Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites and Study Participants
2.2. Influenza Vaccination and Surveillance
2.3. Antibody and Cell-Mediated Immune Response Measures
2.4. Statistical Analysis
3. Results
3.1. Characteristics of LCII and Non-LCII Participants
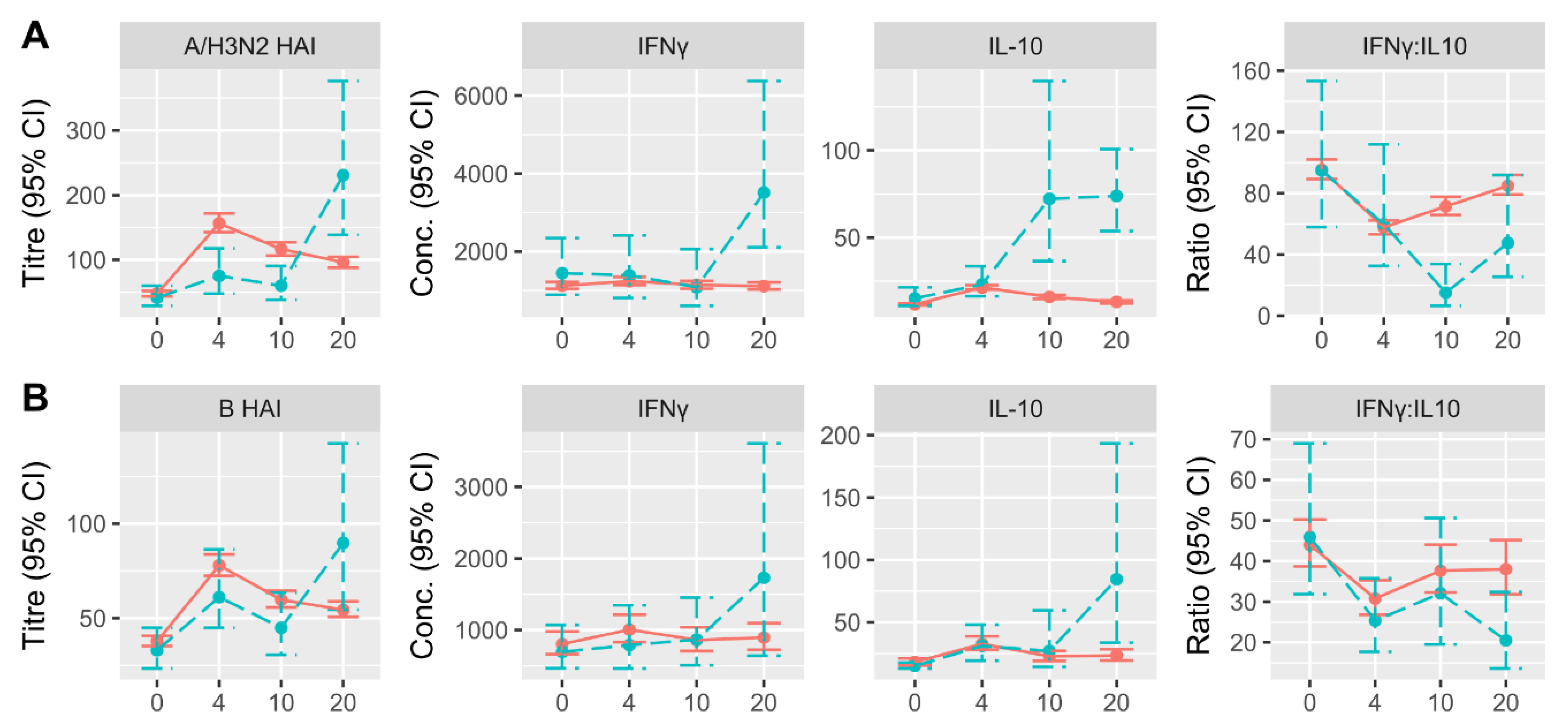
3.2. Changes in HAI and CMI Measures over Time in LCII and Non-LCII Participants
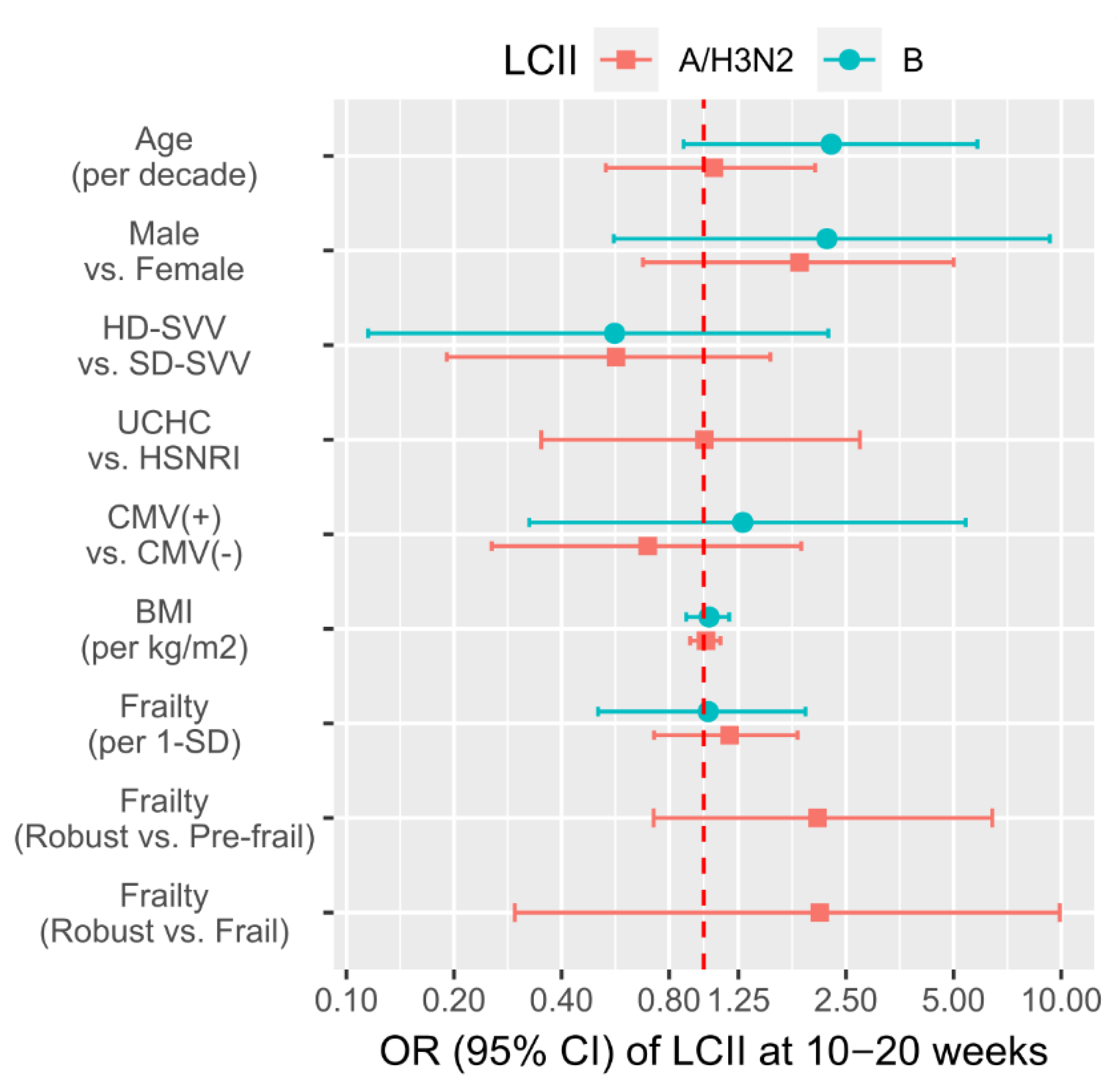
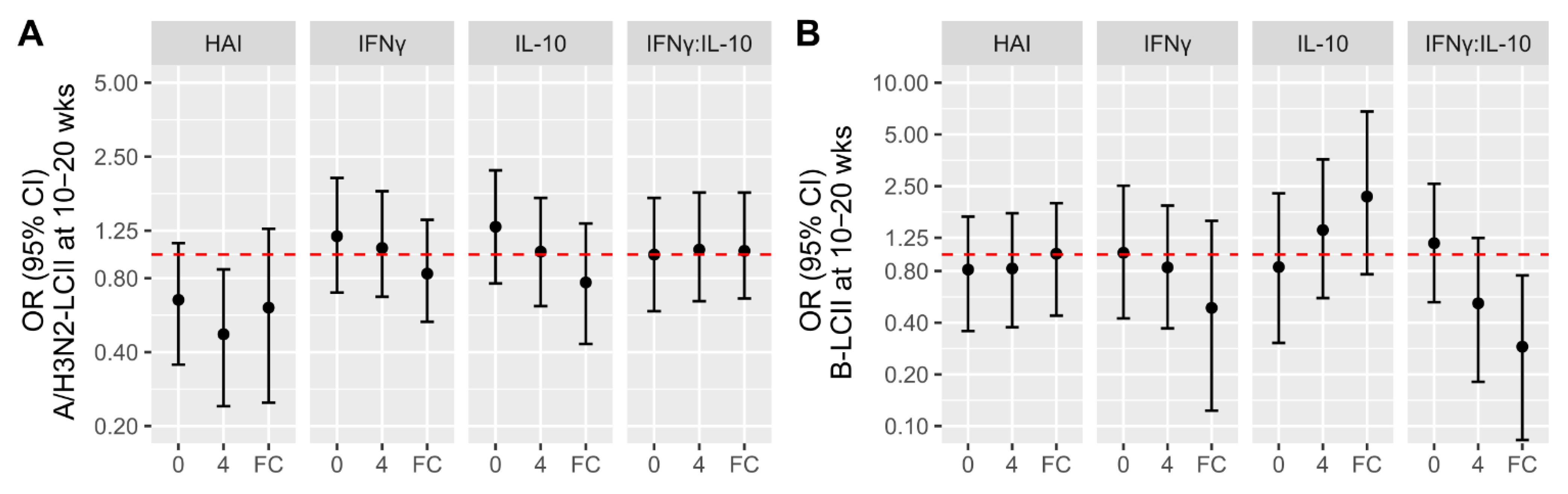
3.3. Associations of Participant Factors with the Likelihood of LCII
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.E.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.M.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.A.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Bridges, C.B.; Cox, N.J.; Fukuda, K. Influenza-associated hospitalizations in the United States. JAMA 2004, 292, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- CDC Weekly, U.S. Influenza Surveillance Report (FluView). Available online: https://www.cdc.gov/flu/weekly/index.htm (accessed on 5 May 2020).
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Loeb, N.; Andrew, M.; Loeb, M.; Kuchel, G.; Haynes, L.; McElhaney, J.; Verschoor, C. Frailty is associated with increased hemagglutinin-inhibition titres in a 4-year randomized trial comparing standard and high dose influenza vaccination. Open Forum Infect. Dis 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Weir, J.P.; Engelhardt, O.; Katz, J.M.; Cox, R.J. Meeting report and review: Immunological assays and correlates of protection for next-generation influenza vaccines. Influenza Other Respir. Viruses 2019. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Gaglani, M.; Martin, E.T.; Middleton, D.; Monto, A.S.; Murthy, K.; Silveira, F.P.; Talbot, H.K.; Zimmerman, R.; Alyanak, E.; et al. Prevention of Influenza Hospitalization Among Adults in the United States, 2015–2016: Results From the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J. Infect. Dis. 2019, 220, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Segaloff, H.E.; Petrie, J.G.; Malosh, R.E.; Cheng, C.K.; McSpadden, E.J.; Ferdinands, J.M.; Lamerato, L.; Lauring, A.S.; Monto, A.S.; Martin, E.T. Severe morbidity among hospitalised adults with acute influenza and other respiratory infections: 2014–2015 and 2015–2016. Epidemiol. Infect. 2018, 146, 1350–1358. [Google Scholar] [CrossRef]
- Andrew, M.K.; Shinde, V.; Ye, L.; Hatchette, T.; Haguinet, F.; Dos Santos, G.; McElhaney, J.E.; Ambrose, A.; Boivin, G.; Bowie, W.; et al. The importance of frailty in the assessment of trivalent inactivated influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J. Infect. Dis 2017, 216, 405–414. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Leroux-Roels, G.; Beran, J.; Devaster, J.-M.; Esen, M.; Launay, O.; McElhaney, J.E.; van Essen, G.A.; Benoit, A.; Claeys, C.; et al. Immunogenicity of AS03-adjuvanted and non-adjuvanted trivalent inactivated influenza vaccines in elderly adults: A Phase 3, randomized trial and post-hoc correlate of protection analysis. Hum. Vaccines Immunother. 2016, 12, 3043–3055. [Google Scholar] [CrossRef]
- Dunning, A.J.; DiazGranados, C.A.; Voloshen, T.; Hu, B.; Landolfi, V.A.; Talbot, H.K. Correlates of Protection against Influenza in the Elderly: Results from an Influenza Vaccine Efficacy Trial. Clin. Vaccine Immunol. CVI 2016, 23, 228–235. [Google Scholar] [CrossRef]
- He, W.; Tan, G.S.; Mullarkey, C.E.; Lee, A.J.; Lam, M.M.W.; Krammer, F.; Henry, C.; Wilson, P.C.; Ashkar, A.A.; Palese, P.; et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc. Natl. Acad. Sci. USA 2016, 113, 11931–11936. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Gooch, K.E.; Marriott, A.C.; Ryan, K.A.; Yeates, P.; Slack, G.S.; Brown, P.J.; Fothergill, R.; Whittaker, C.J.; Carroll, M.W. Heterosubtypic cross-protection correlates with cross-reactive interferon-gamma-secreting lymphocytes in the ferret model of influenza. Sci. Rep. 2019, 9, 2617. [Google Scholar] [CrossRef] [PubMed]
- van de Sandt, C.E.; Kreijtz, J.H.C.M.; de Mutsert, G.; Geelhoed-Mieras, M.M.; Hillaire, M.L.B.; Vogelzang-van Trierum, S.E.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; Rimmelzwaan, G.F. Human cytotoxic T lymphocytes directed to seasonal influenza A viruses cross-react with the newly emerging H7N9 virus. J. Virol. 2014, 88, 1684–1693. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Xie, D.; Hager, W.D.; Barry, M.B.; Wang, Y.; Kleppinger, A.; Ewen, C.; Kane, K.P.; Bleackley, R.C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [Google Scholar] [CrossRef]
- Merani, S.; Kuchel, G.A.; Kleppinger, A.; McElhaney, J.E. Influenza vaccine-mediated protection in older adults: Impact of influenza infection, cytomegalovirus serostatus and vaccine dosage. Exp. Gerontol. 2018, 107, 116–125. [Google Scholar] [CrossRef]
- Kissling, E.; Pozo, F.; Buda, S.; Vilcu, A.-M.; Rizzo, C.; Gherasim, A.; Horváth, J.K.; Brytting, M.; Domegan, L.; Meijer, A.; et al. Effectiveness of influenza vaccine against influenza A in Europe in seasons of different A(H1N1)pdm09 and the same A(H3N2) vaccine components (2016–2017 and 2017–2018). Vaccine X 2019, 3, 100042. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Kou, Y.; Yu, X.; Zheng, Z.; Yang, X.; Wang, H. Interim estimates of divergence date and vaccine strain match of human influenza A(H3N2) virus from systematic influenza surveillance (2010–2015) in Hangzhou, southeast of China. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2015, 40, 17–24. [Google Scholar] [CrossRef]
- Shahid, Z.; Kleppinger, A.; Gentleman, B.; Falsey, A.R.; McElhaney, J.E. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine 2010, 28, 6145–6151. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Hottes, T.S.; McElhaney, J.E.; Janjua, N.Z.; Sabaiduc, S.; Chan, T.; Gentleman, B.; Purych, D.; Gardy, J.; Patrick, D.M.; et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: Higher antibody and lower cell-mediated immune responses with advanced age. J. Infect. Dis. 2011, 203, 158–167. [Google Scholar] [CrossRef]
- McNeil, S.; Johnstone, J.; Rockwood, M.; MacKinnon-Cameron, D.; Wang, H.; Ye, L.; Andrew, M. Impact of Hospitalizaiton Due to Influenza on Frailty in Older Adults: Toward a Better Understanding of Burden of Disease. In Proceedings of the IDWeek 2012: Meeting of the Infectious Diseases Society of America, San Diego, CA, USA, 17–21 October 2012. [Google Scholar]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Hoover, M.; Rotermann, M.; Sanmartin, C.; Bernier, J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013, 24, 10–17. [Google Scholar]
- Haq, K.; Fulop, T.; Tedder, G.; Gentleman, B.; Garneau, H.; Meneilly, G.S.; Kleppinger, A.; Pawelec, G.; McElhaney, J.E. Cytomegalovirus Seropositivity Predicts a Decline in the T Cell But Not the Antibody Response to Influenza in Vaccinated Older Adults Independent of Type 2 Diabetes Status. J. Gerontol. Biol. Sci. Med. Sci. 2017, 72, 1163–1170. [Google Scholar] [CrossRef]
- Gravenstein, S.; Miller, B.; Ershler, W.; Brown, C.; Mast, E.; Circo, R.; Duthie, E.; Drinka, P. Low sensitivity of CDC case definition for H3N2 influenza in elderly nursing-home subjects. Clin. Res. 1990, 38, A547. [Google Scholar]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Lancaster, G.I.; Febbraio, M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Gentleman, B. Cell-Mediated Immune Response to Influenza Using Ex Vivo Stimulation and Assays of Cytokine and Granzyme B Responses. Methods Mol. Biol. 2015, 1343, 121–141. [Google Scholar] [CrossRef]
- Gijzen, K.; Liu, W.M.; Visontai, I.; Oftung, F.; van der Werf, S.; Korsvold, G.E.; Pronk, I.; Aaberge, I.S.; Tütto, A.; Jankovics, I.; et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine 2010, 28, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Verschoor, C.P.; Andrew, M.K.; Haynes, L.; Kuchel, G.A.; Pawelec, G. The immune response to influenza in older humans: Beyond immune senescence. Immun. Ageing A 2020, 17, 10. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Nowalk, M.P.; Chung, J.; Jackson, M.L.; Jackson, L.A.; Petrie, J.G.; Monto, A.S.; McLean, H.Q.; Belongia, E.A.; Gaglani, M.; et al. 2014–2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type. Clin. Infect. Dis. 2016, 63, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Dickinson, J.A.; Winter, A.-L.; Hickman, R.; Chan, T.; Jassem, A.N.; Drews, S.J.; Charest, H.; et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): Interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Eurosurveillance 2018, 23, 18-00035. [Google Scholar] [CrossRef]
- CDC Seasonal Flu Vaccine Effectiveness Studies|CDC. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 14 April 2020).
- McElhaney, J.E.; Herre, J.M.; Lawson, M.L.; Cole, S.K.; Burke, B.L.; Hooton, J.W. Effect of congestive heart failure on humoral and ex vivo cellular immune responses to influenza vaccination in older adults. Vaccine 2004, 22, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.I.; Barr, I.G.; Koh, G.C.H.; Lee, V.J.; Lee, C.P.S.; Shaw, R.; Lin, C.; Yap, J.; Cook, A.R.; Tan, B.H.; et al. Serological Response in RT-PCR Confirmed H1N1-2009 Influenza A by Hemagglutination Inhibition and Virus Neutralization Assays: An Observational Study. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
| A/H3N2-LCII | B-LCII | Negative | |||||
|---|---|---|---|---|---|---|---|
| Either | HAI Only | HAI + PCR | Either | HAI Only | HAI + PCR | Both | |
| All years | 17 | 9 | 8 | 9 | 1 | 8 | 582 |
| 2014/2015 | 7 | 5 | 2 | 0 | 0 | 0 | 99 |
| 2015/2016 | 0 | 0 | 0 | 3 | 1 | 2 | 169 |
| 2016/2017 | 1 | 1 | 0 | 0 | 0 | 0 | 173 |
| 2017/2018 | 9 | 3 | 6 | 6 | 0 | 6 | 141 |
| LCII at 10–20 Weeks | ||||
|---|---|---|---|---|
| Total | Negative | Positive | p-Value | |
| (n = 608) | (n = 582) | (n = 26) | ||
| Age | 76 (7.38) | 76 (7.41) | 77 (6.68) | 0.50 |
| Missing | 1 (0.2%) | 1 (0.2%) | 0 (0%) | |
| Sex | 0.09 | |||
| Female | 408 (67.1%) | 395 (67.9%) | 13 (50.0%) | |
| Male | 200 (32.9%) | 187 (32.1%) | 13 (50.0%) | |
| Dose | 0.21 | |||
| SD | 313 (51.5%) | 296 (50.9%) | 17 (65.4%) | |
| HD | 295 (48.5%) | 286 (49.1%) | 9 (34.6%) | |
| Site | 0.17 | |||
| HSNRI | 354 (58.2%) | 335 (57.6%) | 19 (73.1%) | |
| UCHC | 254 (41.8%) | 247 (42.4%) | 7 (26.9%) | |
| CMV Status | 0.89 | |||
| Negative | 284 (46.7%) | 271 (46.6%) | 13 (50.0%) | |
| Positive | 324 (53.3%) | 311 (53.4%) | 13 (50.0%) | |
| BMI | 28 (4.84) | 28 (4.84) | 29 (4.68) | 0.33 |
| Missing | 3 (0.5%) | 3 (0.5%) | 0 (0%) | |
| Frailty Index (continuous) | 0.11 (0.0735) | 0.11 (0.0736) | 0.12 (0.07) | 0.57 |
| Missing | 2 (0.3%) | 2 (0.3%) | 0 (0%) | |
| Frailty Index (categorical) | 0.40 | |||
| Robust | 303 (49.8%) | 293 (50.3%) | 10 (38.5%) | |
| Pre-frail | 249 (41.0%) | 235 (40.4%) | 14 (53.8%) | |
| Frail | 54 (8.9%) | 52 (8.9%) | 2 (7.7%) | |
| Missing | 2 (0.3%) | 2 (0.3%) | 0 (0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verschoor, C.P.; Andrew, M.K.; Loeb, M.; Pawelec, G.; Haynes, L.; Kuchel, G.A.; McElhaney, J.E. Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults. Vaccines 2021, 9, 25. https://doi.org/10.3390/vaccines9010025
Verschoor CP, Andrew MK, Loeb M, Pawelec G, Haynes L, Kuchel GA, McElhaney JE. Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults. Vaccines. 2021; 9(1):25. https://doi.org/10.3390/vaccines9010025
Chicago/Turabian StyleVerschoor, Chris P., Melissa K. Andrew, Mark Loeb, Graham Pawelec, Laura Haynes, George A. Kuchel, and Janet E. McElhaney. 2021. "Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults" Vaccines 9, no. 1: 25. https://doi.org/10.3390/vaccines9010025
APA StyleVerschoor, C. P., Andrew, M. K., Loeb, M., Pawelec, G., Haynes, L., Kuchel, G. A., & McElhaney, J. E. (2021). Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults. Vaccines, 9(1), 25. https://doi.org/10.3390/vaccines9010025






