p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer—A Comparative Study with Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Lines, Culture Conditions, and Viability Measures
2.3. Tissue Sources, Protein Extraction, and Western Immunoblotting
2.4. Statistics
3. Results
3.1. Increased p38γ and pp38γ in CRC Patients Compared to Normal Subjects
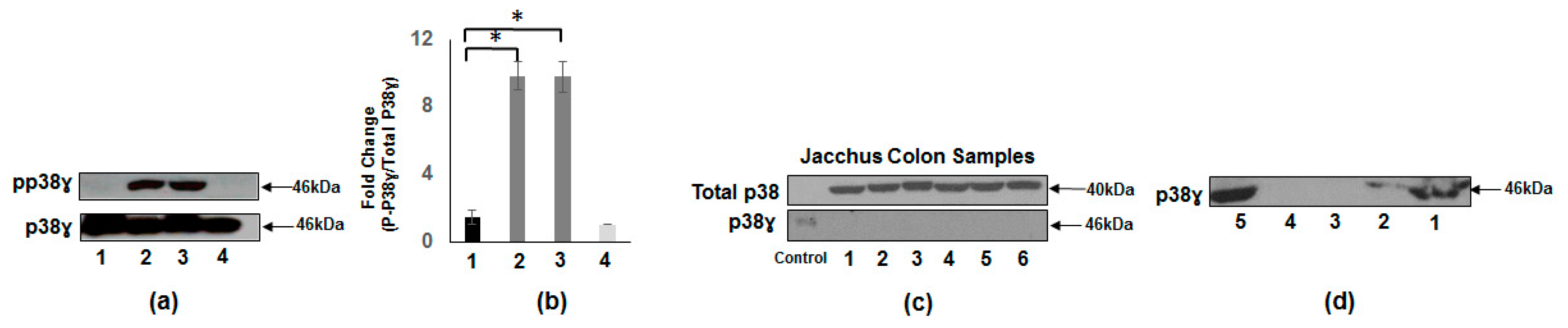
3.2. Presence of p38γ Protein and pp38γ in Preclinical Tissues Compared to Human Colon Cancer Specimens
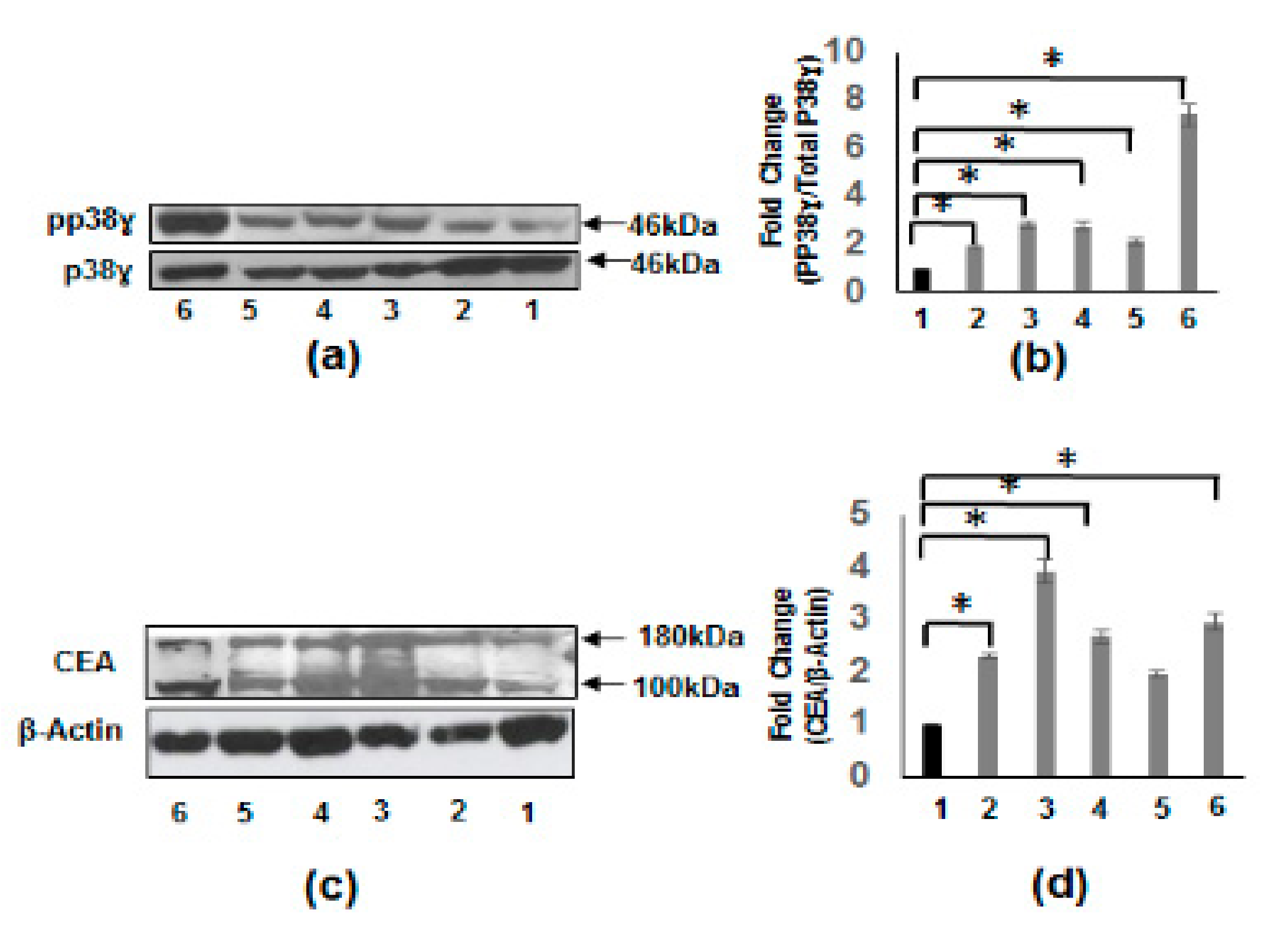
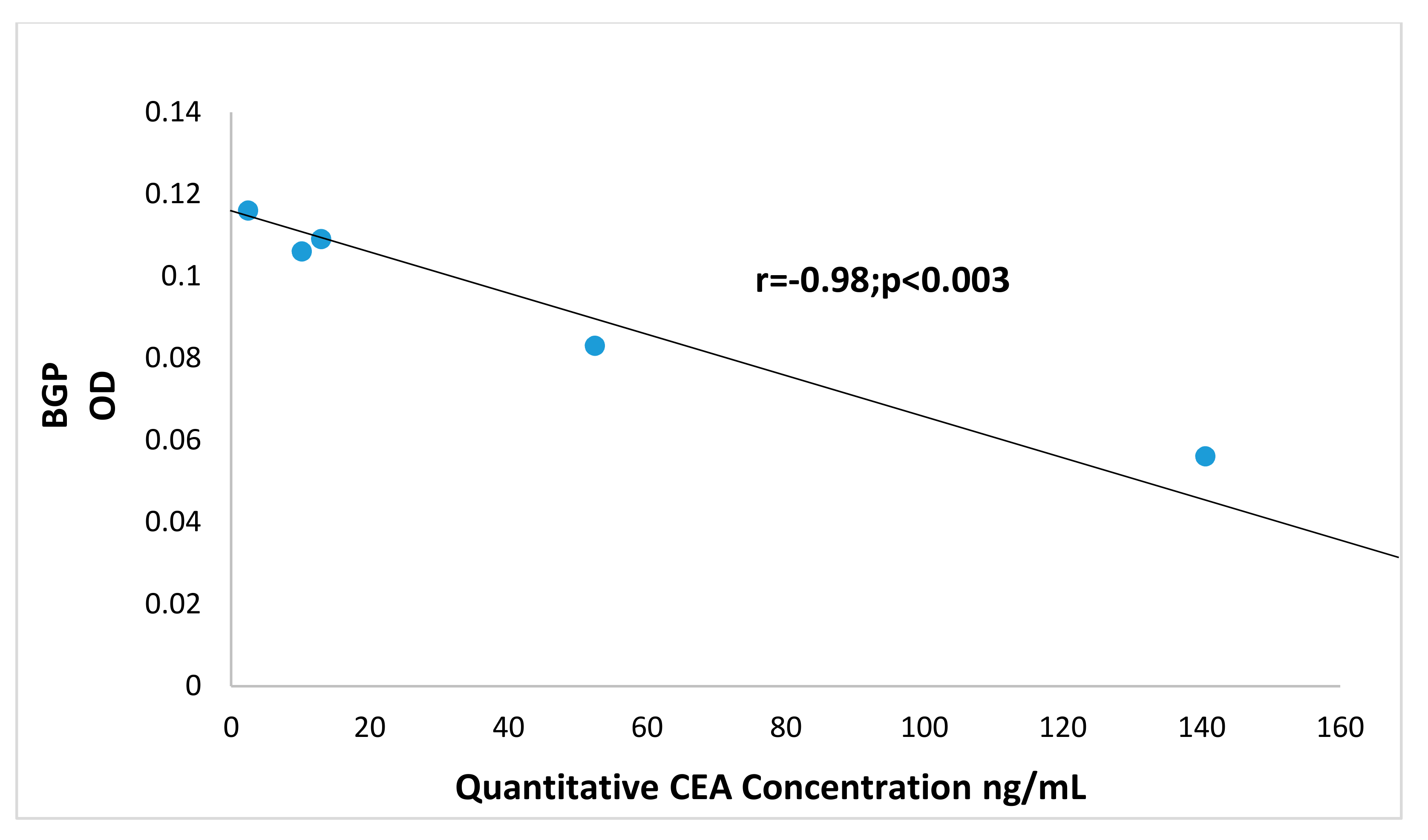
3.3. Increased Carcinoembryonic Antigen (CEA) and Biliary Glycoprotein (BGP) in CRC Patients
3.4. Increased pp38γ and CEA Protein Levels in CRC Cell Lines
3.5. Losmapimod (LOS) and Doramapimod (DORA) Inhibition of CRC Cell Growth
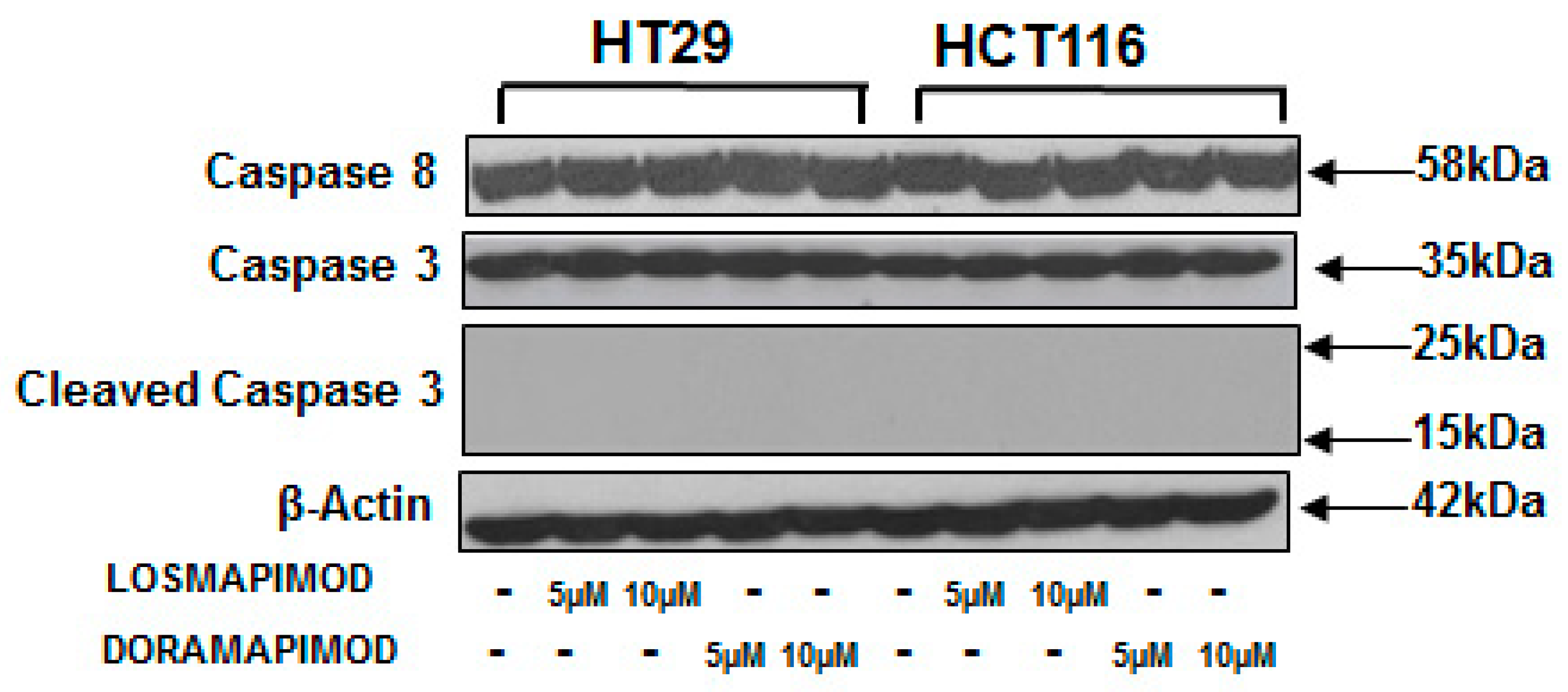
3.6. Inhibition of p38 MAPK by Losmapimod and Doramapimod Has no Effect on Caspase 8 and 3 Activities
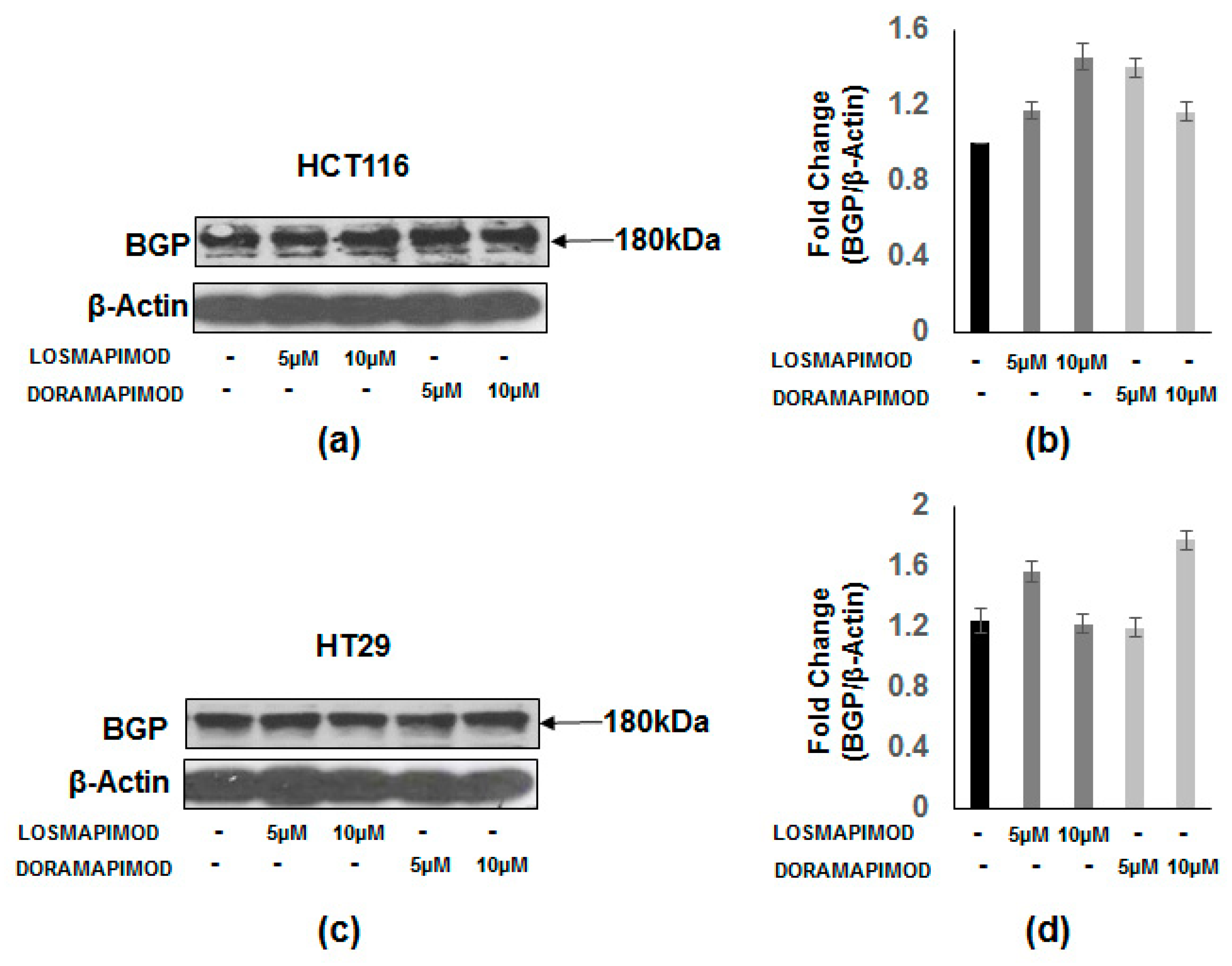
3.7. Inhibition of p38 MAPK by Losmapimod and Doramapimod Has no Effect on BGP Levels
3.8. Expression of Tumor Metastasis-Associated Antigens in Colon Mucosal Extracts of the Cotton Top Tamarin and Common Marmoset (Callitrichidae)
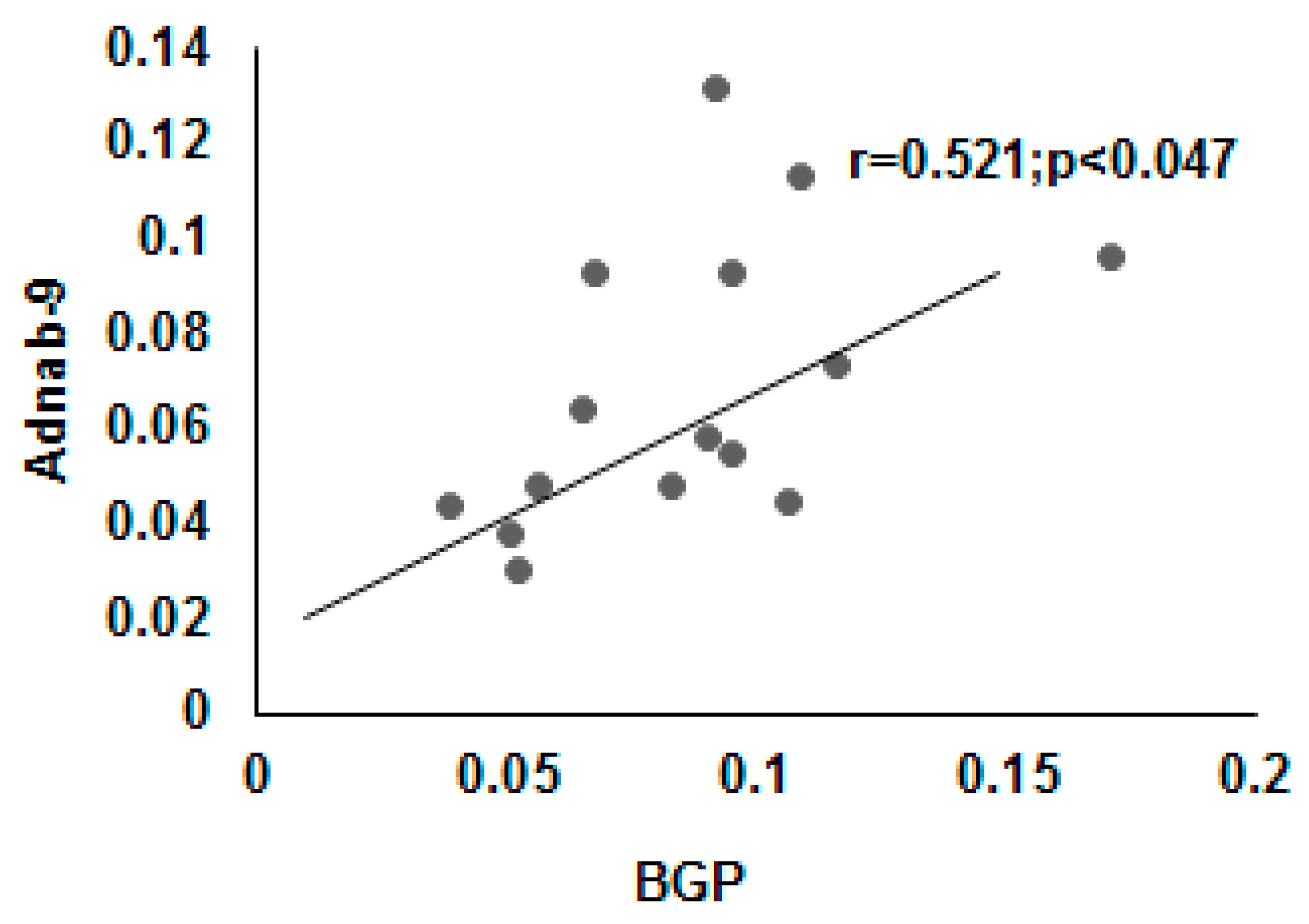
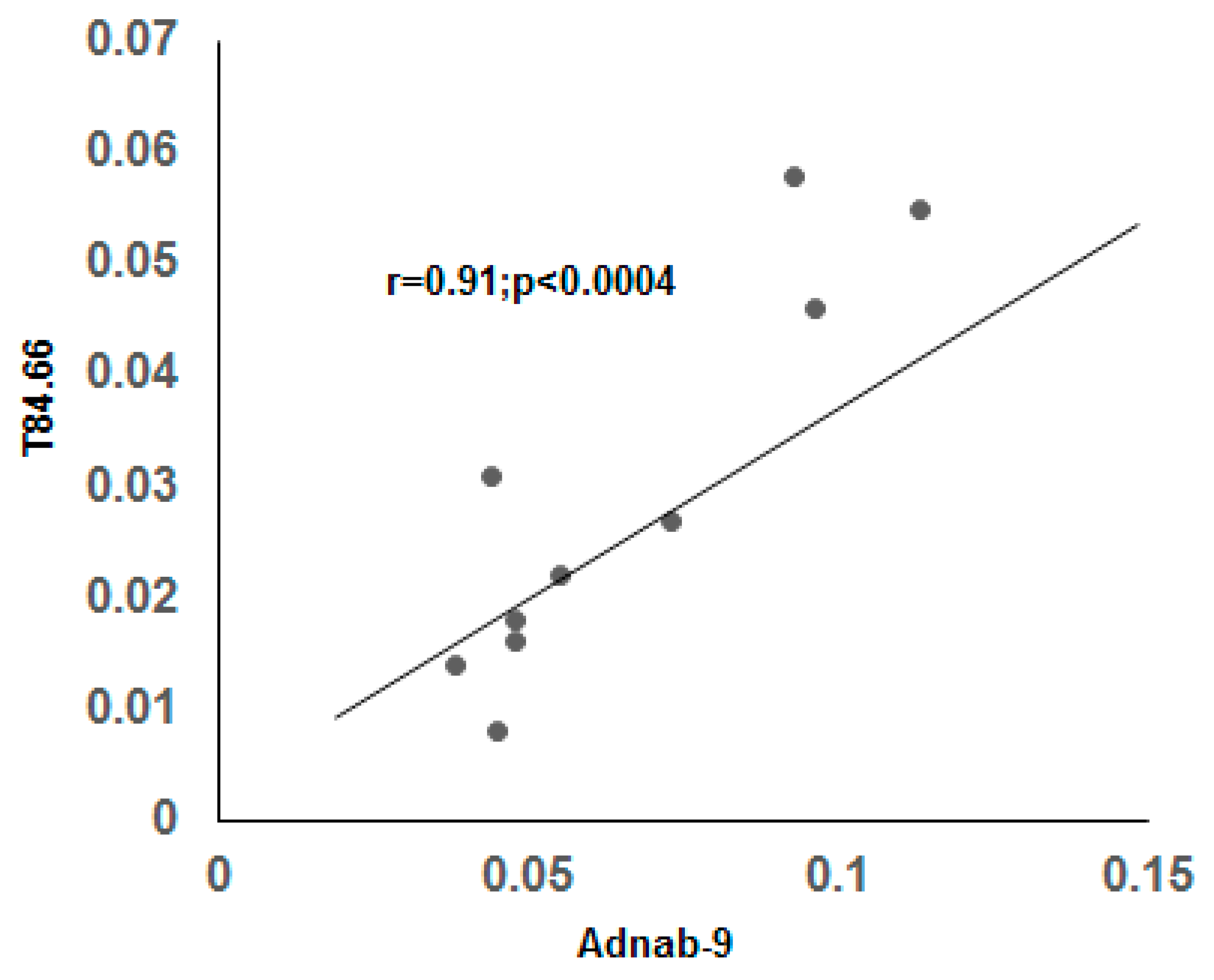
3.9. Correlation of BGP with other Prometastatic Antigens in Preclinical Primate Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Haddad, A.J.; Bani Hani, M.; Pawlik, T.M.; Cunningham, S.C. Colorectal liver metastases. Int. J. Surg. Oncol. 2011, 2011, 285840. [Google Scholar] [CrossRef]
- Kim, G.-A.; Lee, H.C.; Choe, J.; Kim, M.-J.; Lee, M.J.; Chang, H.-S.; Bae, I.Y.; Kim, H.-K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Patel, N.; Zhang, H.-J. The progress of non-alcoholic fatty liver disease as the risk of liver metastasis in colorectal cancer. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.M.; Gould, J.J.; Kubik, J.L.; Talmon, G.A.; Casey, C.A.; Thomas, P.; Tuma, D.J.; McVicker, B.L. Enhanced colorectal cancer metastases in the alcohol-injured liver. Clin. Exp. Metastasis 2017, 34, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Van der Eynden, G.G.; Majeed, A.W.; Illemann, M.; Vermeulen, P.; Bird, N.C.; Høyer-Hansen, G.; Eefsen, R.L.; Reynolds, A.R.; Brodt, P. The Multifaceted Role of the Microenvironment in Liver Metastasis: Biology and Clinical Implications. Cancer Res. 2013, 73, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Tobi, M.; Kim, M.; Zimmer, R.; Hatfield, J.; Kam, M.; Khoury, N.; Carville, A.; Lawson, M.J.; Schiemann, W.P.; Thomas, P. Colorectal cancer in the cotton top tamarin (Saguinus oedipus): How do they evade liver metastasis? Dig. Dis. Sci. 2011, 56, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, Y.; Nudelman, E.; Levery, S.B.; Hakomori, S.; Rauvala, H. Novel fucolipids accumulating in human adenocarcinoma. III. A hybridoma antibody (FH6) defining a human cancer-associated difucoganglioside (VI3NeuAcV3III3Fuc2nLc6). J. Biol. Chem. 1984, 259, 10511–10517. [Google Scholar]
- Tobi, M.; Elitsur, Y.; Moyer, M.P.; Halline, A.; Deutsch, M.; Nochomovitz, L.; Luk, G.D. Mucosal origin and shedding of an early colonic tumor marker defined by Adnab-9 monoclonal antibody. Scand. J. Gastroenterol. 1993, 28, 1025–1034. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef]
- Kuespert, K.; Pils, S.; Hauck, C.R. CEACAMs: Their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 2006, 18, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Aldulaymi, B.; Byström, P.; Berglund, Å.; Christensen, I.J.; Brünner, N.; Nielsen, H.J.; Glimelius, B. High Plasma TIMP-1 and Serum CEA Levels during Combination Chemotherapy for Metastatic Colorectal Cancer Are Significantly Associated with Poor Outcome. Oncology 2010, 79, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ieda, J.; Yokoyama, S.; Tamura, K.; Takifuji, K.; Hotta, T.; Matsuda, K.; Oku, Y.; Nasu, T.; Kiriyama, S.; Yamamoto, N.; et al. Re-expression of CEACAM1 long cytoplasmic domain isoform is associated with invasion and migration of colorectal cancer. Int. J. Cancer 2011, 129, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, T.J.; Mao, W.; Karl, R.C. Biliary glycoprotein is overexpressed in human colon cancer cells with high metastatic potential. J. Gastrointest. Surg. 1997, 1, 292–298. [Google Scholar] [CrossRef]
- Del Reino, P.; Alsina-Beauchamp, D.; Escos, A.; Cerezo-Guisado, M.I.; Risco, A.; Aparicio, N.; Zur, R.; Fernandez-Estevez, M.; Collantes, E.; Montans, J.; et al. Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38gamma and p38delta, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res. 2014, 74, 6150–6160. [Google Scholar] [CrossRef]
- Hou, S.-W.; Zhi, H.-Y.; Pohl, N.; Loesch, M.; Qi, X.-M.; Li, R.-S.; Basir, Z.; Chen, G. PTPH1 dephosphorylates and cooperates with p38gamma MAPK to increase ras oncogenesis through PDZ-mediated interaction. Cancer Res. 2010, 70, 2901–2910. [Google Scholar] [CrossRef]
- Gutkind, J.S. Essential role of p38gamma in K-Ras transformation independent of phosphorylation. Fac. J. Biol. Chem. 2005, 280, 23910–23917. [Google Scholar] [CrossRef]
- Yin, N.; Qi, X.; Tsai, S.; Lu, Y.; Basir, Z.; Oshima, K.; Thomas, J.P.; Myers, C.R.; Stoner, G.; Chen, G. p38gamma MAPK is required for inflammation-associated colon tumorigenesis. Oncogene 2016, 35, 1039–1048. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Chen, N.-C.; Koh, Y.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Pterostilbene Inhibits Adipocyte Conditioned-Medium-Induced Colorectal Cancer Cell Migration through Targeting FABP5-Related Signaling Pathway. J. Agric. Food Chem. 2019, 67, 10321–10329. [Google Scholar] [CrossRef]
- Tomas-Loba, A.; Manieri, E.; Gonzalez-Teran, B.; Mora, A.; Leiva-Vega, L.; Santamans, A.M.; Romero-Becerra, R.; Rodriguez, E.; Pintor-Chocano, A.; Feixas, F.; et al. p38gamma is essential for cell cycle progression and liver tumorigenesis. Nature 2019, 568, 557–560. [Google Scholar] [CrossRef]
- Kim, H.-S.; Berstad, A. Experimental Colitis in Animal Models. Scand. J. Gastroenterol. 1992, 27, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Kuma, Y.; Sabio, G.; Bain, J.; Shpiro, N.; Marquez, R.; Cuenda, A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J. Biol. Chem. 2005, 280, 19472–19479. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, L.; Feng, X.; Wang, C.; Zhang, Y.; Wang, L.; Ding, Y.; Zhao, T. Losmapimod Protected Epileptic Rats From Hippocampal Neuron Damage Through Inhibition of the MAPK Pathway. Front. Pharmacol. 2019, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Ren, Z.; Frank, J.A.; Yang, X.H.; Zhang, Z.; Ke, Z.J.; Shi, X.; Luo, J. Chronic ethanol exposure enhances the aggressiveness of breast cancer: The role of p38gamma. Oncotarget 2016, 7, 3489–3505. [Google Scholar] [CrossRef]
- Tobi, M.; Chintalapani, S.; Kithier, K.; Clapp, N. Carcinoembryonic antigen family of adhesion molecules in the cotton top tamarin (Saguinus oedipus). Cancer Lett. 2000, 157, 45–50. [Google Scholar] [CrossRef]
- Venkatesh, J.; Sekhar, S.C.; Cheriyan, V.T.; Muthu, M.; Meister, P.; Levi, E.; Dzinic, S.; Gauld, J.W.; Polin, L.A.; Rishi, A.K. Antagonizing binding of cell cycle and apoptosis regulatory protein 1 (CARP-1) to the NEMO/IKKgamma protein enhances the anticancer effect of chemotherapy. J. Biol. Chem. 2020, 295, 3532–3552. [Google Scholar] [CrossRef]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Vatter, S.W.L.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nat. Cell Biol. 1994, 372, 739–746. [Google Scholar] [CrossRef]
- Qi, X.; Xie, C.; Hou, S.; Li, G.; Yin, N.; Dong, L.; Lepp, A.; Chesnik, M.A.; Mirza, S.P.; Szabo, A.; et al. Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer. Oncotarget 2014, 5, 4269–4282. [Google Scholar] [CrossRef][Green Version]
- Boucher, D.; Cournoyer, D.; Stanners, C.P.; Fuks, A. Studies on the control of gene expression of the carcinoembryonic antigen family in human tissue. Cancer Res. 1989, 49, 847–852. [Google Scholar]
- Neumaier, M.; Paululat, S.; Chan, A.; Matthaes, P.; Wagener, C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc. Natl. Acad. Sci. USA 1993, 90, 10744–10748. [Google Scholar] [CrossRef]
- Yang, S.Y.; Miah, A.; Sales, K.M.; Fuller, B.; Seifalian, A.M.; Winslet, M. Inhibition of the p38 MAPK pathway sensitises human colon cancer cells to 5-fluorouracil treatment. Int. J. Oncol. 2011, 38, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Qin, X.; Wang, X.; Liu, F.; White, E.; Zheng, X.F. PP2AC Level Determines Differential Programming of p38-TSC-mTOR Signaling and Therapeutic Response to p38-Targeted Therapy in Colorectal Cancer. EBioMedicine 2015, 2, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Tobi, M.; Thomas, P.; Ezekwudo, D. Avoiding hepatic metastasis naturally: Lessons from the cotton top tamarin (Saguinus oedipus). World J. Gastroenterol. 2016, 22, 5479–5494. [Google Scholar] [CrossRef] [PubMed]
- Tobi, M.; Chintalapani, S.; Kithier, K.; Clapp, N. Gastrointestinal tract antigenic profile of cotton-top tamarin, Saguinus oedipus, is similar to that of humans with inflammatory bowel disease. Dig. Dis. Sci. 2000, 45, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Tobi, M.; Kalia, V.; Chintalapani, S.; Kithier, K.; Henke, M.; Clapp, N. An antigenic Profile in Cotton-Top Tamarins-Saguinus oedipus: Model for Human Inflammatory Bowel Disease and Colorectal Cancer. In The Cotton-Top Tamarin: A Primate Model for the Study of Colon Carcinogenesis; Clapp, N., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1993; pp. 113–125. [Google Scholar]
- Han, J.; Lee, J.D.; Bibbs, L.; Ulevitch, R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994, 265, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Han, J. The p38 signal transduction pathway Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nat. Cell Biol. 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Weston, C.R.; Lambright, D.G.; Davis, R.J. Signal transduction. MAP kinase signaling specificity. Science 2002, 296, 2345–2347. [Google Scholar] [CrossRef]
- Qi, X.; Pohl, N.M.; Loesch, M.; Hou, S.; Li, R.; Qin, J.Z.; Cuenda, A.; Chen, G. p38alpha antagonizes p38gamma activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J. Biol. Chem. 2007, 282, 31398–31408. [Google Scholar] [CrossRef]
- Qi, X.; Tang, J.; Loesch, M.; Pohl, N.; Alkan, S.; Chen, G. p38gamma mitogen-activated protein kinase integrates signaling crosstalk between Ras and estrogen receptor to increase breast cancer invasion. Cancer Res. 2006, 66, 7540–7547. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Liu, G.; Kreike, B.; Chen, W.; Sethi, S.; Miller, F.R.; Wu, G. p38gamma mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly (ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia 2011, 13, 472–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosenthal, D.T.; Iyer, H.; Escudero, S.; Bao, L.; Wu, Z.; Ventura, A.C.; Kleer, C.G.; Arruda, E.M.; Garikipati, K.; Merajver, S.D. p38gamma promotes breast cancer cell motility and metastasis through regulation of RhoC GTPase, cytoskeletal architecture, and a novel leading edge behavior. Cancer Res. 2011, 71, 6338–6349. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [PubMed]
- Clapp, N.; Nardi, R.V.; Tobi, M. Future Directions for Colon Research using Cotton-Top Tamarins. In The Cotton-Top Tamarin: A Primate Model for the Study of Colon Carcinogenesis; Clapp, N., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1993; pp. 319–324. [Google Scholar]
- Cohen, B.; Mosbach, E.H.; Henke, M.A.; Clapp, N.K. Fecal steroids in tamarins & marmosets. In The Cotton-Top Tamarin: A Primate Model for the Study of Colon Carcinogenesis; Clapp, N., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1993; pp. 241–251. [Google Scholar]
- Miller, A.D.; Kramer, J.A.; Lin, K.C.; Knight, H.; Martinot, A.; Mansfield, K.G. Small Intestinal Adenocarcinoma in Common Marmosets (Callithrix jacchus). Veter. Pathol. 2010, 47, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Ilantzis, C.; Jothy, S.; Alpert, L.C.; Draber, P.; Stanners, C.P. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab. Investig. 1997, 76, 703–716. [Google Scholar] [PubMed]
- Singer, B.B.; Scheffrahn, I.; Kammerer, R.; Suttorp, N.; Ergun, S.; Slevogt, H. Deregulation of the CEACAM Expression Pattern Causes Undifferentiated Cell Growth in Human Lung Adenocarcinoma Cells. PLoS ONE 2010, 5, e8747. [Google Scholar] [CrossRef]
- Garrett, E.P.; Kurtz, S.R. Clinical utility of oncofetal proteins and hormones as tumor markers. Med Clin. N. Am. 1986, 70, 1295–1306. [Google Scholar] [CrossRef]
- Steele, G.; Zamcheck, N. The use of carcinoembryonic antigen in the clinical management of patients with colorectal cancer. Cancer Detect. Prev. 1985, 8, 421–427. [Google Scholar]
- Thomas, P.; Forse, R.A.; Bajenova, O. Carcinoembryonic antigen (CEA) and its receptor hnRNP M are mediators of metastasis and the inflammatory response in the liver. Clin. Exp. Metastasis 2011, 28, 923–932. [Google Scholar] [CrossRef]
- Toth, A.C.; Thomas, P.; Broitman, A.S.; Zamcheck, N. Receptor-mediated endocytosis of carcinoembryonic antigen by rat liver Kupffer cells. Cancer Res. 1985, 45, 392–397. [Google Scholar]
- McVicker, B.L.; Tuma, D.J.; Lazure, K.E.; Thomas, P.; Casey, C.A. Alcohol, Carcinoembryonic Antigen Processing and Colorectal Liver Metastases. In Biological Basis of Alcohol-Induced Cancer. Advances in Experimental Medicine and Biology; Vasiliou, V., Zakhari, S., Seitz, H., Hoek, J., Eds.; Springer: Cham, Switzerland, 2014; Volume 815, pp. 295–311. [Google Scholar]
- Lv, Y.; Zhang, H.-J. Effect of Non-alcoholic Fatty Liver Disease on the Risk of Synchronous Liver Metastasis: Analysis of 451 Consecutive Patients of Newly Diagnosed Colorectal Cancer. Front. Oncol. 2020, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Yun, H.S.; Chang, W.I.; Kim, J.Y.; Kim, Y.-H.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Chang, D.K. Influence of non-alcoholic fatty liver disease on the prognosis in patients with colorectal cancer. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Finerty, S.; Mackett, M.; Arrand, J.; Watkins, P.; Tarlton, J.; Morgan, A. Immunization of cottontop tamarins and rabbits with a candidate vaccine against the Epstein-Barr virus based on the major viral envelope glycoprotein gp340 and alum. Vaccine 1994, 12, 1180–1184. [Google Scholar] [CrossRef]
- Smith, D.R.; Johnston, S.C.; Piper, A.; Botto, M.; Donnelly, G.; Shamblin, J.; Albariño, C.G.; Hensley, L.E.; Schmaljohn, C.; Nichol, S.T.; et al. Attenuation and efficacy of live-attenuated Rift Valley fever virus vaccine candidates in non-human primates. PLoS Negl. Trop. Dis. 2018, 12, e0006474. [Google Scholar] [CrossRef]
- van den Boorn, J.G.; Hartmann, G. Turning Tumors into Vaccines: Co-opting the Innate Immune System. Immunity 2013, 39, 27–37. [Google Scholar] [CrossRef]
- Falzarano, D.; de Wit, E.; Feldmann, F.; Rasmussen, A.L.; Okumura, A.; Peng, X.; Thomas, M.J.; van Doremalen, N.; Haddock, E.; Nagy, L.; et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014, 10, e1004250. [Google Scholar] [CrossRef]
- Umasuthan, N.; Bathige, S.; Noh, J.K.; Lee, J. Gene structure, molecular characterization and transcriptional expression of two p38 isoforms (MAPK11 and MAPK14) from rock bream (Oplegnathus fasciatus). Fish Shellfish. Immunol. 2015, 47, 331–343. [Google Scholar] [CrossRef]
- Duarte, S.; Momier, D.; Baqué, P.; Casanova, V.; Loubat, A.; Samson, M.; Guigonis, J.-M.; Staccini, P.; Saint-Paul, M.-C.; De Lima, M.P.; et al. Preventive Cancer Stem Cell-Based Vaccination Reduces Liver Metastasis Development in a Rat Colon Carcinoma Syngeneic Model. Stem Cells 2013, 31, 423–432. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talwar, H.; McVicker, B.; Tobi, M. p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer—A Comparative Study with Humans. Vaccines 2020, 8, 720. https://doi.org/10.3390/vaccines8040720
Talwar H, McVicker B, Tobi M. p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer—A Comparative Study with Humans. Vaccines. 2020; 8(4):720. https://doi.org/10.3390/vaccines8040720
Chicago/Turabian StyleTalwar, Harvinder, Benita McVicker, and Martin Tobi. 2020. "p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer—A Comparative Study with Humans" Vaccines 8, no. 4: 720. https://doi.org/10.3390/vaccines8040720
APA StyleTalwar, H., McVicker, B., & Tobi, M. (2020). p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer—A Comparative Study with Humans. Vaccines, 8(4), 720. https://doi.org/10.3390/vaccines8040720



