Nanoparticles (PLGA and Chitosan)-Entrapped ADP-Ribosylation Factor 1 of Haemonchus contortus Enhances the Immune Responses in ICR Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Materials
2.3. Animals
2.4. Preparation of Recombinant Protein of H. contortus (rHcARF1)
2.5. Preparation of Antigen-Loaded PLGA and CS NP
2.6. Characterization of Antigen-Loaded NP
2.6.1. Loading Capacity (LC), Encapsulation Efficiency (EE), and Cumulative Release
2.6.2. Scanning Electron Microscopy for Morphology, Size, and Zeta Potential Measurements of Antigen-Loaded NP
2.6.3. Integrity of the Antigen–NP Complex
2.7. Immunization Protocol
2.8. Observation of Gross lesions
2.9. Antibody Assays
2.10. Antigen-Specific Cytokines Determination by ELISA
2.11. Lymphocyte Proliferation Assay
2.12. Flow Cytometry
2.13. Statistical Analysis
3. Results
3.1. Characterization of Antigen-Loaded NP
3.1.1. Shape and Zeta Potential of NP
3.1.2. Antigen Integrity Was Not Affected by Vaccine Formulation Procedures
3.1.3. Cumulative Release Assay of Antigen
3.1.4. The Loading Capacity (LC) and Encapsulation Efficiency (EE) of Antigen-Loaded NP
3.2. Safety Assessment of Vaccination
3.3. Evaluation of Antigen-Specific Serum Antibodies Induced by Nanovaccine
3.4. Modulation of rHcARF1-Specific Cytokine Production
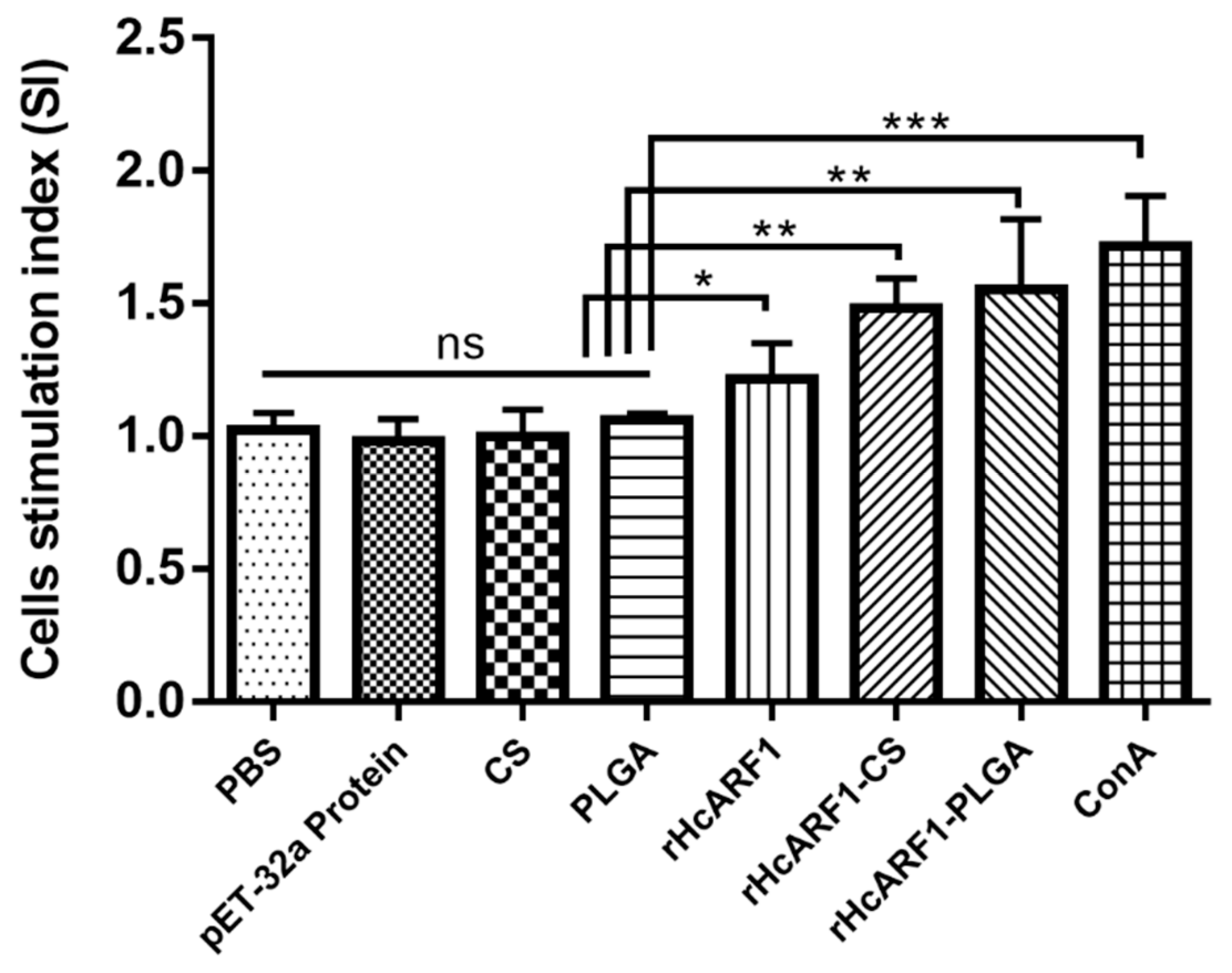
3.5. Lymphocyte Proliferation Induced by Antigen and Antigen-Loaded Nanoparticles
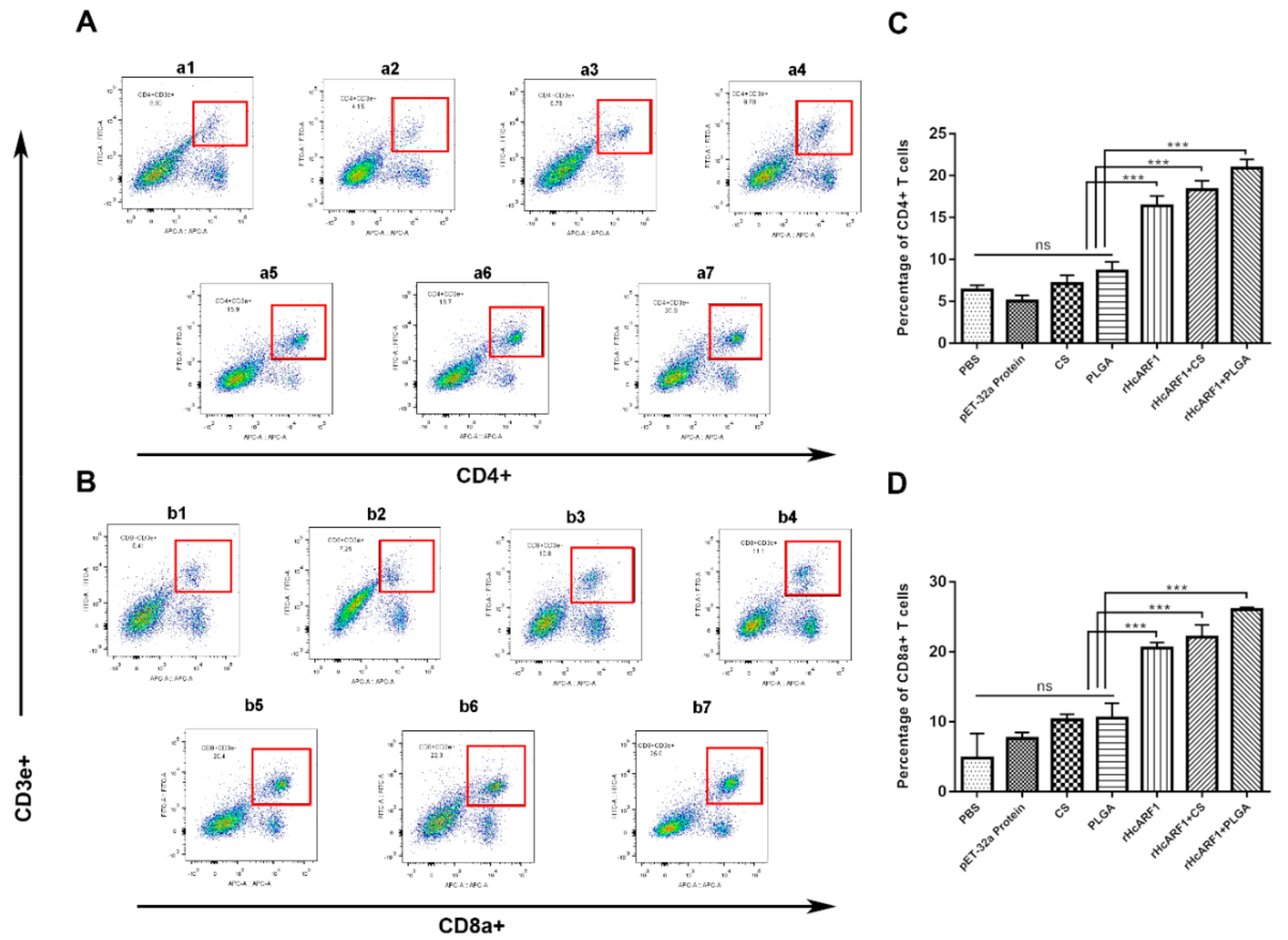
3.6. Antigen and Antigen-Loaded Nanovaccine Promoted CD4+ and CD8+ T Cells Stimulation
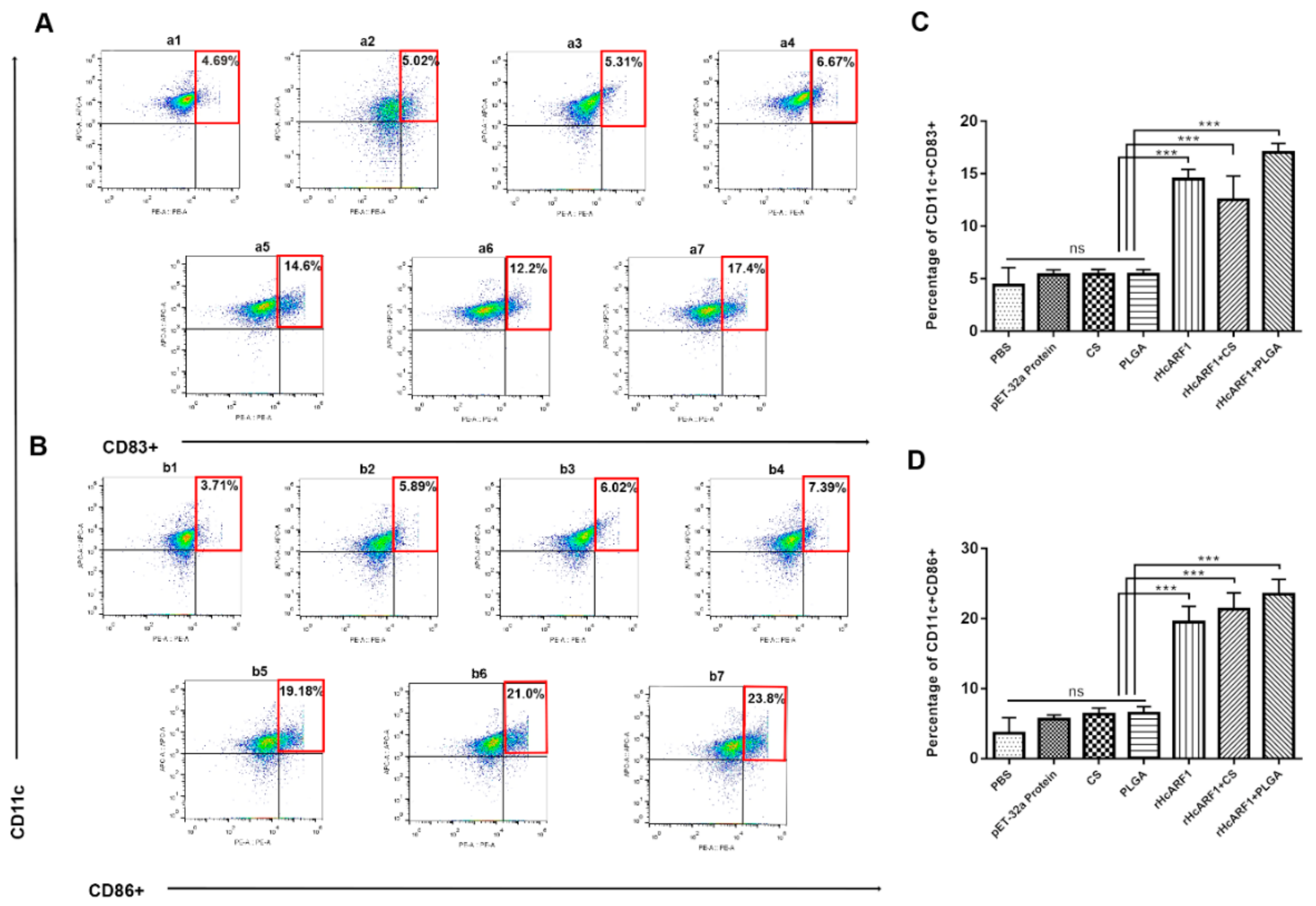
3.7. Antigen and Antigen-Loaded Nanovaccine Induced DC Phenotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Material
Abbreviations
| HcARF1 | Haemonchus contortus (H. contortus), H. contortus ADP-ribosylation factor 1 |
| rHcARF1 | recombinant HcARF1 |
| PLGA | poly (lactic-co-glycolic acid) |
| CS | chitosan |
| NP | nanoparticles |
| ICR | Institute of Cancer Research |
| CFA | complete Freund’s adjuvant |
| ESPs | excretory and secretory products |
| ARF6 | ADP-ribosylation factor 6 |
| APC | antigen-presenting cells |
| DC | dendritic cells |
| ICR | Institute of Cancer Research |
| Mw | molecular weight |
| PBS | phosphate buffer saline |
| PVA | polyvinyl alcohol |
| RT | room temperature |
| LC | loading capacity |
| EE | encapsulation efficiency |
| SEM | scanning electron microscope |
| CM | culture medium |
| NRK | normal rat kidney |
References
- Ehsan, M.; Wang, W.; Gadahi, J.A.; Hasan, M.W.; Lu, M.; Wang, Y.; Liu, X.; Haseeb, M.; Yan, R.; Xu, L.; et al. The Serine/Threonine-Protein Phosphatase 1 from Haemonchus contortus Is Actively Involved in Suppressive Regulatory Roles on Immune Functions of Goat Peripheral Blood Mononuclear Cells. Front. Immunol. 2018, 9, 1627. [Google Scholar] [CrossRef]
- Wang, C.; Li, F.; Zhang, Z.; Yang, X.; Ahmad, A.A.; Li, X.; Du, A.; Hu, M. Recent research progress in China on Haemonchus contortus. Front. Microbiol. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Jasmer, D.P.; Lahmers, K.K.; Brown, W.C. Haemonchus contortus intestine: A prominent source of mucosal antigens. Parasite Immunol. 2007, 29, 139–151. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Wang, S.; Bo, G.; Ehsan, M.; Yan, R.F.; Song, X.K.; Xu, L.X.; Li, X.R. Proteomic analysis of the excretory and secretory proteins of Haemonchus contortus (HcESP) binding to goat PBMCs in vivo revealed stage-specific binding profiles. PLoS ONE 2016, 11, e0159796. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus small GTPase ADP-ribosylation factor 1 (HcARF1) modulate the cell mediated immune response in vitro. Oncotarget 2017, 8, 112211–112221. [Google Scholar] [CrossRef]
- Nie, Z. Arf GAPs and membrane traffic. J. Cell Sci. 2006, 119, 1203–1211. [Google Scholar] [CrossRef]
- Balch, W.E.; Kahn, R.A.; Schwaninger, R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J. Biol. Chem. 1992, 267, 13053–13061. [Google Scholar]
- Puertollano, R.; Randazzo, P.A.; Presley, J.F.; Hartnell, L.M.; Bonifacino, J.S. The GGAs promote ARF-dependent recruitment of Clatrin to the TGN. Cell 2001, 105, 93–102. [Google Scholar] [CrossRef]
- Schlienger, S.; Ramirez, R.A.M.; Claing, A. ARF1 regulates adhesion of MDA-MB-231 invasive breast cancer cells through formation of focal adhesions. Cell. Signal. 2015, 27, 403–415. [Google Scholar] [CrossRef]
- Sanda, M.; Ohara, N.; Kamata, A.; Hara, Y.; Tamaki, H.; Sukegawa, J.; Yanagisawa, T.; Fukunaga, K.; Kondo, H.; Sakagami, H. Vezatin, a potential target for ADP-ribosylation factor 6, regulates the dendritic formation of hippocampal neurons. Neurosci. Res. 2010, 67, 126–136. [Google Scholar] [CrossRef]
- Liu, W.; Han, F.; Zhang, X. Ran GTPase regulates hemocytic phagocytosis of shrimp by interaction with myosin. J. Proteome Res. 2009, 8, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zong, R.; Xu, J.; Zhang, X. Antiviral phagocytosis is regulated by a novel rab-dependent complex in shrimp Penaeus japonicus. J. Proteome Res. 2008, 7, 424–431. [Google Scholar] [CrossRef]
- Ding, Z.F.; Ren, J.; Tan, J.M.; Wang, Z.; Yin, S.W.; Huang, Y.; Huang, X.; Wang, W.; Lan, J.F.; Ren, Q. Characterization of two novel ADP ribosylation factors from giant freshwater prawn Macrobrachium rosenbergii and their responses to WSSV challenge. Dev. Comp. Immunol. 2015, 48, 204–209. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Mangu, V.R.; Zandkarimi, H.; Prasad, M.; Baisakh, N. Structure, organization and evolution of ADP-ribosylation factors in rice and foxtail millet, and their expression in rice. Sci. Rep. 2016, 6, 24008. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Mine, A.; Taniguchi, T.; Kaido, M.; Mise, K.; Taniguchi, H.; Okuno, T. ADP Ribosylation Factor 1 Plays an Essential Role in the Replication of a Plant RNA Virus. J. Virol. 2013, 87, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Xu, L.; Yan, R.; Song, X.; Li, X. Vaccination of goats with glyceraldehyde-3-phosphate dehydrogenase DNA vaccine induced partial protection against Haemonchus contortus. Vet. Immunol. Immunopathol. 2012, 149, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Sun, W.; Song, X.; Xu, L.; Li, X. Vaccination of goats with DNA vaccine encoding Dim-1 induced partial protection against Haemonchus contortus: A preliminary experimental study. Res. Vet. Sci. 2013, 95, 189–199. [Google Scholar] [CrossRef]
- Fawzi, E.M.; González-Sánchez, M.E.; Corral, M.J.; Alunda, J.M.; Cuquerella, M. Vaccination of lambs with the recombinant protein rHc23 elicits significant protection against Haemonchus contortus challenge. Vet. Parasitol. 2015, 211, 54–59. [Google Scholar] [CrossRef]
- Meier, L.; Torgerson, P.R.; Hertzberg, H. Vaccination of goats against Haemonchus contortus with the gut membrane proteins H11/H-gal-GP. Vet. Parasitol. 2016, 229, 15–21. [Google Scholar] [CrossRef]
- Danesh-Bahreini, M.A.; Shokri, J.; Samiei, A.; Kamali-Sarvestani, E.; Barzegar-Jalali, M.; Mohammadi-Samani, S. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomed. 2011, 6, 835–842. [Google Scholar] [CrossRef]
- Melén, K.; Kakkola, L.; He, F.; Airenne, K.; Vapalahti, O.; Karlberg, H.; Mirazimi, A.; Julkunen, I. Production, purification and immunogenicity of recombinant Ebola virus proteins—A comparison of Freund’s adjuvant and adjuvant system 03. J. Virol. Methods 2017, 242, 35–45. [Google Scholar] [CrossRef]
- Jiao, X.D.; Cheng, S.; Hu, Y.H.; Sun, L. Comparative study of the effects of aluminum adjuvants and Freund’s incomplete adjuvant on the immune response to an Edwardsiella tarda major antigen. Vaccine 2010, 28, 1832–1837. [Google Scholar] [CrossRef]
- Wieber, A.; Selzer, T.; Kreuter, J. Characterisation and stability studies of a hydrophilic decapeptide in different adjuvant drug delivery systems: A comparative study of PLGA nanoparticles versus chitosan-dextran sulphate microparticles versus DOTAP-liposomes. Int. J. Pharm. 2011, 421, 151–159. [Google Scholar] [CrossRef]
- Ho, N.I.; Huis In ’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Singh, M.; Gupta, R.K. Poly(lactide-co-glycolide) microparticles for the development of single- dose controlled-release vaccines. Adv. Drug Deliv. Rev. 1998, 32, 225–246. [Google Scholar] [CrossRef]
- Hook, S.; McBurney, W.; Saupe, A.; Rades, T.; Gordon, S. Comparison of chitosan nanoparticles and chitosan hydrogels for vaccine delivery. J. Pharm. Pharmacol. 2008, 60, 1591–1600. [Google Scholar]
- Singh, M.; Singh, A.; Talwar, G.P. Controlled Delivery of Diphtheria Toxoid Using Biodegradable Poly(D,L-Lactide) Microcapsules. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1991, 8, 958–961. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shaffie, K.; Moustafa, A.; Saleh, N.; Nasr, H. Effect of Polyvinyl Alcohol of Different Molecular Weights as Protective Colloids on the Kinetics of the Emulsion Polymerization of Vinyl Acetate. J. Am. Sci. 2010, 6, 1202–1212. [Google Scholar]
- Garinot, M.; Fiévez, V.; Pourcelle, V.; Stoffelbach, F.; des Rieux, A.; Plapied, L.; Theate, I.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J. Control. Release 2007, 120, 195–204. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, G.; Shi, X.M.; Gao, T.T.; Li, W.; Zhao, Y.; Zhang, F.Q.; Wu, J.; Cui, X.; Wang, Y.F. Preparation and Efficacy of a Live Newcastle Disease Virus Vaccine Encapsulated in Chitosan Nanoparticles. PLoS ONE 2012, 7, e53314. [Google Scholar] [CrossRef]
- Debnath, S.K.; Saisivam, S.; Debanth, M.; Omri, A. Development and evaluation of Chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS ONE 2018, 13, e0190976. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y.; Huang, R.; Gao, L. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015–5022. [Google Scholar] [CrossRef]
- Luo, L.; Qin, T.; Huang, Y.; Zheng, S.; Bo, R.; Liu, Z.; Xing, J.; Hu, Y.; Liu, J.; Wang, D. Exploring the immunopotentiation of chinese yam polysaccharide poly(Lactic-co-glycolic acid) nanoparticles in an ovalbumin vaccine formulation in vivo. Drug Deliv. 2017, 24, 1099–1111. [Google Scholar] [CrossRef]
- Salvador, A.; Sandgren, K.J.; Liang, F.; Thompson, E.A.; Koup, R.A.; Pedraz, J.L.; Hernandez, R.M.; Loré, K.; Igartua, M. Design and evaluation of surface and adjuvant modified PLGA microspheres for uptake by dendritic cells to improve vaccine responses. Int. J. Pharm. 2015, 496, 371–381. [Google Scholar] [CrossRef]
- Liang, X.; Duan, J.; Li, X.; Zhu, X.; Chen, Y.; Wang, X.; Sun, H.; Kong, D.; Li, C.; Yang, J. Improved vaccine-induced immune responses: Via a ROS-triggered nanoparticle-based antigen delivery system. Nanoscale 2018, 10, 9489–9503. [Google Scholar] [CrossRef]
- Gilavand, F.; Marzban, A.; Ebrahimipour, G.; Soleimani, N.; Goudarzi, M. Designation of chitosan nano-vaccine based on MxiH antigen of Shigella flexneri with increased immunization capacity. Carbohydr. Polym. 2020, 232, 115813. [Google Scholar] [CrossRef]
- Zupančič, E.; Curato, C.; Paisana, M.; Rodrigues, C.; Porat, Z.; Viana, A.S.; Afonso, C.A.M.; Pinto, J.; Gaspar, R.; Moreira, J.N.; et al. Rational design of nanoparticles towards targeting antigen-presenting cells and improved T cell priming. J. Control. Release 2017, 258, 182–195. [Google Scholar] [CrossRef]
- Han, K.; Xu, L.; Yan, R.; Song, X.; Li, X. Molecular cloning, expression and characterization of enolase from adult Haemonchus contortus. Res. Vet. Sci. 2012, 92, 259–265. [Google Scholar] [CrossRef]
- Sun, G.G.; Wang, Z.Q.; Liu, C.Y.; Jiang, P.; Liu, R.D.; Wen, H.; Qi, X.; Wang, L.; Cui, J. Early serodiagnosis of trichinellosis by ELISA using excretory-secretory antigens of Trichinella spiralis adult worms. Parasites Vectors 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Xu, Y.; Wang, M.; Chen, J.; Huang, S.Y.; Gao, Q.; Zhu, X.Q. Vaccination with Toxoplasma gondii calcium-dependent protein kinase 6 and rhoptry protein 18 encapsulated in poly(lactide-co-glycolide) microspheres induces long-term protective immunity in mice. BMC Infect. Dis. 2016, 16, 168. [Google Scholar] [CrossRef]
- Nikbakht, M.; Pakbin, B.; Nikbakht Brujeni, G. Evaluation of a new lymphocyte proliferation assay based on cyclic voltammetry; an alternative method. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Das, G.; Augustine, M.M.; Das, J.; Bottomly, K.; Ray, P.; Ray, A. An important regulatory role for CD4+CD8αα T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2003, 100, 5324–5329. [Google Scholar] [CrossRef]
- Szczepanik, M.; Bryniarski, K.; Tutaj, M.; Ptak, M.; Skrzeczynska, J.; Askenase, P.W.; Ptak, W. Epicutaneous immunization induces αβ T-cell receptor CD4 CD8 double-positive non-specific suppressor T cells that inhibit contact sensitivity via transforming growth factor-β. Immunology 2005, 115, 42–54. [Google Scholar] [CrossRef]
- Orsini, G.; Legitimo, A.; Failli, A.; Massei, F.; Biver, P.; Consolini, R. Enumeration of human peripheral blood dendritic cells throughout the life. Int. Immunol. 2012, 24, 347–356. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Bacon, A.; Makin, J.; Sizer, P.J.; Jabbal-Gill, I.; Hinchcliffe, M.; Illum, L.; Chatfield, S.; Roberts, M. Carbohydrate biopolymers enhance antibody responses to mucosally delivered vaccine antigens. Infect. Immun. 2000, 68, 5764–5770. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef]
- Gogev, S.; De Fays, K.; Versali, M.F.; Gautier, S.; Thiry, E. Glycol chitosan improves the efficacy of intranasally administrated replication defective human adenovirus type 5 expressing glycoprotein D of bovine herpesvirus 1. Vaccine 2004, 22, 1946–1953. [Google Scholar] [CrossRef]
- Jabbal-Gill, I.; Fisher, A.N.; Rappuoli, R.; Davis, S.S.; Illum, L. Stimulation of mucosal and systemic antibody responses against Bordetella pertussis filamentous haemagglutinin and recombinant pertussis toxin after nasal administration with chitosan in mice. Vaccine 1998, 16, 2039–2046. [Google Scholar] [CrossRef]
- Moore, A.; McGuirk, P.; Adams, S.; Jones, W.C.; Paul McGee, J.; O’Hagan, D.T.; Mills, K.H.G. Immunization with a soluble recombinant HIV protein entrapped in biodegradable microparticles induces HIV-specific CD8+ cytotoxic T lymphocytes and CD4+ Th1 cells. Vaccine 1995, 13, 1741–1749. [Google Scholar] [CrossRef]
- Silva, J.M.; Videira, M.; Gaspar, R.; Préat, V.; Florindo, H.F. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J. Control. Release 2013, 168, 179–199. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Feczkó, T.; Tóth, J.; Dósa, G.; Gyenis, J. Optimization of protein encapsulation in PLGA nanoparticles. Chem. Eng. Process. Process Intensif. 2011, 50, 757–765. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Proc. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable Particles as Vaccine Delivery Systems: Size Matters. AAPS J. 2012, 15, 85–94. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Kudelko, M.; Brault, J.B.; Kwok, K.; Li, M.Y.; Pardigon, N.; Peiris, J.S.M.; Bruzzone, R.; Despre, P.; Nal, B.; Wang, P.G. Class II ADP-ribosylation factors are required for efficient secretion of dengue viruses. J. Biol. Chem. 2012, 287, 767–777. [Google Scholar] [CrossRef]
- Schoelermann, J.; Burtey, A.; Allouni, Z.E.; Gerdes, H.H.; Cimpan, M.R. Contact-dependent transfer of TiO2 nanoparticles between mammalian cells. Nanotoxicology 2016, 10, 1–12. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. THE IL-4 RECEPTOR: Signaling Mechanisms and Biologic Functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Wan, Y.Y. GATA3: A master of many trades in immune regulation. Trends Immunol. 2014, 35, 233–242. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Tian, L.; Chen, Y. Cytokine-Mediated Immunopathogenesis of Hepatitis B Virus Infections. Clin. Rev. Allergy Immunol. 2016, 50, 41–54. [Google Scholar] [CrossRef]
- Pulendran, B.; Smith, J.L.; Caspary, G.; Brasel, K.; Pettit, D.; Maraskovsky, E.; Maliszewski, C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 1036–1041. [Google Scholar] [CrossRef]
- Maldonado-López, R.; De Smedt, T.; Michel, P.; Godfroid, J.; Pajak, B.; Heirman, C.; Thielemans, K.; Leo, O.; Urbain, J.; Moser, M. CD8α+ and CD8α- Subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999, 189, 587–592. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, Z.; Lin, F.; Zou, Z.S.; Xu, R.N.; Jin, L.; Fu, J.L.; Shi, F.; Shi, M.; Wang, H.F.; et al. Interleukin-17-producing CD4+ T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010, 51, 81–91. [Google Scholar] [CrossRef]
- Li, P.; Asokanathan, C.; Liu, F.; Khaing, K.K.; Kmiec, D.; Wei, X.; Song, B.; Xing, D.; Kong, D. PLGA nano/micro particles encapsulated with pertussis toxoid (PTd) enhances Th1/Th17 immune response in a murine model. Int. J. Pharm. 2016, 513, 183–190. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Day, M.J. Immunoglobulin G subclass distribution in canine leishmaniosis: A review and analysis of pitfalls in interpretation. Vet. Parasitol. 2007, 147, 2–8. [Google Scholar] [CrossRef]
- Handman, E. Leishmaniasis: Current status of vaccine development. Clin. Microbiol. Rev. 2001, 14, 229–243. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, P.; Du, T.; Han, K.; Wang, D.; Ye, J.; Xiao, S.; Ye, X.; Wang, Y. Preparation of Modified Konjac Glucomannan Nanoparticles and their Application as Vaccine Adjuvants to Promote Ovalbumin-Induced Immune Response in Mice. Pharm. Res. 2018, 35, 105. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4+T cells in CD8+T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Howard, C.J.; Charleston, B.; Stephens, S.A.; Sopp, P.; Hope, J.C. The role of dendritic cells in shaping the immune response. Anim. Health Res. Rev. 2004, 5, 191–195. [Google Scholar] [CrossRef]
- Mellman, I. Dendritic Cells: Master Regulators of the Immune Response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Saluja, S.S.; Hanlon, D.J.; Sharp, F.A.; Hong, E.; Khalil, D.; Robinson, E.; Tigelaar, R.; Fahmy, T.M.; Edelson, R.L. Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen. Int. J. Nanomed. 2014, 9, 5231–5246. [Google Scholar] [CrossRef]

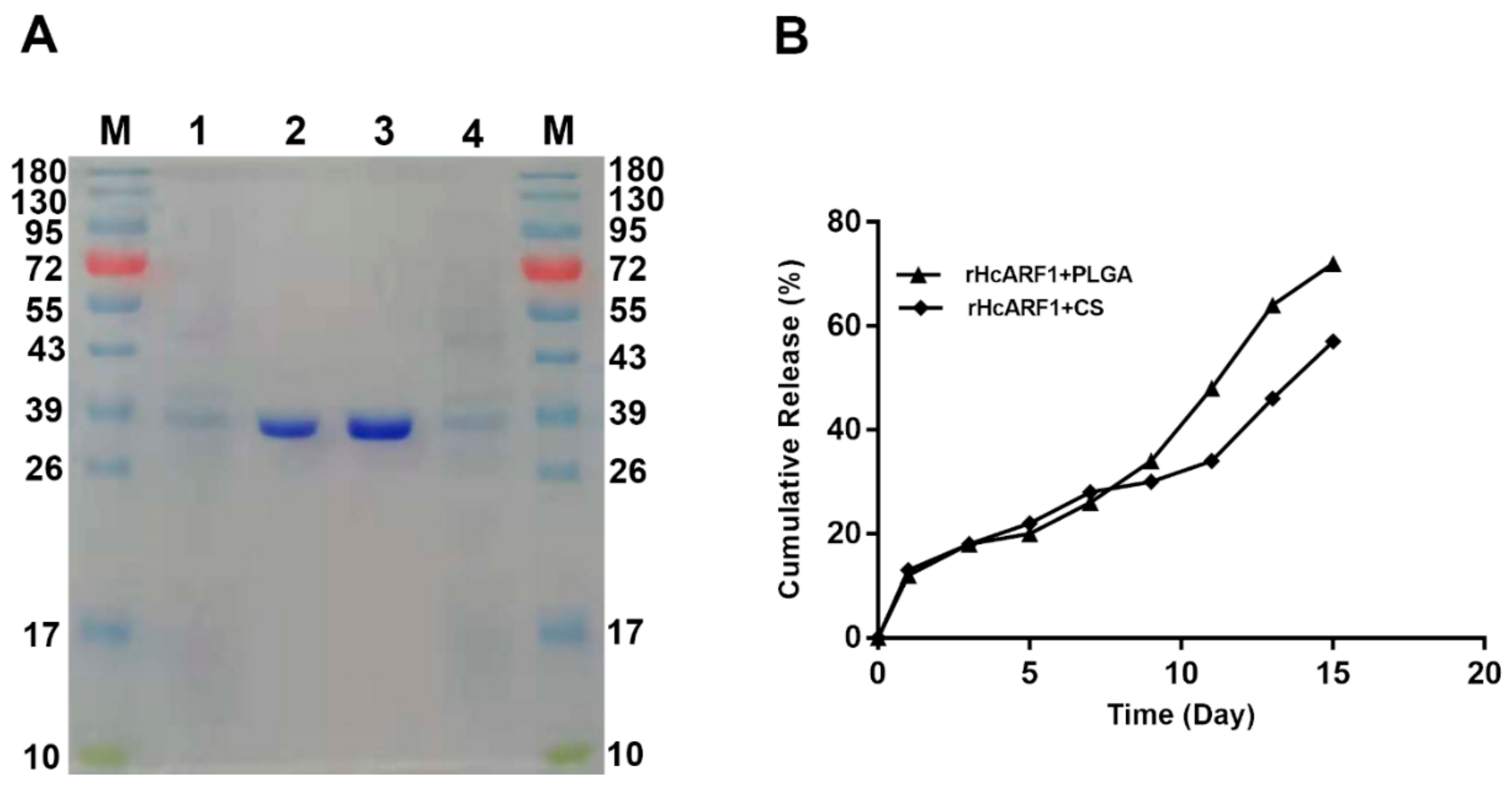
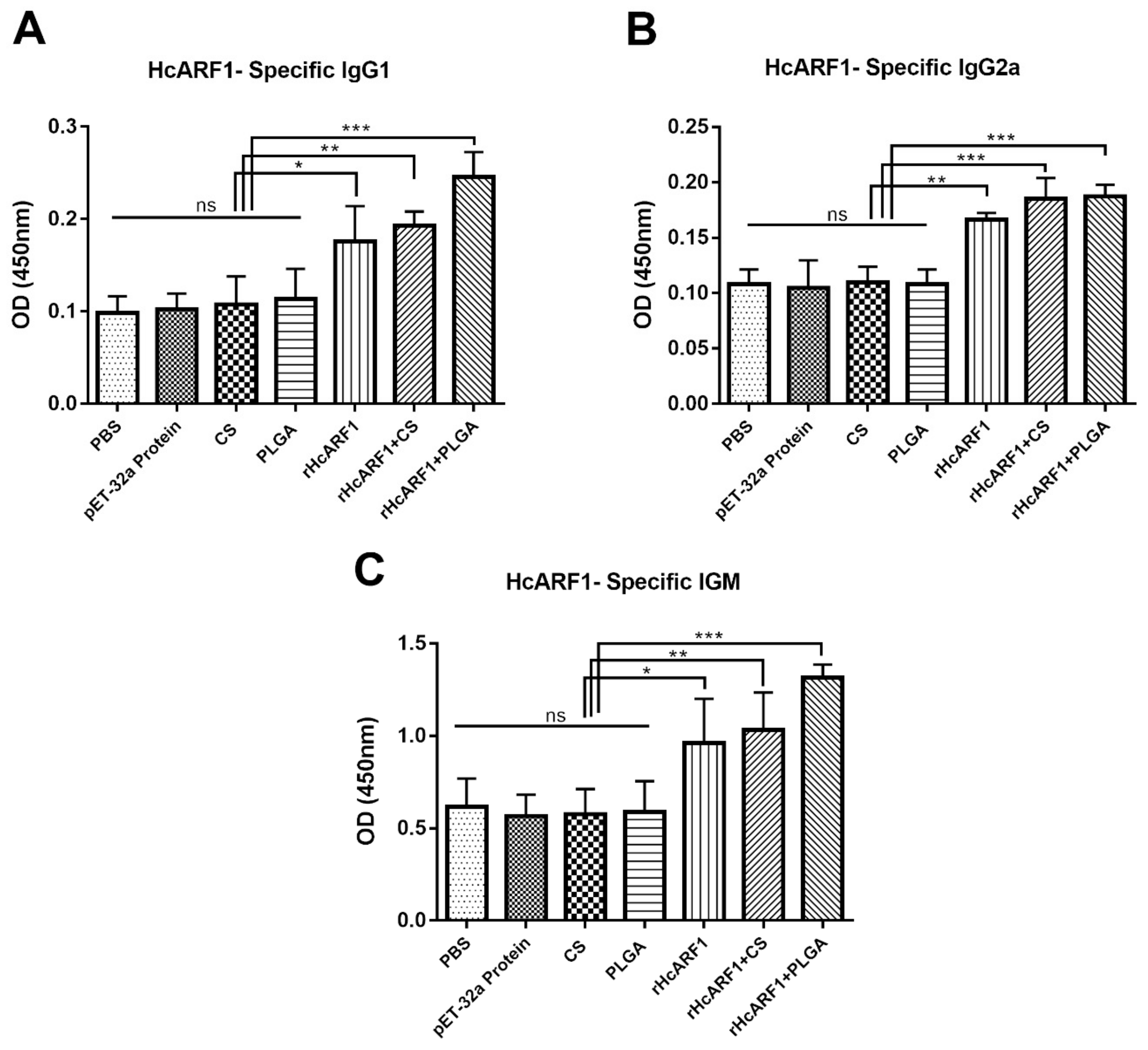
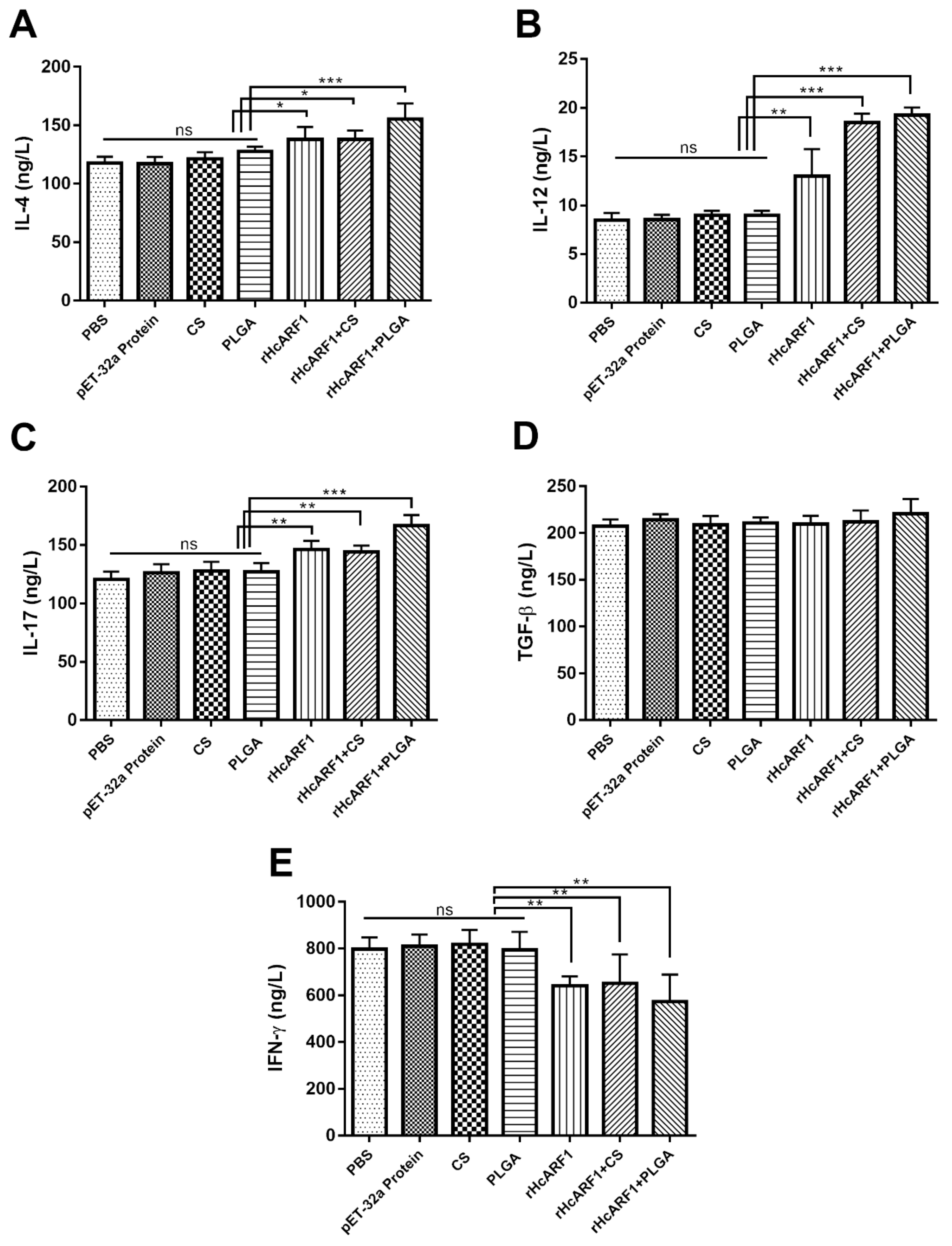
| Groups | Inoculations | Injection at 0 Day | Purpose of Injection |
|---|---|---|---|
| 1 | PBS | 1 | Blank control |
| 2 | pET-32a Protein | 1 | Negative control |
| 3 | Chitosan (CS) | 1 | Immunogenicity of chitosan nanoparticles |
| 4 | PLGA | 1 | Immunogenicity of PLGA nanoparticles |
| 5 | rHcARF1 | 1 | Immunogenicity of rHcARF1 |
| 6 | rHcARF1-CS | 1 | Immunogenicity of rHcARF1 with chitosan nanoparticle |
| 7 | rHcARF1-PLGA | 1 | Immunogenicity of rHcARF1 with PLGA nanoparticle |
| NPs | Size (nm) | LC a | EE b | Zeta Potential (mV) |
|---|---|---|---|---|
| rHcARF1 + PLGA | 100 ± 10 | 28.8 | 81.33 | 24 ± 1.8 |
| rHcARF1 + CS | 260 ± 35 | 40.6 | 76 | 18 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.W.; Haseeb, M.; Ehsan, M.; Gadahi, J.A.; Naqvi, M.A.-u.-H.; Wang, Q.Q.; Liu, X.; Lakho, S.A.; Yan, R.; Xu, L.; et al. Nanoparticles (PLGA and Chitosan)-Entrapped ADP-Ribosylation Factor 1 of Haemonchus contortus Enhances the Immune Responses in ICR Mice. Vaccines 2020, 8, 726. https://doi.org/10.3390/vaccines8040726
Hasan MW, Haseeb M, Ehsan M, Gadahi JA, Naqvi MA-u-H, Wang QQ, Liu X, Lakho SA, Yan R, Xu L, et al. Nanoparticles (PLGA and Chitosan)-Entrapped ADP-Ribosylation Factor 1 of Haemonchus contortus Enhances the Immune Responses in ICR Mice. Vaccines. 2020; 8(4):726. https://doi.org/10.3390/vaccines8040726
Chicago/Turabian StyleHasan, Muhammad Waqqas, Muhammad Haseeb, Muhammad Ehsan, Javaid Ali Gadahi, Muhammad Ali-ul-Husnain Naqvi, Qiang Qiang Wang, Xinchao Liu, Shakeel Ahmed Lakho, Ruofeng Yan, Lixin Xu, and et al. 2020. "Nanoparticles (PLGA and Chitosan)-Entrapped ADP-Ribosylation Factor 1 of Haemonchus contortus Enhances the Immune Responses in ICR Mice" Vaccines 8, no. 4: 726. https://doi.org/10.3390/vaccines8040726
APA StyleHasan, M. W., Haseeb, M., Ehsan, M., Gadahi, J. A., Naqvi, M. A.-u.-H., Wang, Q. Q., Liu, X., Lakho, S. A., Yan, R., Xu, L., Song, X., & Li, X. (2020). Nanoparticles (PLGA and Chitosan)-Entrapped ADP-Ribosylation Factor 1 of Haemonchus contortus Enhances the Immune Responses in ICR Mice. Vaccines, 8(4), 726. https://doi.org/10.3390/vaccines8040726










