Abstract
Little is known about the risk of febrile seizures (FS) after vaccination with measles-containing vaccines (MCVs) in middle- and low-income countries. This self-controlled case series study aimed to evaluate the risk of FSs in Chinese children using data from the Ningbo Regional Health Information Platform. The observation period was 0–12 and 13–24 months of age for the MR and MMR vaccines, respectively. The relative incidences (RIs) within 0–6 days, 7–13 days, 14–27 days, and 28–42 days after vaccination with MCVs were estimated. The remaining observation period was the control period. The RIs within 0–6 days, 7–13 days, 14–27 days, and 28–42 days after MR vaccination were 1.11 [95% confidence interval (CI) 0.33 to 3.70], 0.80 (95% CI 0.23 to 2.86), 1.67 (95% CI 0.81 to 3.42), and 1.02 (95% CI 0.49 to 2.14), respectively. The corresponding RIs after MMR vaccination were 0.99 (95% CI 0.56 to 1.75), 1.17 (95% CI 0.68 to 2.01), 0.87 (95% CI 0.54 to 1.39), and 0.85 (95% CI 0.54 to 1.34), respectively. This study suggests that China’s vaccination schedule for MCVs, as suggested by the World Health Organization (WHO) for countries with a high risk of measles mortality and ongoing transmission, does not increase the risk of FSs.
1. Introduction
As the most type of seizure disorder among children, particularly those younger than two years old, febrile seizures (FSs) affect approximately 2–5% of children [1]. Infections, especially respiratory tract infections, vaccine administration, etc., are causes of FSs [2].
According to a recent Cochrane systematic review [3], all cohort or self-controlled case series (SCCS) studies published before 2 May 2019, have observed an increased risk of FSs within the risk window of 7–14 days after MMR vaccination [4,5,6,7,8], and no evidence supported an increased risk of FSs in 14 or more days after MMR vaccination [5,6]. In terms of the risk of FSs within one week after MMR vaccination, the results from two cohort studies were controversial [4,5]. Notably, the existing studies on the association between measles-containing vaccines (MCVs) and FSs were all from high-income countries in Europe, America, and Australia, and no such studies came from middle- and low-income countries. However, the vaccination schedule of MCVs varied between high-income countries and middle- and low-income countries. For example, China’s Expanded Program on Immunization (EPI) requires that the first dose of an MCV (i.e., the measles-rubella (MR) vaccine before June 2020 and the MMR vaccine after June 2020) be given at eight months of age, and the second dose of an MCV (i.e., the MMR vaccine) be given at 18 months of age [9], which is in line with the vaccination schedule recommended by the World Health Organization (WHO) for countries with a high risk of measles mortality and ongoing transmission [10]. However, the vaccination schedules of MCVs in most developed countries were in line with the schedule recommended by the WHO for countries with low levels of measles transmission [10], as the first dose of an MCV (i.e., MMR in most developed countries) is given in the second year of life [11,12,13]. Although in China, the first dose of the MMR vaccine is also given in the second year of life when the peak of FSs occurrence exists [14]; the first dose of an MCV is the MR vaccine and not the MMR vaccine in China. Therefore, the risk of FSs after MMR vaccination in the second year of life may be different under China’s vaccination schedule for MCVs. Additionally, the medical sources in most middle- and low-income countries are limited; therefore, the diagnosis, treatment, and outcome of FSs in middle- and low-income countries may be different from those in high-income countries [15].
In China, considering the different vaccination schedules, large population sizes, unique genetic backgrounds, etc., the evidence of vaccine safety should be of great value to global vaccine safety; however, no active surveillance of the association between MCVs and FSs has been conducted in China until now. The Ningbo Regional Health Information Platform (NRHIP) is a united and standardized medical information network in Ningbo, China. In 2016, the NRHIP reached the top level in the Standardization and Maturity Measurement of Regional Health Information Interconnection by the National Health Commission of China [16]. Therefore, this study is the first to use the SCCS design to evaluate the risk of FSs after vaccination with MCVs in Chinese children using the NRHIP.
2. Materials and Methods
2.1. Data Source
Ningbo, a city in Zhejiang Province, is an important port city on the southeast coast of China. The population in Ningbo was over 8.5 million in 2019. In 2011, the Health Commission of Ningbo began to design and develop the NRHIP to form a systematic and standardized medical information network. By 2015, almost all the health-related activities of more than 87% of Ningbo residents were covered by the NRHIP. The detailed introduction of the NRHIP can be found in a previous study [16]. In this study, the databases of immunization registers and electronic medical records (EMRs) on the NRHIP from 1 January 2016 to 31 December 2019 were used, from which the cases aged 0–24 months were born from 2014 to 2019. The study was approved by the Ethical Review Committee of the Center for Disease Control and Prevention of Ningbo (IRB. No: 202002). The requirement for informed consent was waived.
2.2. Study Population
In China, the timing of vaccine administration should be based on the EPI, which requires that children aged eight months to one year should be vaccinated with the MR vaccine and that children aged 18 months to two years should be vaccinated with the MMR vaccine [9,17]. Therefore, this study was restricted to inpatient FS patients aged 0–24 months (0–730 days). Analysis was further restricted to the first inpatient FS episodes of patients aged 0–24 months with vaccination records in the immunization register database. Exclusion criteria due to the violation of the EPI were as follows: children who received ≥two doses of the MR vaccine; children who did not receive the MR vaccine before the MMR vaccine; children who received ≥two doses of the MMR vaccine; children who received the MR vaccine at ages younger than 8 months or older than 12 months; or children who received the MMR vaccine at ages younger than 18 months or older than 24 months. FS cases were identified by using the International Classification of Diseases version 10 (ICD-10) code (i.e., R56) or specific Chinese diagnostic terms of FSs (i.e., “Re Xing Jing Jue” and “Gao Re Jing Jue”).
2.3. Case Validation
To evaluate the accuracy of the inpatient FS diagnoses on the NRHIP, the hospital medical charts of 287 inpatient FS patients aged 0–24 months old were reviewed by two pediatricians independently. The judgment criteria were based on three levels of the Brighton diagnostic certainty of generalized convulsive seizure and Brighton Collaboration case definition for fever [18,19]. Any disagreement between the pediatricians was resolved by consulting another senior expert. The positive predictive value was calculated.
2.4. Statistical Analysis
The adapted SCCS method for event-dependent exposures (which allows delayed MR or MMR vaccination after the occurrence of FSs) [20] was used to estimate the relative incidences (RIs) of FSs within 0–6 days, 7–13 days, 14–27 days, 28–42 days, and 0–42 days after MR or MMR vaccination [6]. The observation periods for the MR vaccine and MMR vaccine were 0–12 months of age and 13–24 months of age, respectively. The observation period excluding the 0–42 days after receiving an MR or MMR vaccination was defined as an unexposed control period. In the SCCS method, each case acts as its own control by comparing the risk of FS occurrence in exposed periods after MR or MMR vaccination and in unexposed control periods; therefore, time-invariant covariates can be controlled automatically [21]. The potential confounding from age was adjusted by using one-month age bands. Moreover, two sensitivity analyses were conducted: (1) adjustment for age using wider intervals (four-month age groups) and (2) excluding children with a high risk of FSs, i.e., those with a personal history of seizure disorder, infection, neoplasm, central nervous system injury, encephalopathy, or other neurological conditions (Table A1 in Appendix A) [22]; therefore, no children had experienced previous convulsions. Subgroup analysis by sex was performed.
RIs and 95% confidence intervals (CIs) were calculated using conditional Poisson regression. A two-sided p value less than 0.05 indicated statistical significance. All statistical analyses were performed with Stata software, version 15.0, and R software, version 3.6.2.
2.5. Sample Size
To detect an RI of 1.5 or more within 0 to 42 days after the MCV vaccination, under the assumption of a one-year observation time, 100% vaccine coverage (since vaccination with MCVs on time is considered a societal and obligatory duty in China, coverage with MCVs is high among Chinese children [23]), 80% power and a type 1 alpha level of 0.05, 387 FS episodes were needed.
3. Results
3.1. Characteristics of the Children with FSs
A total of 837 children (males: 517; females: 320) were enrolled in this study (Figure 1 and Table 1), with a male to female ratio of 1.6. The median onset age of all first FS episodes was 515 days (interquartile range (IQR) 403 to 614).
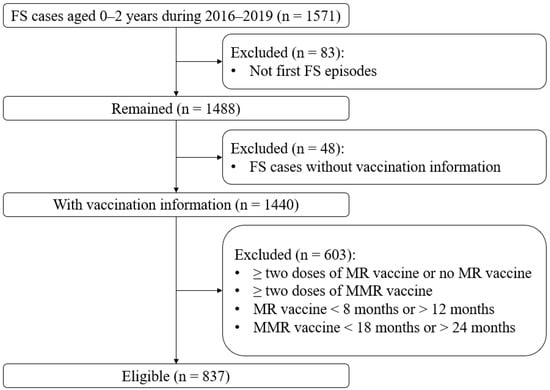
Figure 1.
Flowchart of the study participants. Abbreviations: FSs, febrile seizures; MMR, measles-mumps-rubella; MR, measles-rubella.

Table 1.
Characteristics of the children with FSs included in this study.
3.2. Case Validation
Among the 287 chart-reviewed FS cases, 260 met level 1 or 2 of the Brighton diagnostic certainty of generalized convulsive seizure and Brighton Collaboration case definition for fever [18,19]. The positive predictive value of the inpatient FS diagnoses among the patients aged 0–24 months on the NRHIP was 91%.
3.3. Risk of FSs following MR and MMR Vaccination
The sample size for evaluating the risk of FSs after MR vaccination was 146. The sample size for evaluating the risk of FSs after MMR vaccination was 691. The relative incidences within 0–6 days, 7–13 days, 14–27 days, 28–42 days, and 0–42 days after MR vaccination were 1.11 (95% CI 0.33 to 3.70), 0.80 (95% CI 0.23 to 2.86), 1.67 (95% CI 0.81 to 3.42), 1.02 (95% CI 0.49 to 2.14), and 1.17 (95% CI 0.65 to 2.11), respectively (Table 2). Due to the small sample size (considering that the SCCS model cannot converge), no sensitivity analysis or subgroup analysis was performed to evaluate the risk of FSs after MR vaccination.

Table 2.
Relative incidence of FSs after MR vaccination.
The risk of FSs within 0–6 days after MMR vaccination was not higher than the background risk (RI 0.99; 95% CI 0.56 to 1.75) and was similar to the risk of FSs 7–13 days (RI 1.17; 95% CI 0.68 to 2.01), 14–27 days (RI 0.87; 95% CI 0.54 to 1.39) and 28–42 days (RI 0.85; 95% CI 0.54 to 1.34) after MMR vaccination (Table 3). The results of the sensitivity analyses (Table 3) and subgroup analyses (Table 4) by sex also indicated that there was not an increased risk of FSs within the 42 days after MMR vaccination.

Table 3.
Relative incidence of FSs after MMR vaccination.

Table 4.
Relative incidence of FSs after MMR vaccination by sex.
4. Discussion
This is the first study to assess the risk of FSs after receiving an MR and MMR vaccination in China. No significant association between FSs and vaccination with MCVs in any risk period (0–6 days, 7–13 days, 14–27 days, 28–42 days) among children was found. There was no increased risk of FSs within 7–14 days after MMR vaccination, which is different from the results in European, American, or Australian studies [4,5,6,7,8].
One explanation for the lack of increased FS risk after receiving an MR and MMR vaccination in China may be that the MMR vaccination schedule during the study period in China was different from that in developed countries. In China, the EPI requires that the MR vaccine be given at eight months of age and that the MMR vaccine be given at 18 months of age [9]. However, in the United States, the first dose is given at 12–15 months of age, and the second dose is given at 4–6 years of age [11]. In most European countries, the first dose of the MMR vaccine is given at 12–15 months of age, and the second dose is given at 3–12 years of age [12]. In Australia, the MMR vaccine is recommended to be given at 12 months of age, and the measles, mumps, rubella, and varicella (MMRV) vaccine is recommended to be given at 18 months of age [13]. Therefore, the lack of an increased risk of FSs after receiving an MMR vaccination in the second year of life in Chinese children may be because the vast majority of persons receiving the second dose of MCVs may have already been fully protected by the first dose, which can help neutralize the vaccine virus immediately and completely [24,25,26]. However, we still did not observe an increased risk of FSs after MR vaccination among those aged ≤one year, which may suggest that giving the first dose of an MCV in the first year of life would not increase the risk of FSs. However, the possibility cannot be excluded because the sample size for the MR vaccine was too small to detect the potential risk.
Another explanation may be that the technology of MCV production varies between China and Europe, America, or Australia. First, different virus strains that are used in vaccines may affect the safety of the vaccines [27]. In terms of the measles vaccine strains produced worldwide, most are derived from the Edmonston strain, but the strain used in China was the Shanghai-191 strain. For the rubella vaccine strain, most vaccines used worldwide contain the RA 27/3 strain, but the vaccine produced in China contains the BRD2 strain. Second, the varied excipients may also play a role; for example, most vaccines produced worldwide contain neomycin, except for those produced in China.
Moreover, the misconception of contraindications to immunization is severe in low- or middle-income countries such as China and Africa [28,29], which can lead to healthy vaccinee effects, making it hard to detect adverse events following vaccination [30]). Although to improve vaccination coverage, since the 1980s, the WHO has recommended that some minor illnesses, such as slight fever, mild respiratory infections, and diarrhea, should not be considered as contraindications [31], the problem of false contraindications still cannot be ignored in China. The problem of missed opportunities for immunization caused by false contraindications was more obvious for MCVs than for other vaccines, since the rate of adverse events following immunization caused by MCVs were multiple of other vaccines [28].
The racial or genetic differences between populations in China and the developed countries of Europe or America may also play a role. On the one hand, different immune responses toward MCVs may be taken into consideration, since it was reported that fever is related to a stronger measles antibody response, which is independent of age and the type of MCVs [32]. According to the latest systematic review on MMR vaccine safety and efficacy, the risk ratios (RRs) of the effectiveness of the first dose of the MMR vaccine against measles among children from many cohort studies in European countries were below 0.02 [3], but a study conducted in the Anhui Province of China reported an RR of 0.44 among children aged ≤5 years [17]. Therefore, the immune response toward the MMR vaccine of Chinese children may be lower than that of children from developed countries, which can lead to a lower risk of fever after MMR vaccination, further lowering the risk of FSs among Chinese children. On the other hand, genetic factors have garnered attention recently, as distinct variants may affect the occurrence of FSs after MMR vaccination, or MMR vaccines may be a stimulus to trigger FSs in susceptible individuals [33].
This was the first study in low- and middle-income countries to assess the risk of FSs after vaccination with MCVs by using a population-based regional electronic medical database. However, there were still some limitations. First, due to the lack of medical charts on the NRHIP, we took the visit to the hospital for FSs as the onset time of FSs. However, since FS is an acute disease [34], with the manifestations that can be extremely frightening for parents, parents would take their children to the hospital in a timely manner, and the time interval between FS onset and the visit to the hospital would be short. Second, we cannot distinguish between the primary diagnosis and secondary diagnosis of FSs. Since the primary diagnosis can better reflect the purpose of visiting a doctor, it may, to some extent, affect our results. However, the positive predictive value of the diagnoses of the inpatient FS cases used in this study was up to 91%. Third, we only considered inpatient FS cases, and inpatient FSs may be more severe than outpatient FSs. However, most previous studies also did not include outpatient FS cases [6,7].
5. Conclusions
It is reassuring that no increased risk of FSs after MCV vaccination was found among Chinese children, suggesting that China’s vaccination schedule for MCVs (i.e., giving the first dose of an MCV in the first year of life and the second dose in the second year of life), as suggested by the WHO for countries with a high risk of measles mortality and ongoing transmission, does not increase the risk of FSs after vaccination with MCVs.
Author Contributions
Conceptualization, L.X., Z.L. and S.Z.; methodology, L.X., Z.L. and S.Z.; software, L.X.; validation, L.X. and Z.L.; formal analysis, L.X.; investigation, L.X., N.L., L.Z., R.M., T.F. and Z.L.; resources, N.L. and L.Z.; data curation, N.L. and L.Z.; writing—original draft preparation, L.X.; writing—review and editing, all authors; visualization, L.X.; supervision, Z.L. and S.Z.; project administration, Z.L. and S.Z.; funding acquisition, L.X. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Advanced Project of the Beijing Natural Science Foundation (grant number, BMU2019GJJXK003) and the Innovation Fund for Outstanding Doctoral Candidates of Peking University Health Science Center (China) (grant number, not applicable). The funders had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Review Committee of the Center for Disease Control and Prevention of Ningbo (IRB. No: 202002).
Informed Consent Statement
The requirement for informed consent was waived because no identifiable individual information was used in this study.
Data Availability Statement
Due to the Ningbo government’s restrictions, deidentified individual participant data will not be made available.
Acknowledgments
We thank Keli Li (from the Center for Immunization, Chinese Center for Disease Control and Prevention) for her constructive suggestions for our study. We thank Jian Chen, Yan He, Lihua Zhu, and Zongxiu Xu (from the Ningbo Women and Children’s Hospital) for their contribution to the case validation.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The ICD-10 codes used to identify children who may have a high risk of febrile seizures.
Table A1.
The ICD-10 codes used to identify children who may have a high risk of febrile seizures.
| Category of Condition | Condition | ICD-10 Code |
|---|---|---|
| Diseases of the nervous system: Inflammatory diseases of the central nervous system | Bacterial meningitis, not classified elsewhere | G00 |
| Meningitis in bacterial diseases classified elsewhere | G01 | |
| Meningitis in other infectious and parasitic diseases classified elsewhere | G02 | |
| Meningitis due to other and unspecified causes | G03 | |
| Encephalitis, myelitis and encephalomyelitis | G04 | |
| Encephalitis, myelitis and encephalomyelitis in diseases classified elsewhere | G05 | |
| Intracranial and intraspinal abscess and granuloma | G06 | |
| Intracranial and intraspinal abscess and granuloma in diseases classified elsewhere | G07 | |
| Intracranial and intraspinal phlebitis and thrombophlebitis | G08 | |
| Sequelae of inflammatory diseases of the central nervous system | G09 | |
| Diseases of the nervous system: Systemic atrophies primarily affecting the central nervous system | Huntington’s disease | G10 |
| Spinal muscular atrophy and related syndromes | G12 | |
| Diseases of the nervous system: Extrapyramidal and movement disorders | Other degenerative diseases of the basal ganglia | G23 |
| Dystonia | G24 | |
| Other extrapyramidal and movement disorders | G25 | |
| Extrapyramidal and movement disorders in diseases classified elsewhere | G26 | |
| Diseases of the nervous system: Other degenerative diseases of the nervous system | Other degenerative diseases of nervous system, not classified elsewhere | G31 |
| Other degenerative disorders of nervous system in diseases classified elsewhere | G32 | |
| Diseases of the nervous system: Episodic and paroxysmal disorders | Epilepsy | G40 |
| Diseases of the nervous system: Other disorders of the nervous system | Disorders of the autonomic nervous system | G90 |
| Hydrocephalus | G91 | |
| Toxic encephalopathy | G92 | |
| Other disorders of the brain | G93 | |
| Other disorders of the brain in diseases classified elsewhere | G94 | |
| Other disorders of the central nervous system | G96 | |
| Postprocedural disorders of the nervous system, not classified elsewhere | G97 | |
| Other disorders of the nervous system, not classified elsewhere | G98 | |
| Other disorders of the nervous system in diseases classified elsewhere | G99 | |
| Metabolic disorders | Sphingolipid metabolism disorders and other lipid storage disorders | E75 |
| Infectious and parasitic diseases | Tuberculosis of the nervous system | A17 |
| Atypical virus infections of the central nervous system | A81 | |
| Mosquito-borne viral encephalitis | A83 | |
| Tick-borne viral encephalitis | A84 | |
| Other viral encephalitis, not classified elsewhere | A85 | |
| Unspecified viral encephalitis | A86 | |
| Viral meningitis | A87 | |
| Other viral infections of the central nervous system, not classified elsewhere | A88 | |
| Unspecified viral infection of the central nervous system | A89 | |
| Other mosquito-borne viral fevers | A92 | |
| Other arthropod-borne viral fevers, not classified elsewhere | A93 | |
| Unspecified arthropod-borne viral fever | A94 | |
| Neoplasms | Malignant neoplasm of the meninges | C70 |
| Malignant neoplasm of the brain | C71 | |
| Malignant neoplasm of the spinal cord, cranial nerves and other parts of the central nervous system | C72 | |
| Benign neoplasm of the meninges | D32 | |
| Benign neoplasm of the brain and other parts of the central nervous system | D33 | |
| Injuries to the head | Fracture of the skull and facial bones | S02 |
| Intracranial injury | S06 | |
| Crushing injury of the head | S07 | |
| Traumatic amputation of part of the head | S08 | |
| Other and unspecified injuries of the head | S09 |
References
- Sawyer, M.H.; Simon, G.; Byington, C. Vaccines and febrile seizures: Quantifying the risk. Pediatrics 2016, 138, e20160976. [Google Scholar] [CrossRef] [Green Version]
- Principi, N.; Esposito, S. Vaccines and febrile seizures. Expert Rev. Vaccines 2013, 12, 885–892. [Google Scholar] [CrossRef]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst. Rev. 2020, 4, Cd004407. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Hviid, A.; Madsen, K.M.; Wohlfahrt, J.; Thorsen, P.; Schendel, D.; Melbye, M.; Olsen, J. MMR vaccination and febrile seizures: Evaluation of susceptible subgroups and long-term prognosis. JAMA 2004, 292, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Barlow, W.E.; Davis, R.L.; Glasser, J.W.; Rhodes, P.H.; Thompson, R.S.; Mullooly, J.P.; Black, S.B.; Shinefield, H.R.; Ward, J.I.; Marcy, S.M.; et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N. Engl. J. Med. 2001, 345, 656–661. [Google Scholar] [CrossRef]
- Macartney, K.; Gidding, H.F.; Trinh, L.; Wang, H.; Dey, A.; Hull, B.; Orr, K.; McRae, J.; Richmond, P.; Gold, M.; et al. Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatr. 2017, 171, 992–998. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.L.; Jacobsen, S.J.; Klein, N.P.; Naleway, A.L.; Kharbanda, E.O.; Glanz, J.M.; Jackson, L.A.; Weintraub, E.S.; McLean, H.Q. Similar relative risks of seizures following measles containing vaccination in children born preterm compared to full-term without previous seizures or seizure-related disorders. Vaccine 2019, 37, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.N.; Bryant, N.J.; Andrews, N.J.; Bowley, J.S.; Ohrling, A.; Verity, C.M.; Ross, E.M.; Miller, E. Risk of serious neurologic disease after immunization of young children in Britain and Ireland. Pediatrics 2007, 120, 314–321. [Google Scholar] [CrossRef] [PubMed]
- A Picture to Understand the Children’s Immunization Program of National Immunization Program. Available online: http://www.chinacdc.cn/jkzt/ymyjz/zswd_10960/201904/t20190426_201664.html (accessed on 2 January 2020).
- Summary of WHO Position Papers—Recommended Routine Immunizations for Children. Available online: https://www.who.int/immunization/policy/Immunization_routine_table2.pdf (accessed on 20 June 2021).
- Recommended Child and Adolescent Immunization Schedule for Ages 18 Years or Younger, United States. 2020. Available online: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html (accessed on 9 January 2021).
- Vaccine Schedules in All Countries of the European Union. Available online: https://vaccine-schedule.ecdc.europa.eu/ (accessed on 9 January 2021).
- National Immunisation Program Schedule. Available online: https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule (accessed on 9 January 2021).
- Patel, N.; Ram, D.; Swiderska, N.; Mewasingh, L.D.; Newton, R.W.; Offringa, M. Febrile seizures. BMJ 2015, 351, h4240. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.M.; Abubakar, A.; Stein, A.; Marsh, K.; Newton, C. Prevalence, causes, and behavioral and emotional comorbidities of acute symptomatic seizures in Africa: A critical review. Epilepsia Open 2017, 2, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, L.; Yang, Y.; Meng, R.; Fang, T.; Dong, Y.; Li, N.; Xu, G.; Zhan, S. Active surveillance of adverse events following human papillomavirus vaccination: Feasibility pilot study based on the Regional Health Care Information Platform in the city of Ningbo, China. J. Med. Internet Res. 2020, 22, e17446. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, Y.; Tang, J.; Jia, H.; Qin, W.; Su, Y.; Wang, H.; Hao, L. Assessment of mumps-containing vaccine effectiveness during an outbreak: Importance to introduce the 2-dose schedule for China. Hum. Vaccine Immunother. 2018, 14, 1392–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhoeffer, J.; Menkes, J.; Gold, M.S.; de Souza-Brito, G.; Fisher, M.C.; Halsey, N.; Vermeer, P. Generalized convulsive seizure as an adverse event following immunization: Case definition and guidelines for data collection, analysis, and presentation. Vaccine 2004, 22, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Marcy, S.M.; Kohl, K.S.; Dagan, R.; Nalin, D.; Blum, M.; Jones, M.C.; Hansen, J.; Labadie, J.; Lee, L.; Martin, B.L.; et al. Fever as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004, 22, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Farrington, C.P.; Whitaker, H.J.; Hocine, M.N. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics 2009, 10, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, H.J.; Farrington, C.P.; Spiessens, B.; Musonda, P. Tutorial in biostatistics: The self-controlled case series method. Stat. Med. 2006, 25, 1768–1797. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.E.; Dover, D.C.; Simmonds, K.A.; Svenson, L.W. Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: A population-based cohort study. CMAJ 2014, 186, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Rodewald, L.; Yang, J.; Qin, Y.; Pang, M.; Feng, L.; Yu, H. The landscape of vaccines in China: History, classification, supply, and price. BMC Infect. Dis. 2018, 18, 502. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.L.; Marcuse, E.; Black, S.; Shinefield, H.; Givens, B.; Schwalbe, J.; Ray, P.; Thompson, R.S.; Chen, R. MMR2 immunization at 4 to 5 years and 10 to 12 years of age: A comparison of adverse clinical events after immunization in the Vaccine Safety Datalink project. The Vaccine Safety Datalink Team. Pediatrics 1997, 100, 767–771. [Google Scholar] [CrossRef]
- Chen, R.T.; Moses, J.M.; Markowitz, L.E.; Orenstein, W.A. Adverse events following measles-mumps-rubella and measles vaccinations in college students. Vaccine 1991, 9, 297–299. [Google Scholar] [CrossRef]
- Deng, L.; Wood, N.; Danchin, M. Seizures following vaccination in children: Risks, outcomes and management of subsequent revaccination. Aust. J. Gen. Pract. 2020, 49, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Andrews, N.; Stowe, J.; Grant, A.; Waight, P.; Taylor, B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. Am. J. Epidemiol. 2007, 165, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, J.; Liu, Y.; Xu, Y.; Che, X.; Gu, W.; Du, J.; Zhang, X.; Xu, E. Survey of contraindications in children’s routine vaccination in Hangzhou, China. Hum. Vaccin. Immunother. 2017, 13, 1539–1543. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Maleq, N.; Guillermet, E.; Colombini, A.; Gessner, B.D. A systematic literature review of missed opportunities for immunization in low- and middle-income countries. Vaccine 2014, 32, 6870–6879. [Google Scholar] [CrossRef]
- Hawken, S.; Potter, B.K.; Little, J.; Benchimol, E.I.; Mahmud, S.; Ducharme, R.; Wilson, K. The use of relative incidence ratios in self-controlled case series studies: An overview. BMC Med. Res. Methodol. 2016, 16, 126. [Google Scholar] [CrossRef] [Green Version]
- Galazka, A.M.; Lauer, B.A.; Henderson, R.H.; Keja, J. Indications and contraindications for vaccines used in the Expanded Programme on Immunization. Bull. World Health Organ. 1984, 62, 357–366. [Google Scholar]
- Carazo Perez, S.; Bureau, A.; De Serres, G. Post-immunisation fever and the antibody response to measles-containing vaccines. Epidemiol. Infect. 2018, 146, 1584–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feenstra, B.; Pasternak, B.; Geller, F.; Carstensen, L.; Wang, T.; Huang, F.; Eitson, J.L.; Hollegaard, M.V.; Svanstrom, H.; Vestergaard, M.; et al. Common variants associated with general and MMR vaccine-related febrile seizures. Nat. Genet. 2014, 46, 1274–1282. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.K.; Hon, K.L.; Leung, T.N. Febrile seizures: An overview. Drugs Context 2018, 7, 212536. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
