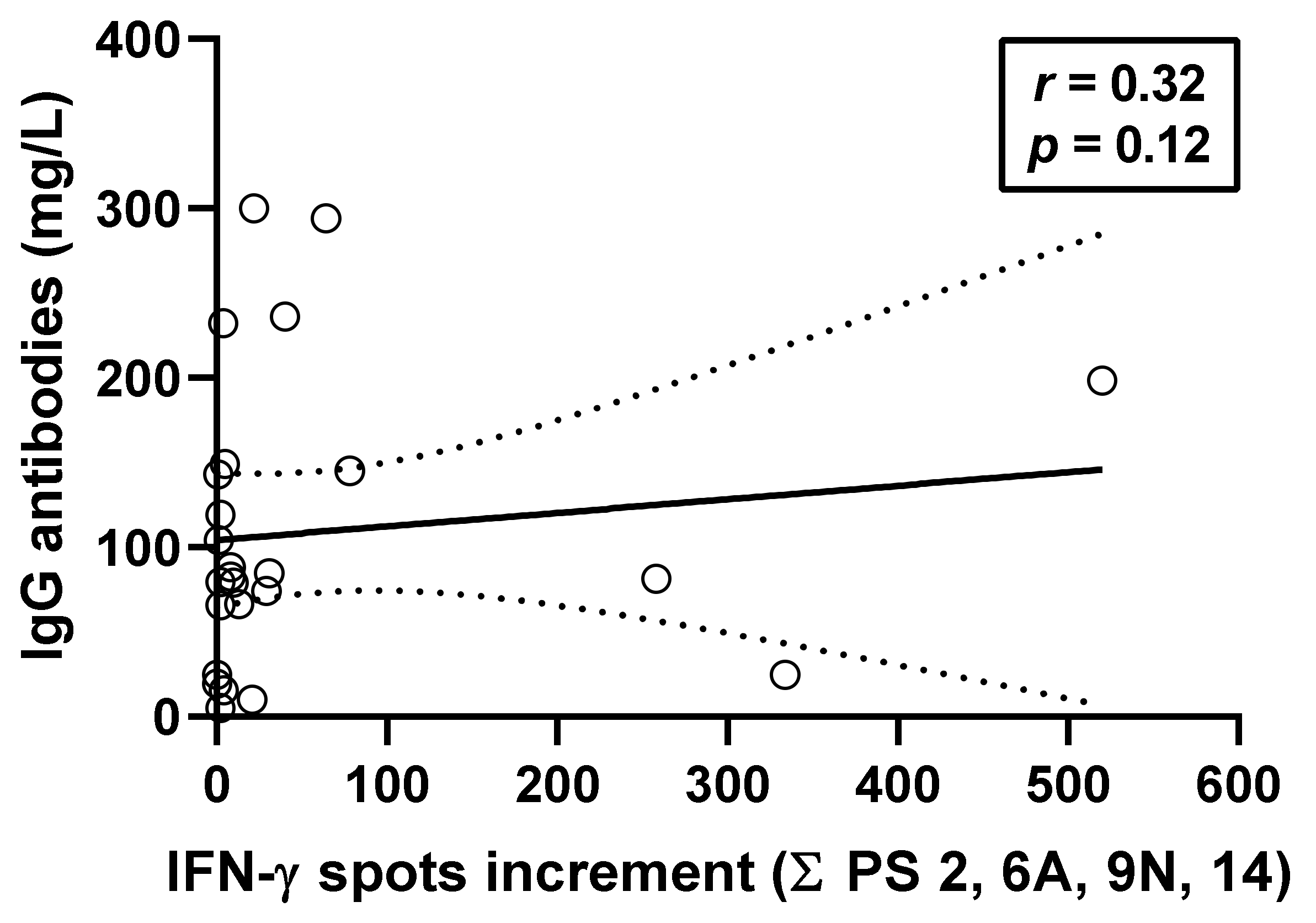
3.4. Correlation between Pneumococcus-Specific ELISpot Responses and Specific Antibodies
3.5. Correlation between Pneumococcus-Specific ELISpot Responses and Patient Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simell, B.; Auranen, K.; Kayhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage, G. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Vaccine Program Office. Adult Immunization Plans. Available online: http://www.hhs.gov/nvpo/national-adult-immunization-plan/ (accessed on 5 October 2021).
- Arora, S.; Kipp, G.; Bhanot, N.; Sureshkumar, K.K. Vaccinations in kidney transplant recipients: Clearing the muddy waters. World J. Transpl. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012, 61, 816–819. [Google Scholar]
- Robert-Koch-Institut. Wissenschaftliche Begründung für die Aktualisierung der Empfehlungen zur Indikationsimpfung gegen Pneumokokken für Risikogruppen. Epid. Bull. 2016, 37, 385–406. [Google Scholar]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency: Assessment Report for Prevenar 13. Available online: https://www.ema.europa.eu/en/documents/assessment-report/prevenar-13-epar-public-assessment-report_en.pdf (accessed on 5 October 2021).
- Soininen, A.; Seppala, I.; Nieminen, T.; Eskola, J.; Kayhty, H. IgG subclass distribution of antibodies after vaccination of adults with pneumococcal conjugate vaccines. Vaccine 1999, 17, 1889–1897. [Google Scholar] [CrossRef]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Stefanetti, G.; Berti, F.; Kasper, D.L. Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. USA 2019, 116, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Niehues, T.; Bogdan, C.; Hecht, J.; Mertens, T.; Wiese-Posselt, M.; Zepp, F. Impfen bei Immundefizienz. Bundesgesundheitsblatt Gesundh. Gesundh. 2017, 60, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Tobudic, S.; Plunger, V.; Sunder-Plassmann, G.; Riegersperger, M.; Burgmann, H. Randomized, single blind, controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in renal transplant recipients. PLoS ONE 2012, 7, e46133. [Google Scholar] [CrossRef]
- Dendle, C.; Stuart, R.L.; Mulley, W.R.; Holdsworth, S.R. Pneumococcal vaccination in adult solid organ transplant recipients: A review of current evidence. Vaccine 2018, 36, 6253–6261. [Google Scholar] [CrossRef]
- Hoffman, T.W.; Meek, B.; Rijkers, G.T.; Grutters, J.C.; van Kessel, D.A. Pneumococcal Conjugate Vaccination Followed by Pneumococcal Polysaccharide Vaccination in Lung Transplant Candidates and Recipients. Transpl. Direct 2020, 6, e555. [Google Scholar] [CrossRef]
- Oesterreich, S.; Lindemann, M.; Goldblatt, D.; Horn, P.A.; Wilde, B.; Witzke, O. Humoral response to a 13-valent pneumococcal conjugate vaccine in kidney transplant recipients. Vaccine 2020, 38, 3339–3350. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Enriquez, N.; Lemaitre, B.; Cadau, G.; Giostra, E.; Hadaya, K.; Meyer, P.; Gasche-Soccal, P.M.; Berney, T.; van Delden, C.; et al. Pneumococcal immunity and PCV13 vaccine response in SOT-candidates and recipients. Vaccine 2021, 39, 3459–3466. [Google Scholar] [CrossRef]
- Wuorimaa, T.; Kayhty, H.; Eskola, J.; Bloigu, A.; Leroy, O.; Surcel, H.M. Activation of cell-mediated immunity following immunization with pneumococcal conjugate or polysaccharide vaccine. Scand. J. Immunol. 2001, 53, 422–428. [Google Scholar] [CrossRef]
- Rabian, C.; Tschope, I.; Lesprit, P.; Katlama, C.; Molina, J.M.; Meynard, J.L.; Delfraissy, J.F.; Chene, G.; Levy, Y.; Group, A.P.S. Cellular CD4 T cell responses to the diphtheria-derived carrier protein of conjugated pneumococcal vaccine and antibody response to pneumococcal vaccination in HIV-infected adults. Clin. Infect. Dis. 2010, 50, 1174–1183. [Google Scholar] [CrossRef] [Green Version]
- Gazi, U.; Karasartova, D.; Sahiner, I.T.; Gureser, A.S.; Tosun, O.; Derici, M.K.; Dolapci, M.; Taylan Ozkan, A. The effect of splenectomy on the levels of PCV-13-induced memory B- and T cells. Int. J. Clin. Pract. 2018, 72, e13077. [Google Scholar] [CrossRef]
- Karasartova, D.; Gazi, U.; Tosun, O.; Gureser, A.S.; Sahiner, I.T.; Dolapci, M.; Ozkan, A.T. Anti-Pneumococcal Vaccine-Induced Cellular Immune Responses in Post-Traumatic Splenectomized Individuals. J. Clin. Immunol. 2017, 37, 388–396. [Google Scholar] [CrossRef]
- Glennie, S.J.; Sepako, E.; Mzinza, D.; Harawa, V.; Miles, D.J.; Jambo, K.C.; Gordon, S.B.; Williams, N.A.; Heyderman, R.S. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS ONE 2011, 6, e25610. [Google Scholar] [CrossRef] [Green Version]
- Sepako, E.; Glennie, S.J.; Jambo, K.C.; Mzinza, D.; Iwajomo, O.H.; Banda, D.; van Oosterhout, J.J.A.W.N.; Gordon, S.B.; Heyderman, R.S. Incomplete recovery of pneumococcal CD4 T cell immunity after initiation of antiretroviral therapy in HIV-infected malawian adults. PLoS ONE 2014, 9, e100640. [Google Scholar] [CrossRef]
- Baril, L.; Dietemann, J.; Essevaz-Roulet, M.; Beniguel, L.; Coan, P.; Briles, D.E.; Guy, B.; Cozon, G. Pneumococcal surface protein A (PspA) is effective at eliciting T cell-mediated responses during invasive pneumococcal disease in adults. Clin. Exp. Immunol. 2006, 145, 277–286. [Google Scholar] [CrossRef]
- Jaat, F.G.; Hasan, S.F.; Perry, A.; Cookson, S.; Murali, S.; Perry, J.D.; Lanyon, C.V.; De Soyza, A.; Todryk, S.M. Anti-bacterial antibody and T cell responses in bronchiectasis are differentially associated with lung colonization and disease. Respir. Res. 2018, 19, 106. [Google Scholar] [CrossRef]
- Groneck, L.; Schrama, D.; Fabri, M.; Stephen, T.L.; Harms, F.; Meemboor, S.; Hafke, H.; Bessler, M.; Becker, J.C.; Kalka-Moll, W.M. Oligoclonal CD4+ T cells promote host memory immune responses to Zwitterionic polysaccharide of Streptococcus pneumoniae. Infect. Immun. 2009, 77, 3705–3712. [Google Scholar] [CrossRef] [Green Version]
- Holtmeier, W.; Kabelitz, D. gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 2005, 86, 151–183. [Google Scholar] [CrossRef]
- Kirby, A.C.; Newton, D.J.; Carding, S.R.; Kaye, P.M. Evidence for the involvement of lung-specific gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection. Eur. J. Immunol. 2007, 37, 3404–3413. [Google Scholar] [CrossRef] [Green Version]
- Kanevskiy, L.M.; Telford, W.G.; Sapozhnikov, A.M.; Kovalenko, E.I. Lipopolysaccharide induces IFN-gamma production in human NK cells. Front. Immunol. 2013, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, M.; Heinemann, F.M.; Horn, P.A.; Witzke, O. Immunity to pneumococcal antigens in kidney transplant recipients. Transplantation 2010, 90, 1463–1467. [Google Scholar] [CrossRef]
- Goldblatt, D.; Plikaytis, B.D.; Akkoyunlu, M.; Antonello, J.; Ashton, L.; Blake, M.; Burton, R.; Care, R.; Durant, N.; Feavers, I.; et al. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin. Vaccine Immunol. 2011, 18, 1728–1736. [Google Scholar] [CrossRef]
- Lindemann, M.; Barsegian, V.; Siffert, W.; Ferencik, S.; Roggendorf, M.; Grosse-Wilde, H. Role of G protein beta3 subunit C825T and HLA class II polymorphisms in the immune response after HBV vaccination. Virology 2002, 297, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
| Parameter | Median (Range) or Number (No.) |
|---|---|
| Median age (range), years 1 | 53 (23–77) |
| Patient sex (female/male) | 12/26 |
| Median interval TX-vaccination (range), months | 38 (3–395) |
| Median serum creatinine (range), mg/dL | |
| Pre vaccination | 1.6 (0.9–3.6) |
| Month 6 post vacc. | 1.5 (0.6–3.7) |
| Month 12 post vacc. | 1.6 (0.9–3.9) |
| Immunosuppression, no. 1 | |
| Cyclosporine A | 5 |
| Tacrolimus | 28 |
| Mycofenolate mofetil | 21 |
| mTOR inhibitors | 6 |
| Corticosteroids | 36 |
| Belatacept | 2 |
| Kidney transplantation, no. | |
| First | 34 |
| Second | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gäckler, A.; Mülling, N.; Völk, K.; Wilde, B.; Eisenberger, U.; Rohn, H.; Horn, P.A.; Witzke, O.; Lindemann, M. Establishment of an ELISpot Assay to Detect Cellular Immunity against S. pneumoniae in Vaccinated Kidney Transplant Recipients. Vaccines 2021, 9, 1438. https://doi.org/10.3390/vaccines9121438
Gäckler A, Mülling N, Völk K, Wilde B, Eisenberger U, Rohn H, Horn PA, Witzke O, Lindemann M. Establishment of an ELISpot Assay to Detect Cellular Immunity against S. pneumoniae in Vaccinated Kidney Transplant Recipients. Vaccines. 2021; 9(12):1438. https://doi.org/10.3390/vaccines9121438
Chicago/Turabian StyleGäckler, Anja, Nils Mülling, Kim Völk, Benjamin Wilde, Ute Eisenberger, Hana Rohn, Peter A. Horn, Oliver Witzke, and Monika Lindemann. 2021. "Establishment of an ELISpot Assay to Detect Cellular Immunity against S. pneumoniae in Vaccinated Kidney Transplant Recipients" Vaccines 9, no. 12: 1438. https://doi.org/10.3390/vaccines9121438
APA StyleGäckler, A., Mülling, N., Völk, K., Wilde, B., Eisenberger, U., Rohn, H., Horn, P. A., Witzke, O., & Lindemann, M. (2021). Establishment of an ELISpot Assay to Detect Cellular Immunity against S. pneumoniae in Vaccinated Kidney Transplant Recipients. Vaccines, 9(12), 1438. https://doi.org/10.3390/vaccines9121438







