Serum Anti-HPV Antibody Titer as a Marker of Vaccine Effectiveness in Males with Genital Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Semen Analysis
2.3. HPV Detection And Genotyping
2.4. Anti-HPV Antibodies
2.5. Statistical Analysis
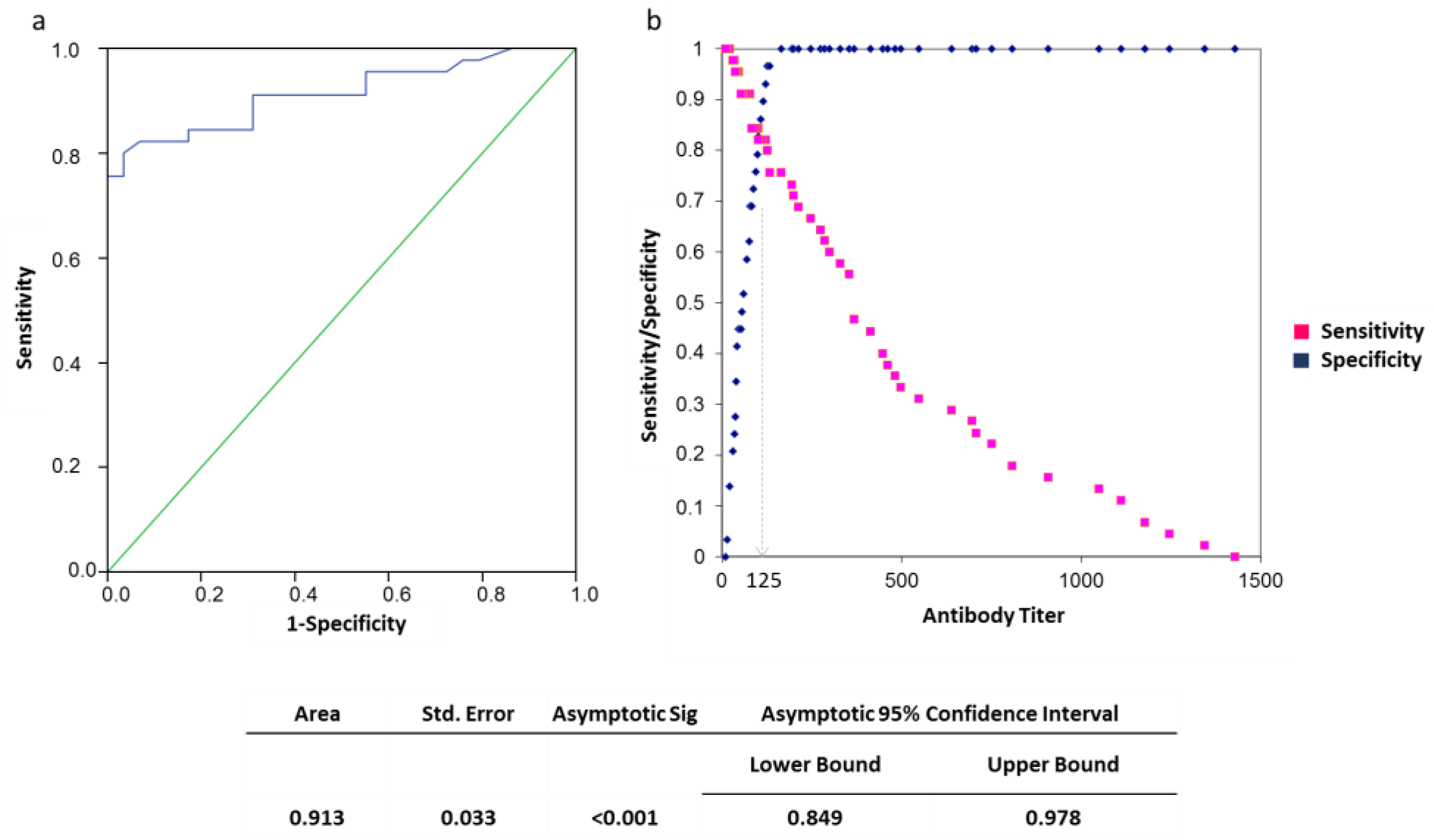
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunne, E.F.; Nielson, C.M.; Stone, K.M.; Markowitz, L.E.; Giuliano, A.R. Prevalence of HPV infection among men: A systematic review of the literature. J. Infect. Dis. 2006, 194, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Álvarez, M.I.; Gómez-Urquiza, J.L.; Husein-El Ahmed, H.; Luis Albendín-García, L.; Juan Gómez-Salgado, J.; Cañadas-De la Fuente, G.A. Prevalnce and risk factors of Human Papillomavirus in male patients: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 2210. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Andino, J.; Buck, C.B.; Ribbeck, K. Adsorption of human papillomavirus 16 to live human sperm. PLoS ONE 2009, 4, e5847. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; De Toni, L.; Barzon, L.; Foresta, C. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil. Steril. 2013, 99, 125–131. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Cannarella, R.; Giacone, F.; Mongioi’, L.; Scalia, G.; Favilla, V.; Russo, G.I.; Cimino, S.; Morgia, G.; et al. High Rate of Detection of Ultrasound Signs of Prostatitis in Patients with HPV-DNA Persistence on Semen: Role of Ultrasound in HPV-related Male Accessory Gland Infection. J. Endocrinol. Investig. 2019, 42, 1459–1465. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bonfig, R.; Wilbert, D.M.; Strohmaier, W.L.; Engelmann, U.H. Risk factors for antisperm antibodies in infertile men. Am. J. Reprod. Immunol. 1994, 31, 69–76. [Google Scholar] [CrossRef]
- Garolla, A.; Engl, B.; Pizzol, D.; Ghezzi, M.; Bertoldo, A.; Bottacin, A.; Noventa, M.; Foresta, C. Spontaneous fertility and in vitro fertilization outcome: New evidence of human papillomavirus sperm infection. Fertil. Steril. 2016, 105, 65–72. [Google Scholar] [CrossRef]
- Markowitz, E.L.; Naleway, A.L.; Klein, N.P.; Lewis, R.M.; Crane, B.; Querec, T.D.; Hsiao, A.; Aukes, L.; Timbol, J.; Weinmann, S.; et al. Human Papillomavirus Vaccine Effectiveness Against HPV Infection: Evaluation of One, Two, and Three Doses. J. Infect. Dis. 2020, 221, 910–918. [Google Scholar] [CrossRef]
- Draper, E.; Bissett, S.L.; Howell-Jones, R.; Waight, P.; Soldan, K.; Jit, M.; Andrews, N.; Miller, E.; Beddows, S. A Randomized, Observer-Blinded Immunogenicity Trial of Cervarix and Gardasil Human Papillomavirus Vaccines in 12–15 Year Old Girls. PLoS ONE 2013, 8, e61825. [Google Scholar] [CrossRef]
- Pinto, L.A.; Kemp, T.J.; Torres, B.N.; Isaacs-Soriano, K.; Ingles, D.; Abrahamsen, M.; Pan, Y.; Lazcano-Ponce, E.; Salmerón, J.; Giuliano, A.R. Quadrivalent Human Papillomavirus (HPV) Vaccine Induces HPV-Specific Antibodies in the Oral Cavity: Results from the Mid-Adult Male Vaccine Trial. J. Infect. Dis. 2016, 214, 1276–1283. [Google Scholar] [CrossRef]
- Malagón, T.; Drolet, M.; Boily, M.C.; Franco, E.L.; Jit, M.; Brisson, J.; Brisson, M. Cross-protective efficacy of two human papillomavirus vaccines: A systematic review and metanalysis. Lancet Infect. Dis. 2012, 12, 781–789. [Google Scholar] [CrossRef]
- Schneider, K.; Grønhøj, C.; Hahn, C.H.; Von Buchwald, C. Therapeutic Human Papillomavirus Vaccines in Head and Neck Cancer: A Systematic Review of Current Clinical Trials. Vaccine 2018, 36, 6594–6605. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, M.; Martí, C.; Torras, I.; Henere, C.; Munmany, M.; Marimon, L.; Saco, A.; Torné, A.; Ordi, J. HPV Vaccination as Adjuvant to Conization in Women with Cervical Intraepithelial Neoplasia: A Study under Real-Life Conditions. Vaccines 2020, 8, 245. [Google Scholar] [CrossRef]
- Foresta, C.; Garolla, A.; Parisi, S.G.; Ghezzi, M.; Bertoldo, A.; Di Nisio, A.; De Toni, L. HPV Prophylactic Vaccination in Males Improves the Clearance of Semen Infection. EBioMedicine 2015, 2, 1487–1493. [Google Scholar] [CrossRef]
- Harder, T.; Wichmann, O.; Klug, S.J.; Van Der Sande, M.A.B.; Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: A systematic review. BMC Med. 2018, 16, 110. [Google Scholar] [CrossRef]
- Karita, H.C.S.; Hauge, K.; Magaret, A.; Mao, C.; Schouten, J.; Grieco, V.; Xi, L.F.; Galloway, D.A.; Madeleine, M.M.; Wald, A. Effect of human papillomavirus vaccine to interrupt recurrence of vulvar and anal neoplasia (VIVA). A trial control. JAMA Netw. Open 2019, 2, e190819. [Google Scholar] [CrossRef]
- Ferguson, M.; Wilkinson, D.E.; Heath, A.; Matejtschuk, P. The first international standard for antibodies to HPV 16. Vaccine 2011, 29, 6520–6526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lin, Z.-J.; Huang, S.-J.; Li, J.; Liu, X.-H.; Guo, M.; Zhang, J.; Xia, N.-S.; Pam, H.-R.; Wu, T.; et al. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum. Vaccines Immunother. 2014, 10, 740–746. [Google Scholar] [CrossRef]
- Insinga, R.P.; Dasbach, E.J.; Myers, E.R. The health and economic burden of genital warts in a set of private health plans in the United States. Clin. Infect. Dis. 2003, 36, 1397–1403. [Google Scholar] [CrossRef]
- Edelstein, Z.R.; Carter, J.J.; Garg, R.; Winer, R.L.; Feng, Q.; Galloway, D.A.; Koutsky, L.A. Serum antibody response following genital {alpha}9 human papillomavirus infection in young men. J. Infect. Dis. 2011, 204, 209–216. [Google Scholar] [CrossRef]
- Han, K.; Shao, X.; Zheng, H.; Wu, C.; Zhu, J.; Zheng, X.; Zhang, Y. Revaccination of non- and low- responders after a standard three dose hepatitis B vaccine schedule. Hum. Vaccines Immunother. 2012, 8, 1845–1849. [Google Scholar] [CrossRef]
| Comparison of Data at T0 (Study Initiation) | ||||
|---|---|---|---|---|
| Parameters | Whole Population N = 379 | Resp. at T1 326/379 (86%) | Non-Resp. at T1 53/379 (14%) | p-Value (Resp. vs. Non-Resp.) |
| Mean age (years ± SEM) | 40.3 ± 0.65 | 39.8 ± 0.48 | 41.5 ± 1.44 | 0.9999 |
| BMI (kg/m2 ± SEM) | 23.9 ± 0.2 | 24.1 ± 0.19 | 23.6 ± 0.41 | 0.3175 |
| Concomitant drug therapy (n/%) | 104/379 (27.4%) | 82/326 (25%) | 22/53 (41.5%) | 0.0194 |
| Genital Warts (n/%) | 123/379 (32.4 %) | 90/326 (27.6%) | 33/53 (62.3%) | 0.0001 |
| HPV Genotypes | ||||
| Vaccine genotypes (n/%) | 227/379 (59.9 %) | 191/326 (58.6%) | 36/53 (67.9%) | 0.2281 |
| Cross-reactive genotypes (n/%) | 152/379 (40.1%) | 135/326 (41.4%) | 17/53 (32.1%) | 0.2281 |
| T0 (Study Initiation) | T1 (6 Months after Vaccination Cycle Ending—12 Months Since Study Initiation) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Whole Population | Resp. | Non-Resp. | p-Value (Resp. vs. Non-Resp.) | Whole Population | Resp. | Non-Resp. | p-Value (Resp. vs. Non-Resp.) |
| Semen FISH-HPV DNA + (n/%) | 166/379 (43.8%) | 129/326 (39.6%) | 37/53 (69.8%) | 0.0001 | 27/379 (7.1%) * | 0 * | 27/53 (50.9%) | 0.0001 |
| Semen ASA + (n/%) | 88/379 (23.1%) | 64/326 (19.6%) | 24/53 (45.3%) | 0.0002 | 54/379 (14.2%) * | 36/326 (11.0%) * | 18/53 (33.9%) | 0.0001 |
| Seroconversion rate (n/%) | 146/379 (38.5%) | 128/326 (39.3%) | 18/53 (33.9%) | 0.5434 | 379/379 (100%) * | 326/326 (100%) * | 53/53 (100%) * | 1.0000 |
| Serum-antibody titer (dilution ± SEM) | 1:72 ± 36.47 | 1:97 ± 34.4 | 1:28 ± 7.28 | 0.4199 | 1:308 ± 42 * | 1:469 ± 58 * | 1:62 ± 7 * | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Toni, L.; Muscianisi, F.; Corsini, C.; Ghezzi, M.; Di Nisio, A.; Foresta, C.; Garolla, A. Serum Anti-HPV Antibody Titer as a Marker of Vaccine Effectiveness in Males with Genital Infection. Vaccines 2020, 8, 743. https://doi.org/10.3390/vaccines8040743
De Toni L, Muscianisi F, Corsini C, Ghezzi M, Di Nisio A, Foresta C, Garolla A. Serum Anti-HPV Antibody Titer as a Marker of Vaccine Effectiveness in Males with Genital Infection. Vaccines. 2020; 8(4):743. https://doi.org/10.3390/vaccines8040743
Chicago/Turabian StyleDe Toni, Luca, Francesco Muscianisi, Christian Corsini, Marco Ghezzi, Andrea Di Nisio, Carlo Foresta, and Andrea Garolla. 2020. "Serum Anti-HPV Antibody Titer as a Marker of Vaccine Effectiveness in Males with Genital Infection" Vaccines 8, no. 4: 743. https://doi.org/10.3390/vaccines8040743
APA StyleDe Toni, L., Muscianisi, F., Corsini, C., Ghezzi, M., Di Nisio, A., Foresta, C., & Garolla, A. (2020). Serum Anti-HPV Antibody Titer as a Marker of Vaccine Effectiveness in Males with Genital Infection. Vaccines, 8(4), 743. https://doi.org/10.3390/vaccines8040743






