Immunological Distinctions between Acellular and Whole-Cell Pertussis Immunizations of Baboons Persist for at Least One Year after Acellular Vaccine Boosting
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
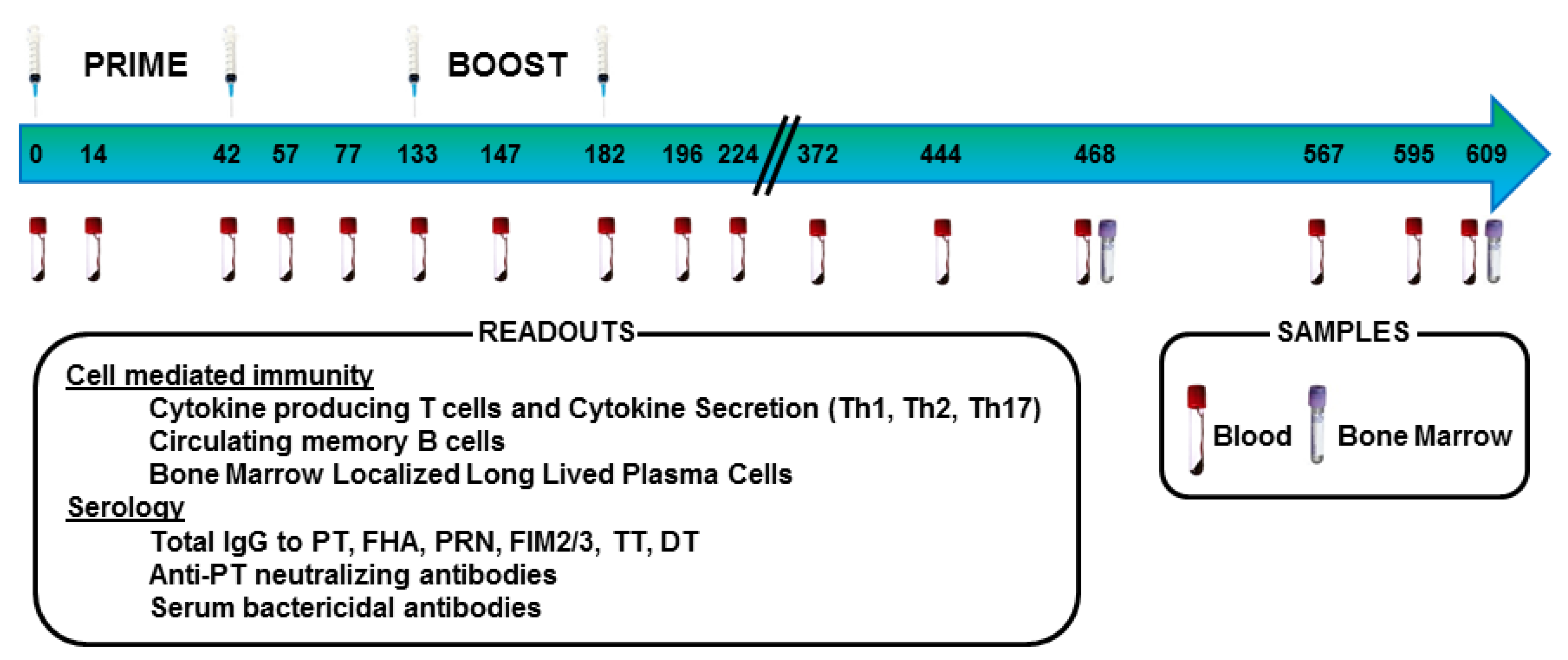
2.2. Animals, Immunization, and Sample Collection
2.3. Collection of Blood, PBMC, and Bone Marrow
2.4. Cytokine Detection in ELISPOT Assays
2.5. Memory B Cell ELISPOT
2.6. Bone Marrow Localized Long Lived Plasma Cells ELISPOT
2.7. Serum IgG Quantification by Multiplex Antibody Assay for the Simultaneous Determination of Antibodies to Bordetella Pertussis Antigens, Diphtheria Toxoid and Tetanus Toxoid
2.8. Serum Bactericidal Activity (SBA) Assay
2.9. Pertussis Toxin Neutralizing Antibody Titration
2.10. Statistical Analysis
3. Results
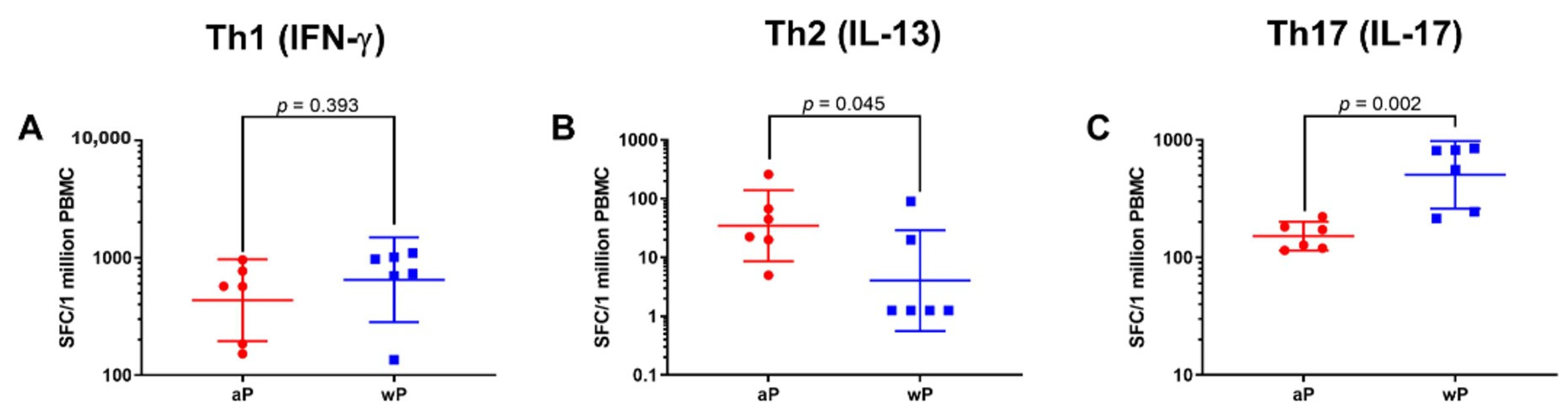
3.1. Animals Primed with wP Vaccine Exhibited a More Th17 Biased Response than Animals Primed with aP Vaccine While Priming with aP Vaccine Led to a More Th2 Biased Response than wP Vaccine Priming
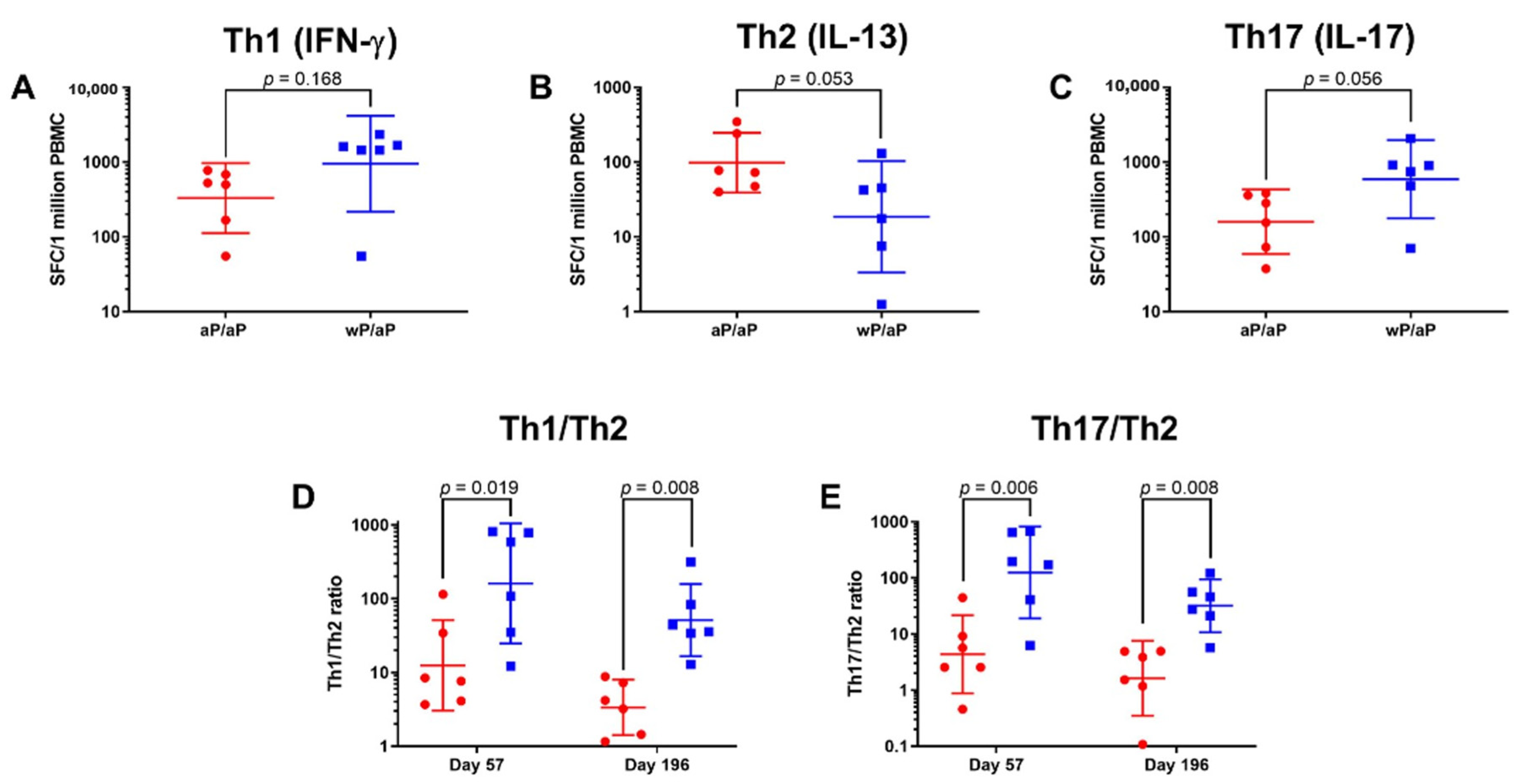
3.2. T Cell Polarization Established by Vaccine Priming Is Maintained Post aP Vaccine Boost
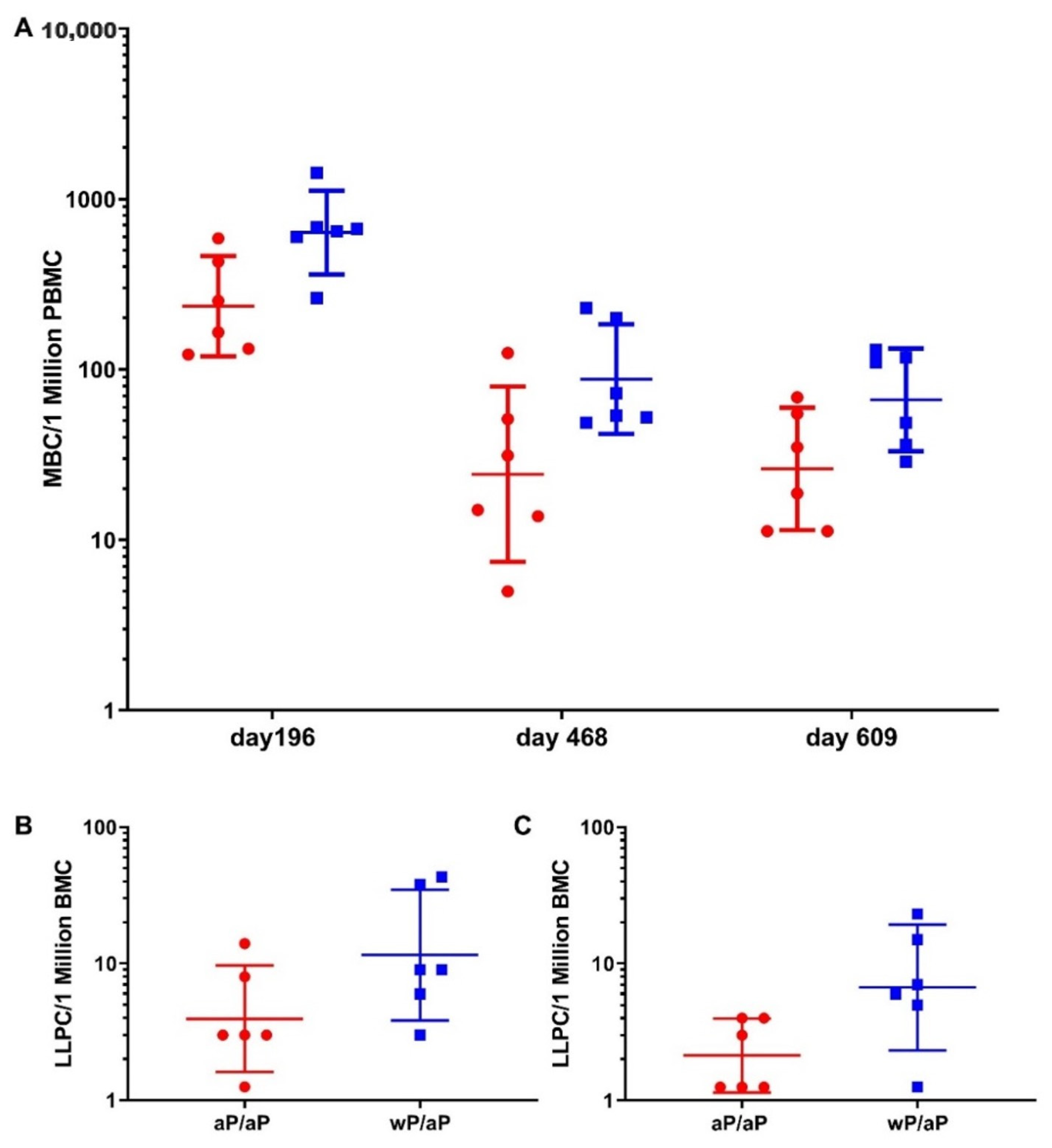
3.3. Priming with wP Vaccine Appeared to Increase the Size of Both the Pertussis Specific Circulating Memory B Cell and Bone Marrow Localized Long Lived Plasma Cell Populations Compared to aP Vaccine
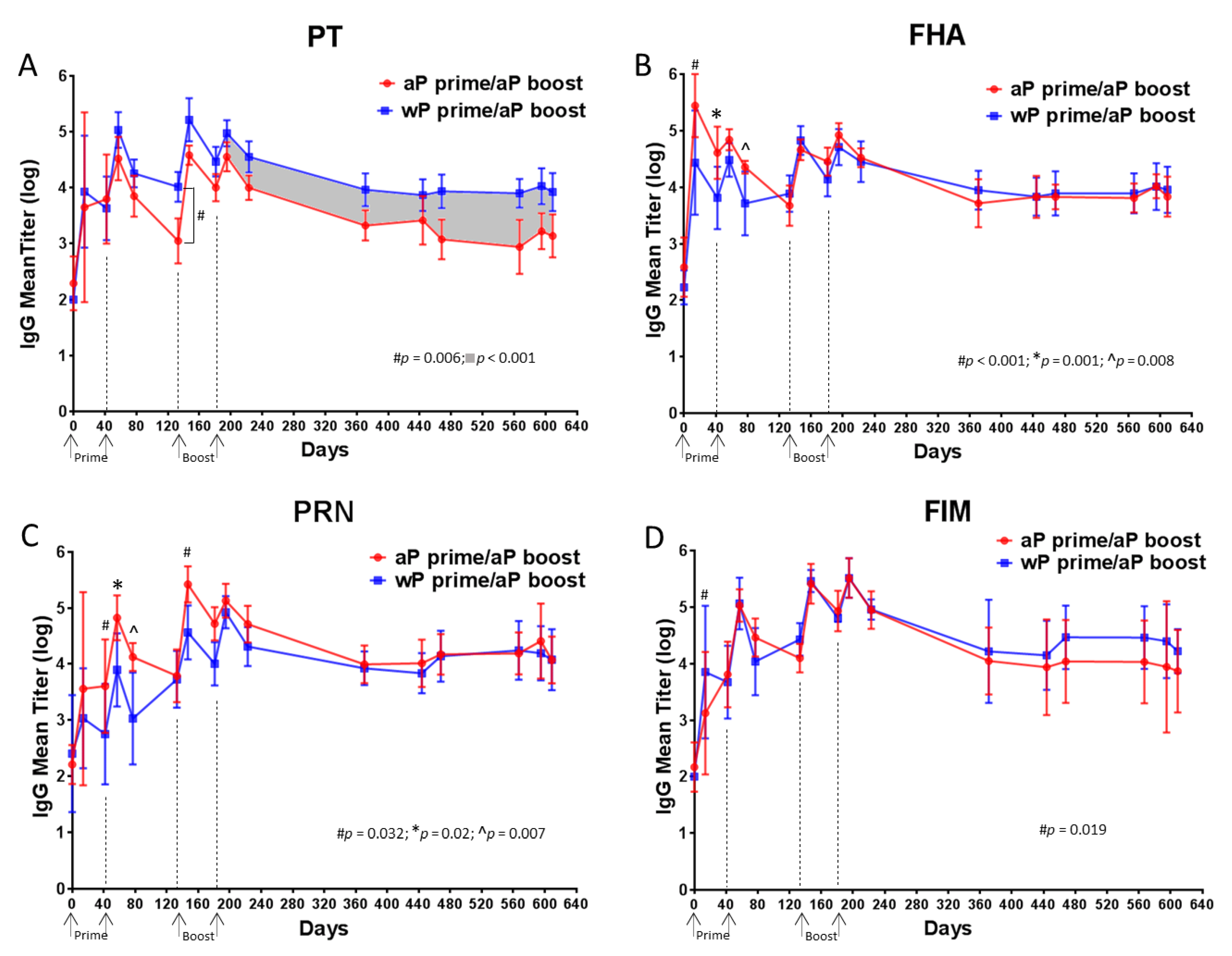
3.4. Higher Anti-PT IgG Response Sustained in wP-Primed Baboons Compared to aP-Primed for 14 Months Following Boost
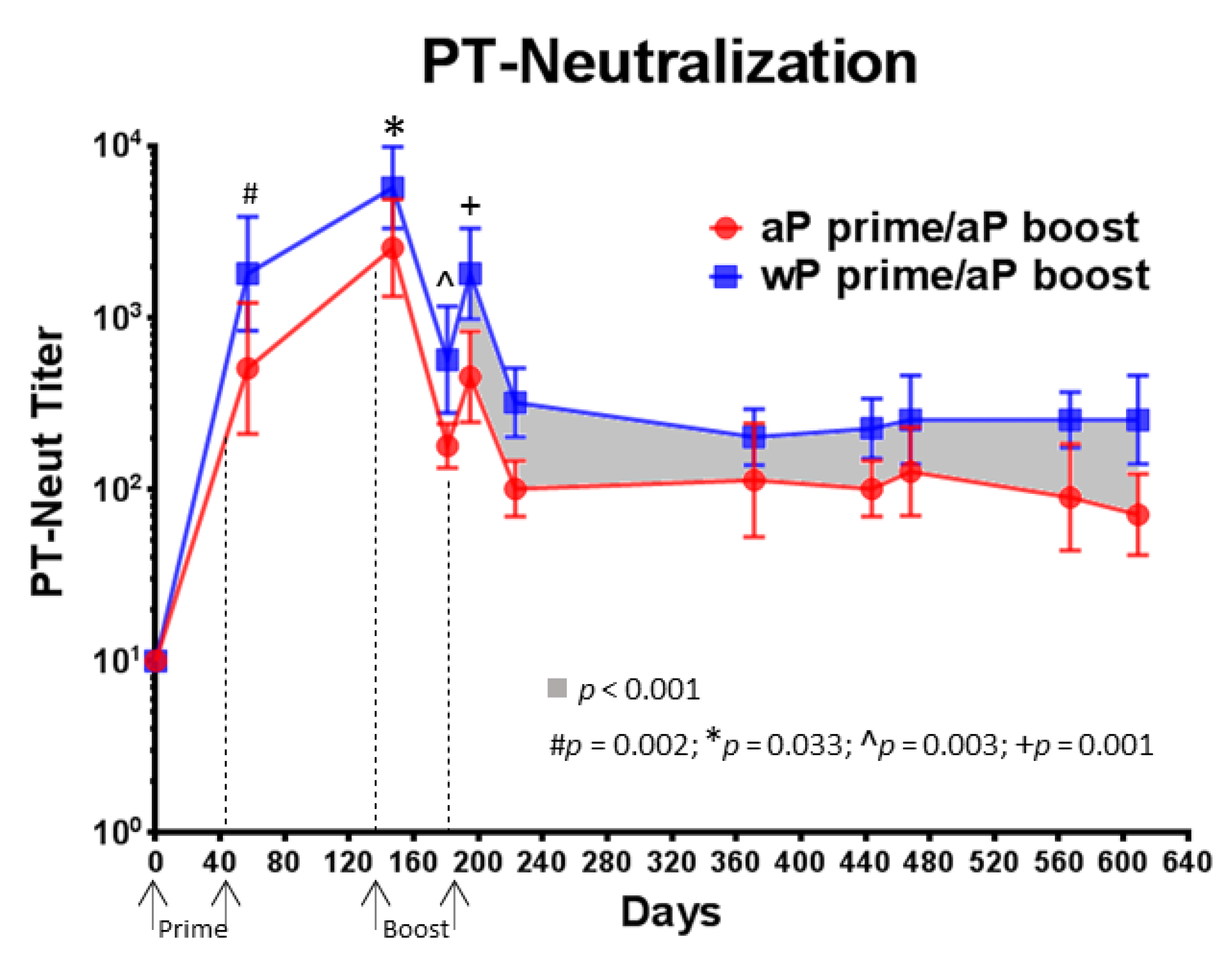
3.5. Compared to aP Vaccine Primed Baboons, wP Vaccine Primed Baboons Had Higher PT-Neutralizing Titers That Were Sustained over 14 Months Following aP Vaccine Boost
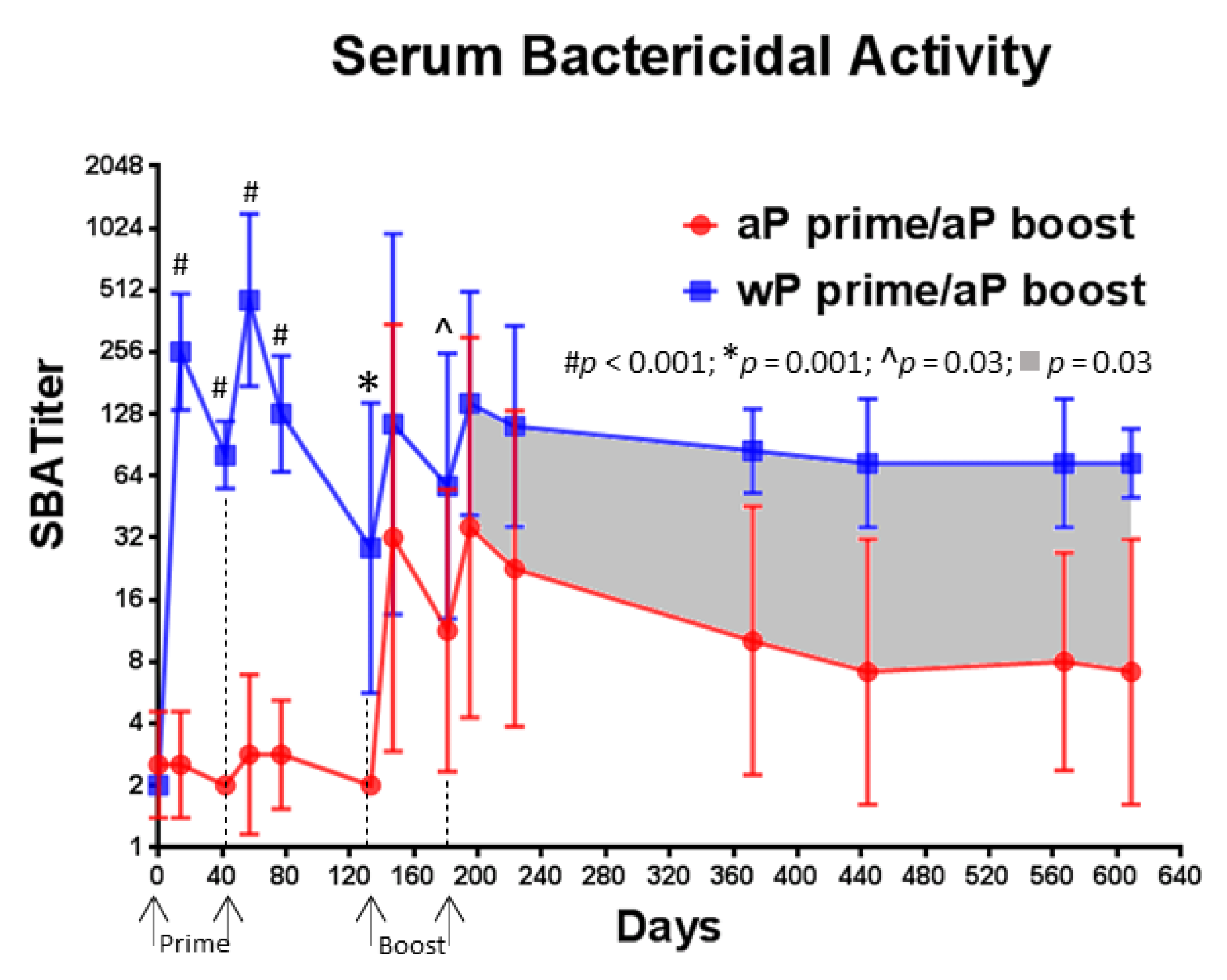
3.6. A Single Immunization with wP Vaccine Is Sufficient to Induce a Serum Bactericidal Antibody Response While Multiple Immunizations with aP Vaccine Are Necessary
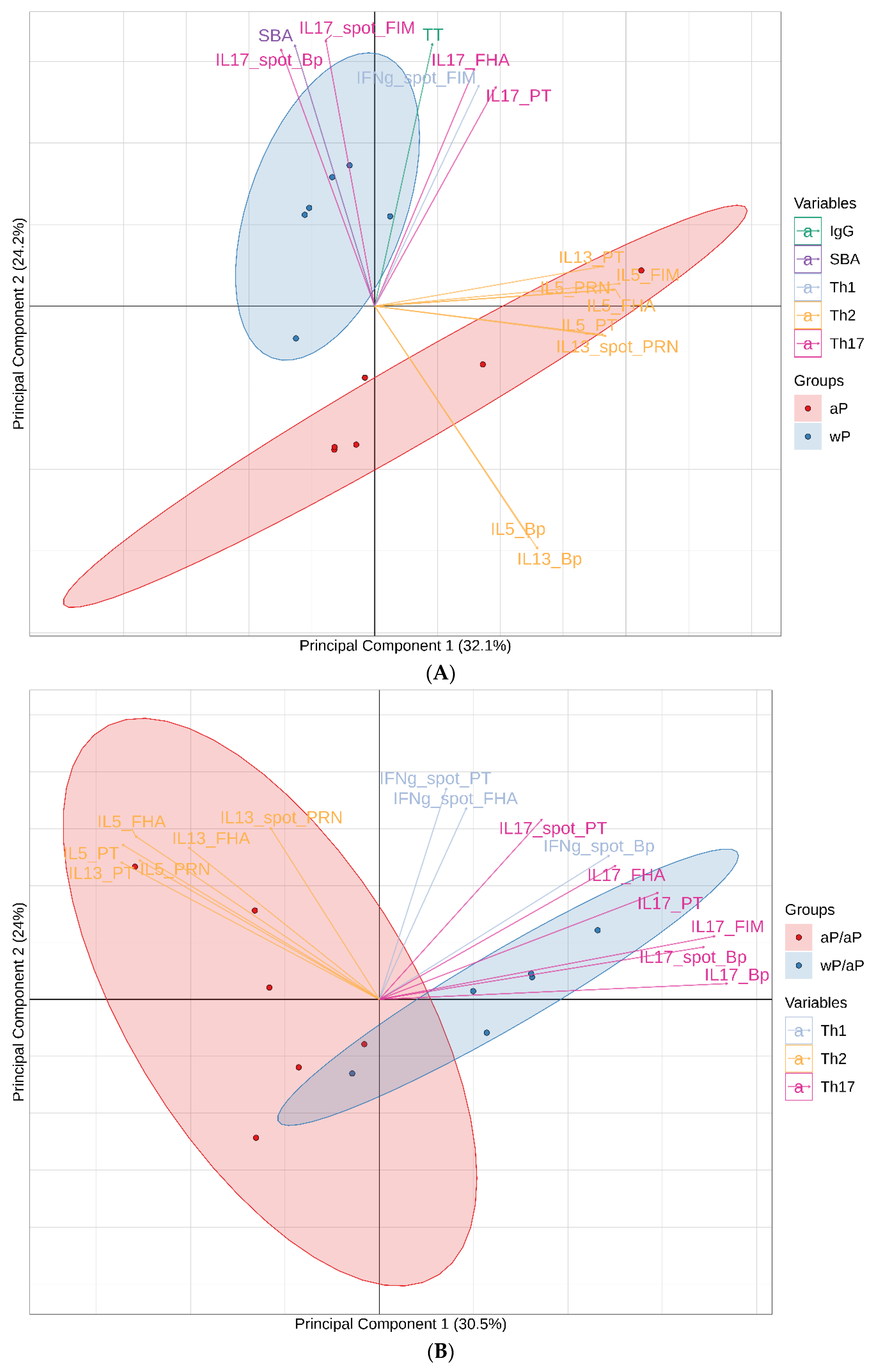
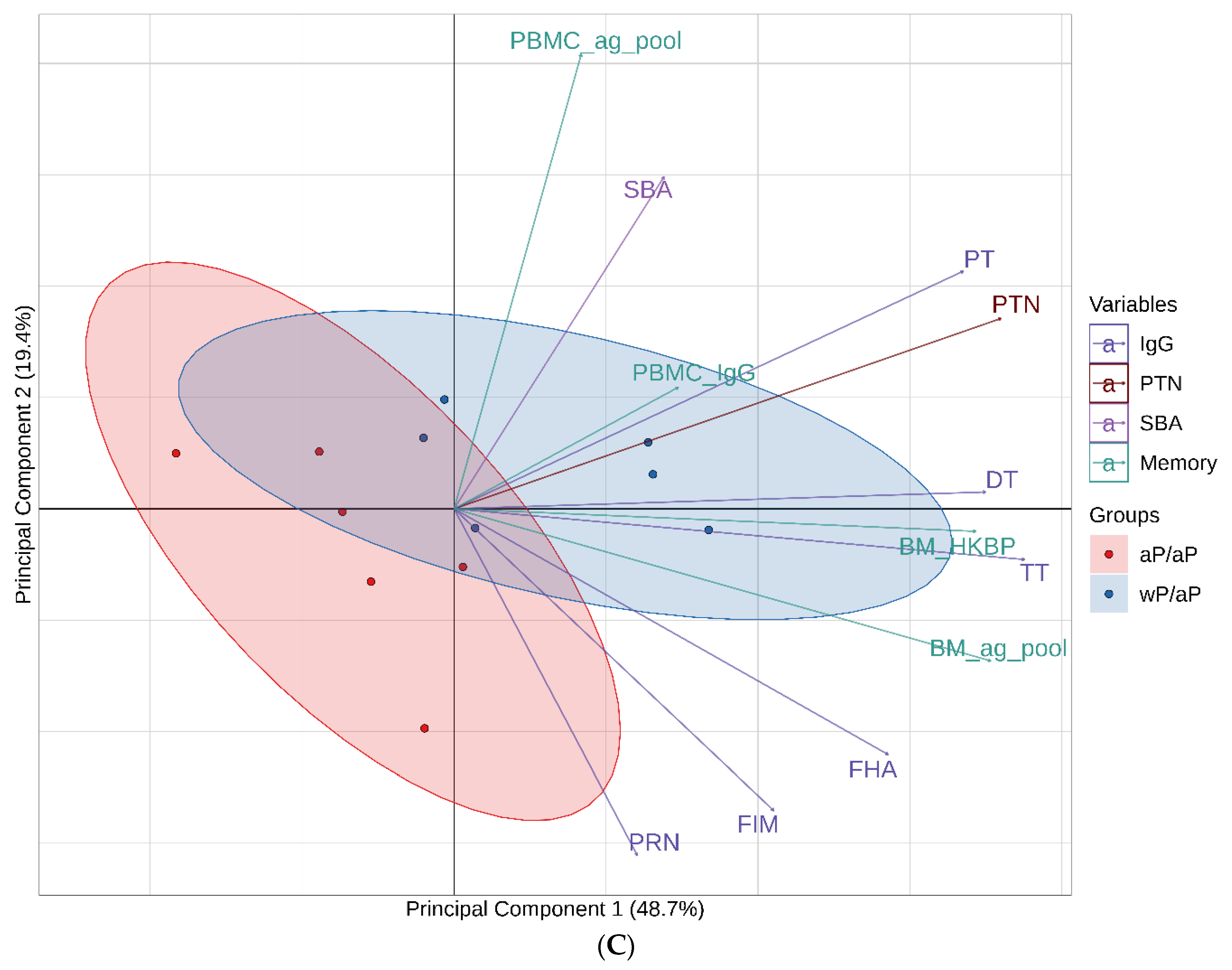
3.7. Principal Component Analysis (PCA) Reveals Immunological Distinction between Animals Primed with aP Vaccine Versus wP Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masseria, C.; Martin, C.K.; Krishnarajah, G.; Becker, L.K.; Buikema, A.; Tan, T.Q. Incidence and Burden of Pertussis Among Infants Less Than 1 Year of Age. Pediatr. Infect. Dis. J. 2017, 36, e54–e61. [Google Scholar] [CrossRef] [PubMed]
- Cornia, P.B.; Hersh, A.L.; Lipsky, B.A.; Newman, T.B.; Gonzales, R. Does this coughing adolescent or adult patient have pertussis? JAMA 2010, 304, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Siriyakorn, N.; Leethong, P.; Tantawichien, T.; Sripakdee, S.; Kerdsin, A.; Dejsirilert, S.; Paitoonpong, L. Adult pertussis is unrecognized public health problem in Thailand. BMC Infect. Dis. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Salmaso, S.; Mastrantonio, P.; Giuliano, M.; Tozzi, A.E.; Anemona, A.; Ciofi degli Atti, M.L.; Giammanco, A.; Panei, P.; Blackwelder, W.C.; et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N. Engl. J. Med. 1996, 334, 341–348. [Google Scholar] [CrossRef]
- Gustafsson, L.; Hallander, H.O.; Olin, P.; Reizenstein, E.; Storsaeter, J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 1996, 334, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Domenech de Celles, M.; Magpantay, F.M.; King, A.A.; Rohani, P. The pertussis enigma: Reconciling epidemiology, immunology and evolution. Proc. Biol. Sci. 2016, 283, 20152309. [Google Scholar] [CrossRef]
- Jackson, D.W.; Rohani, P. Perplexities of pertussis: Recent global epidemiological trends and their potential causes. Epidemiol. Infect. 2014, 142, 672–684. [Google Scholar] [CrossRef]
- Tan, T.; Dalby, T.; Forsyth, K.; Halperin, S.A.; Heininger, U.; Hozbor, D.; Plotkin, S.; Ulloa-Gutierrez, R.; Wirsing von Konig, C.H. Pertussis Across the Globe: Recent Epidemiologic Trends From 2000 to 2013. Pediatr. Infect. Dis. J. 2015, 34, e222–e232. [Google Scholar] [CrossRef]
- Acosta, A.M.; DeBolt, C.; Tasslimi, A.; Lewis, M.; Stewart, L.K.; Misegades, L.K.; Messonnier, N.E.; Clark, T.A.; Martin, S.W.; Patel, M. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics 2015, 135, 981–989. [Google Scholar] [CrossRef]
- Domenech de Celles, M.; Magpantay, F.M.G.; King, A.A.; Rohani, P. The impact of past vaccination coverage and immunity on pertussis resurgence. Sci. Transl. Med. 2018, 10, eaaj1748. [Google Scholar] [CrossRef]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Baxter, R. Waning Tdap Effectiveness in Adolescents. Pediatrics 2016, 137, e20153326. [Google Scholar] [CrossRef]
- Klein, N.P.; Bartlett, J.; Rowhani-Rahbar, A.; Fireman, B.; Baxter, R. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 2012, 367, 1012–1019. [Google Scholar] [CrossRef]
- Liko, J.; Robison, S.G.; Cieslak, P.R. Priming with whole-cell versus acellular pertussis vaccine. N. Engl. J. Med. 2013, 368, 581–582. [Google Scholar] [CrossRef]
- Witt, M.A.; Arias, L.; Katz, P.H.; Truong, E.T.; Witt, D.J. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin. Infect. Dis. 2013, 56, 1248–1254. [Google Scholar] [CrossRef]
- Witt, M.A.; Katz, P.H.; Witt, D.J. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin. Infect. Dis. 2012, 54, 1730–1735. [Google Scholar] [CrossRef]
- Zerbo, O.; Bartlett, J.; Goddard, K.; Fireman, B.; Lewis, E.; Klein, N.P. Acellular Pertussis Vaccine Effectiveness Over Time. Pediatrics 2019, 144, e20183466. [Google Scholar] [CrossRef]
- Storsaeter, J.; Hallander, H.O.; Gustafsson, L.; Olin, P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998, 16, 1907–1916. [Google Scholar] [CrossRef]
- Storsaeter, J.; Hallander, H.O.; Gustafsson, L.; Olin, P. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 2003, 21, 3542–3549. [Google Scholar] [CrossRef]
- Nguyen, A.W.; Wagner, E.K.; Laber, J.R.; Goodfield, L.L.; Smallridge, W.E.; Harvill, E.T.; Papin, J.F.; Wolf, R.F.; Padlan, E.A.; Bristol, A.; et al. A cocktail of humanized anti-pertussis toxin antibodies limits disease in murine and baboon models of whooping cough. Sci. Transl. Med. 2015, 7, 316ra195. [Google Scholar] [CrossRef]
- Warfel, J.M.; Papin, J.F.; Wolf, R.F.; Zimmerman, L.I.; Merkel, T.J. Maternal and neonatal vaccination protects newborn baboons from pertussis infection. J. Infect. Dis. 2014, 210, 604–610. [Google Scholar] [CrossRef]
- Kapil, P.; Papin, J.F.; Wolf, R.F.; Zimmerman, L.I.; Wagner, L.D.; Merkel, T.J. Maternal Vaccination With a Monocomponent Pertussis Toxoid Vaccine Is Sufficient to Protect Infants in a Baboon Model of Whooping Cough. J. Infect. Dis. 2018, 217, 1231–1236. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019, 8, 169–185. [Google Scholar] [CrossRef]
- Leef, M.; Elkins, K.L.; Barbic, J.; Shahin, R.D. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J. Exp. Med. 2000, 191, 1841–1852. [Google Scholar] [CrossRef]
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013, 9, e1003264. [Google Scholar] [CrossRef]
- Warfel, J.M.; Beren, J.; Kelly, V.K.; Lee, G.; Merkel, T.J. Nonhuman primate model of pertussis. Infect. Immun. 2012, 80, 1530–1536. [Google Scholar] [CrossRef]
- Warfel, J.M.; Beren, J.; Merkel, T.J. Airborne transmission of Bordetella pertussis. J. Infect. Dis. 2012, 206, 902–906. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Comparison of Three Whole-Cell Pertussis Vaccines in the Baboon Model of Pertussis. Clin. Vaccine Immunol. 2016, 23, 47–54. [Google Scholar] [CrossRef]
- Hewlett, E.L.; Sauer, K.T.; Myers, G.A.; Cowell, J.L.; Guerrant, R.L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 1983, 40, 1198–1203. [Google Scholar] [CrossRef]
- Isacson, J.; Trollfors, B.; Lagergrd, T.; Taranger, J. Comparison of a toxin neutralization assay and ELISA for determination of pertussis toxin antibodies. Serodiagn. Immun. Infect. Dis. 1997, 8, 163–167. [Google Scholar] [CrossRef]
- Smallridge, W.E.; Rolin, O.Y.; Jacobs, N.T.; Harvill, E.T. Different effects of whole-cell and acellular vaccines on Bordetella transmission. J. Infect. Dis 2014, 209, 1981–1988. [Google Scholar] [CrossRef]
- Solans, L.; Debrie, A.S.; Borkner, L.; Aguilo, N.; Thiriard, A.; Coutte, L.; Uranga, S.; Trottein, F.; Martin, C.; Mills, K.H.G.; et al. IL-17-dependent SIgA-mediated protection against nasal Bordetella pertussis infection by live attenuated BPZE1 vaccine. Mucosal. Immunol. 2018, 11, 1753–1762. [Google Scholar] [CrossRef]
- Ausiello, C.M.; Urbani, F.; la Sala, A.; Lande, R.; Cassone, A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 1997, 65, 2168–2174. [Google Scholar] [CrossRef]
- Mascart, F.; Hainaut, M.; Peltier, A.; Verscheure, V.; Levy, J.; Locht, C. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 2007, 25, 391–398. [Google Scholar] [CrossRef]
- Rowe, J.; Yerkovich, S.T.; Richmond, P.; Suriyaarachchi, D.; Fisher, E.; Feddema, L.; Loh, R.; Sly, P.D.; Holt, P.G. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect. Immun. 2005, 73, 8130–8135. [Google Scholar] [CrossRef]
- Ryan, M.; Murphy, G.; Gothefors, L.; Nilsson, L.; Storsaeter, J.; Mills, K.H. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 1997, 175, 1246–1250. [Google Scholar] [CrossRef]
- Ryan, M.; Murphy, G.; Ryan, E.; Nilsson, L.; Shackley, F.; Gothefors, L.; Oymar, K.; Miller, E.; Storsaeter, J.; Mills, K.H. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 1998, 93, 1–10. [Google Scholar] [CrossRef]
- Bancroft, T.; Dillon, M.B.; da Silva Antunes, R.; Paul, S.; Peters, B.; Crotty, S.; Lindestam Arlehamn, C.S.; Sette, A. Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood. Cell Immunol. 2016, 304, 35–43. [Google Scholar] [CrossRef]
- da Silva Antunes, R.; Babor, M.; Carpenter, C.; Khalil, N.; Cortese, M.; Mentzer, A.J.; Seumois, G.; Petro, C.D.; Purcell, L.A.; Vijayanand, P.; et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J. Clin. Investig. 2018, 128, 3853–3865. [Google Scholar] [CrossRef]
- van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Decker, M.D.; Greenberg, D.P.; Johnson, D.R.; Pool, V. Randomized study of immune responses to two Tdap vaccines among adolescents primed with DTaP and comparison with results among adolescents primed with DTwP. Vaccine 2019, 37, 5003–5008. [Google Scholar] [CrossRef]
- Thierry-Carstensen, B.; Dalby, T.; Stevner, M.A.; Robbins, J.B.; Schneerson, R.; Trollfors, B. Experience with monocomponent acellular pertussis combination vaccines for infants, children, adolescents and adults—A review of safety, immunogenicity, efficacy and effectiveness studies and 15 years of field experience. Vaccine 2013, 31, 5178–5191. [Google Scholar] [CrossRef]
- Taranger, J.; Trollfors, B.; Lagergard, T.; Sundh, V.; Bryla, D.A.; Schneerson, R.; Robbins, J.B. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J. Infect. Dis. 2000, 181, 1010–1013. [Google Scholar] [CrossRef]
- Nguyen, A.W.; DiVenere, A.M.; Papin, J.F.; Connelly, S.; Kaleko, M.; Maynard, J.A. Neutralization of pertussis toxin by a single antibody prevents clinical pertussis in neonatal baboons. Sci. Adv. 2020, 6, eaay9258. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Lattanzi, M.; Solis, C.D.; Contorni, M.; Costantini, M.; Moraschini, L.; Bardelli, M.; Bertholet, S.; Borgogni, E.; Buricchi, F.; et al. A phase I, randomized, controlled, dose-ranging study of investigational acellular pertussis (aP) and reduced tetanus-diphtheria-acellular pertussis (TdaP) booster vaccines in adults. Hum. Vaccin. Immunother. 2018, 14, 45–58. [Google Scholar] [CrossRef]
- Blanchard Rohner, G.; Chatzis, O.; Chinwangso, P.; Rohr, M.; Grillet, S.; Salomon, C.; Lemaitre, B.; Boonrak, P.; Lawpoolsri, S.; Clutterbuck, E.; et al. Boosting Teenagers with Acellular Pertussis Vaccines Containing Recombinant or Chemically Inactivated Pertussis Toxin: A Randomized Clinical Trial. Clin. Infect. Dis. 2019, 68, 1213–1222. [Google Scholar] [CrossRef]
- Kurvers, R.A.; Westra, D.; van Heijst, A.F.; Walk, T.L.; Warris, A.; van de Kar, N.C. Severe infantile Bordetella pertussis pneumonia in monozygotic twins with a congenital C3 deficiency. Eur. J. Pediatr. 2014, 173, 1591–1594. [Google Scholar] [CrossRef]
- Archambault, D.; Rondeau, P.; Martin, D.; Brodeur, B.R. Characterization and comparative bactericidal activity of monoclonal antibodies to Bordetella pertussis lipo-oligosaccharide A. J. Gen. Microbiol. 1991, 137, 905–911. [Google Scholar] [CrossRef]
- Gotto, J.W.; Eckhardt, T.; Reilly, P.A.; Scott, J.V.; Cowell, J.L.; Metcalf, T.N., 3rd; Mountzouros, K.; Gibbons, J.J., Jr.; Siegel, M. Biochemical and immunological properties of two forms of pertactin, the 69,000-molecular-weight outer membrane protein of Bordetella pertussis. Infect. Immun. 1993, 61, 2211–2215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cole, L.E.; Zhang, J.; Pacheco, K.M.; Lhéritier, P.; Anosova, N.G.; Piolat, J.; Zheng, L.; Reveneau, N. Immunological Distinctions between Acellular and Whole-Cell Pertussis Immunizations of Baboons Persist for at Least One Year after Acellular Vaccine Boosting. Vaccines 2020, 8, 729. https://doi.org/10.3390/vaccines8040729
Cole LE, Zhang J, Pacheco KM, Lhéritier P, Anosova NG, Piolat J, Zheng L, Reveneau N. Immunological Distinctions between Acellular and Whole-Cell Pertussis Immunizations of Baboons Persist for at Least One Year after Acellular Vaccine Boosting. Vaccines. 2020; 8(4):729. https://doi.org/10.3390/vaccines8040729
Chicago/Turabian StyleCole, Leah E., Jinrong Zhang, Kristl M. Pacheco, Philippe Lhéritier, Natalie G. Anosova, Julie Piolat, Lingyi Zheng, and Nathalie Reveneau. 2020. "Immunological Distinctions between Acellular and Whole-Cell Pertussis Immunizations of Baboons Persist for at Least One Year after Acellular Vaccine Boosting" Vaccines 8, no. 4: 729. https://doi.org/10.3390/vaccines8040729
APA StyleCole, L. E., Zhang, J., Pacheco, K. M., Lhéritier, P., Anosova, N. G., Piolat, J., Zheng, L., & Reveneau, N. (2020). Immunological Distinctions between Acellular and Whole-Cell Pertussis Immunizations of Baboons Persist for at Least One Year after Acellular Vaccine Boosting. Vaccines, 8(4), 729. https://doi.org/10.3390/vaccines8040729




