Development and Assessment of a Pooled Serum as Candidate Standard to Measure Influenza A Virus Group 1 Hemagglutinin Stalk-Reactive Antibodies
Abstract
1. Introduction
2. Materials and Methods
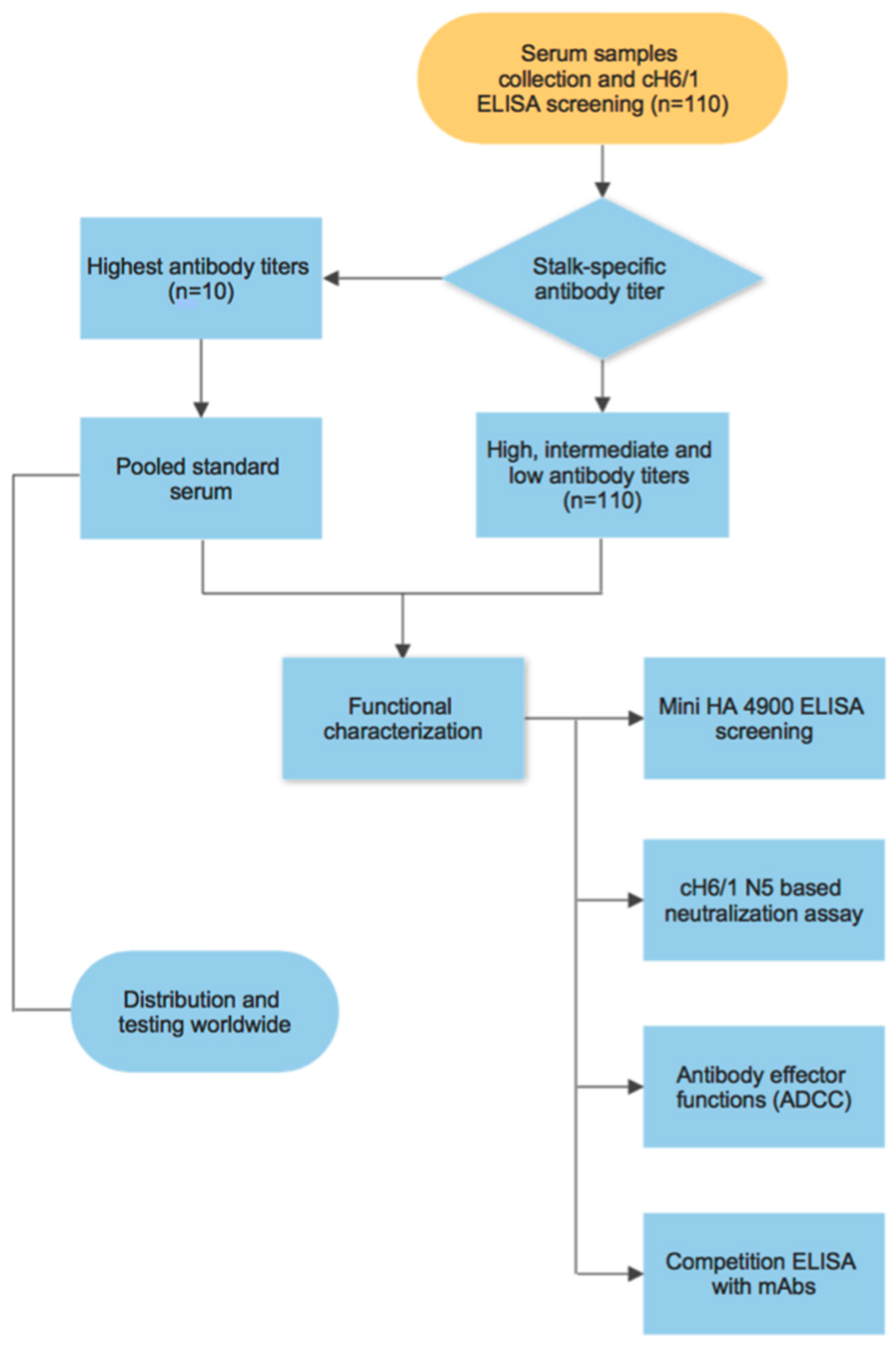
3. Results
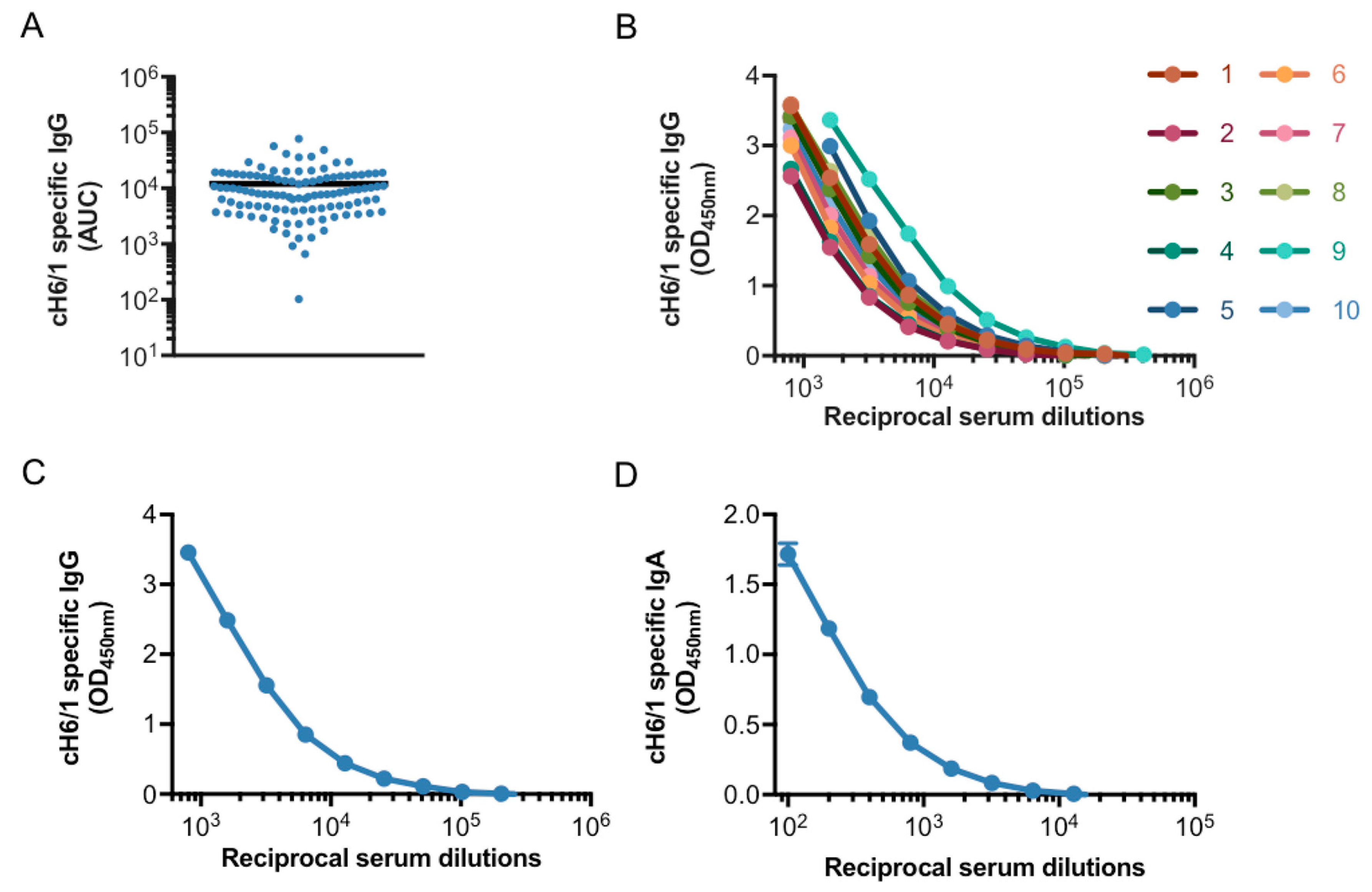
3.1. Generation of a Pooled Serum Containing High Levels of Stalk-Specific Antibodies
3.2. Characterization of Stalk-Specific Antibodies Contained in Serum Samples
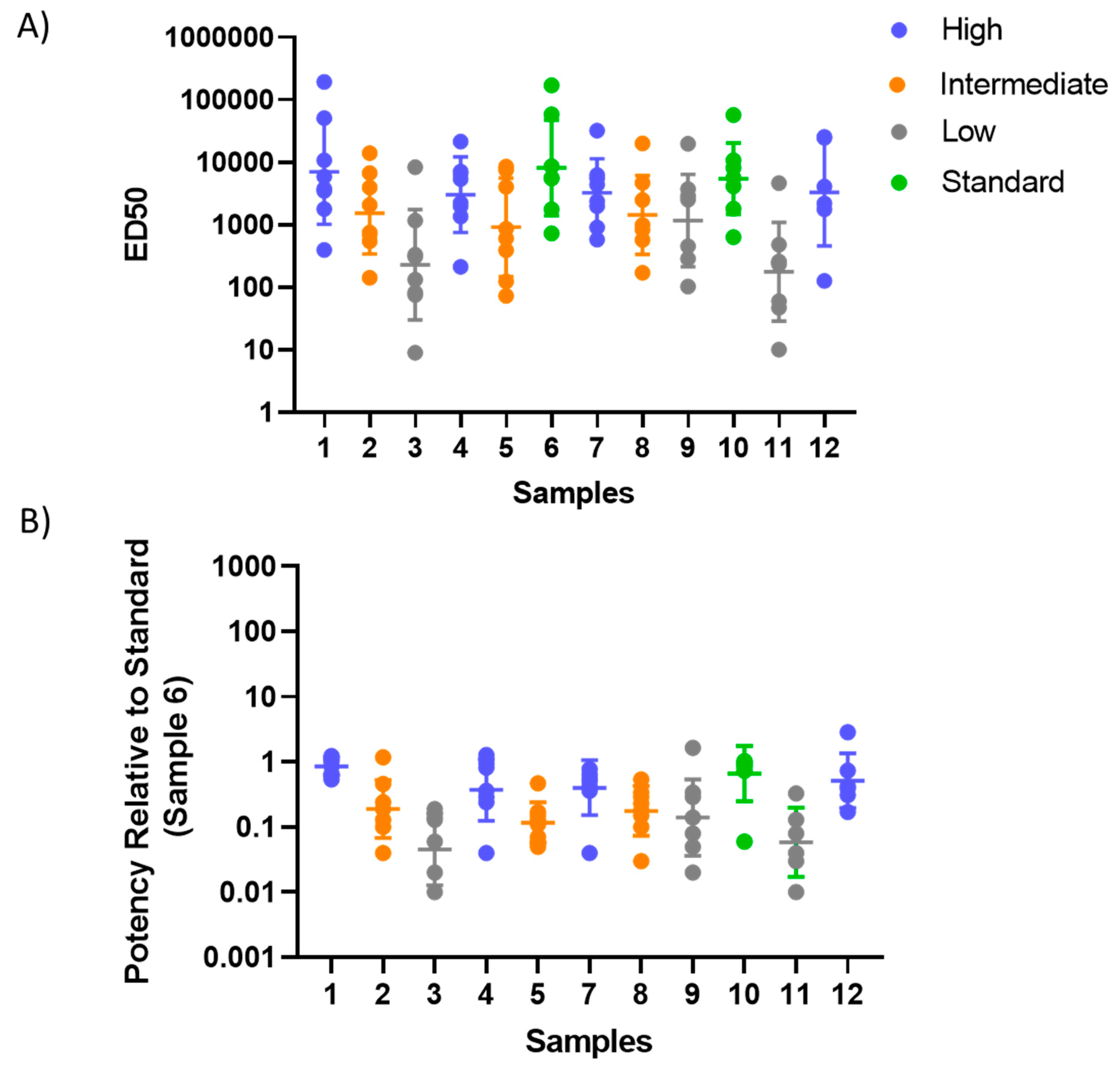
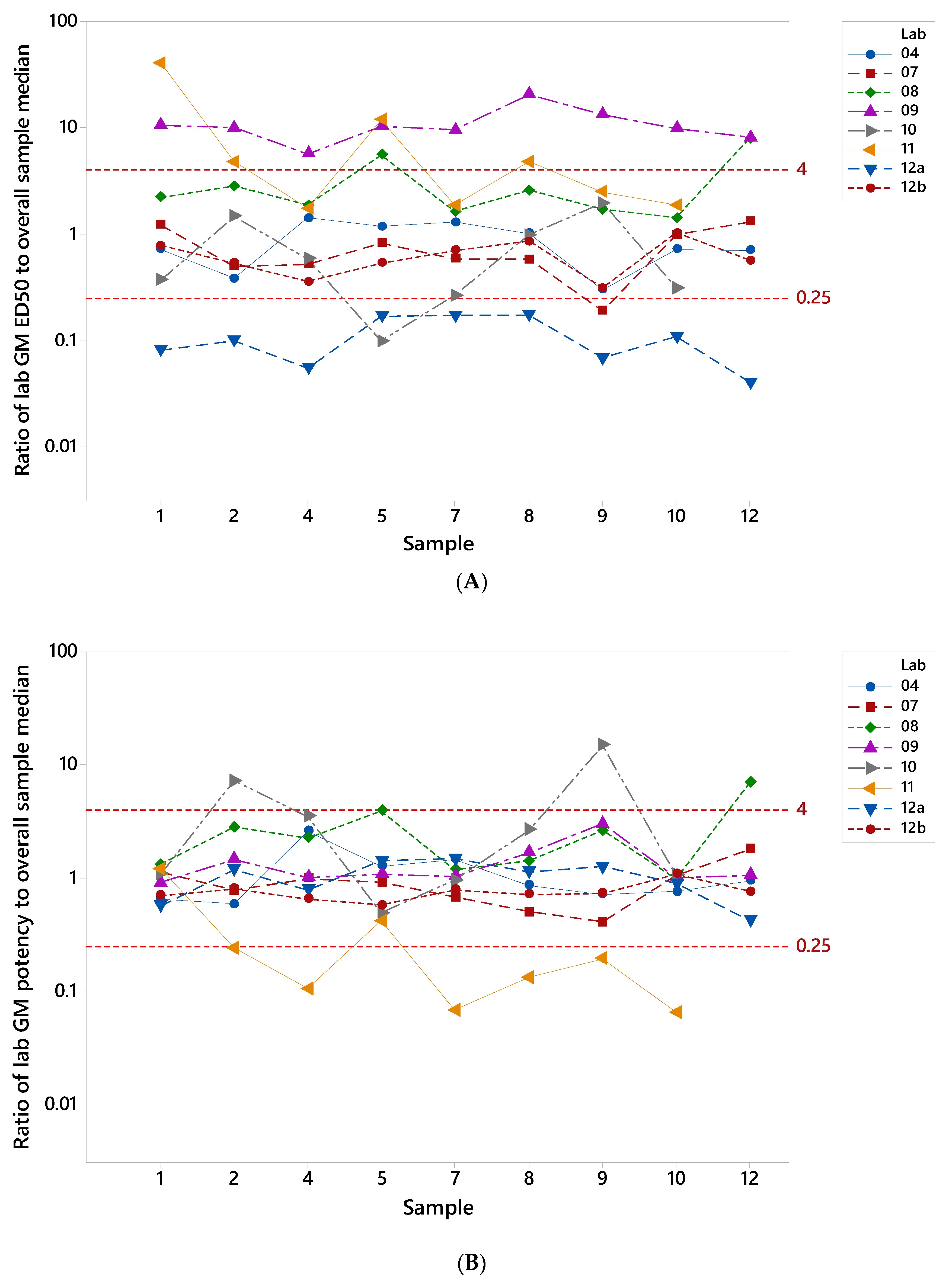
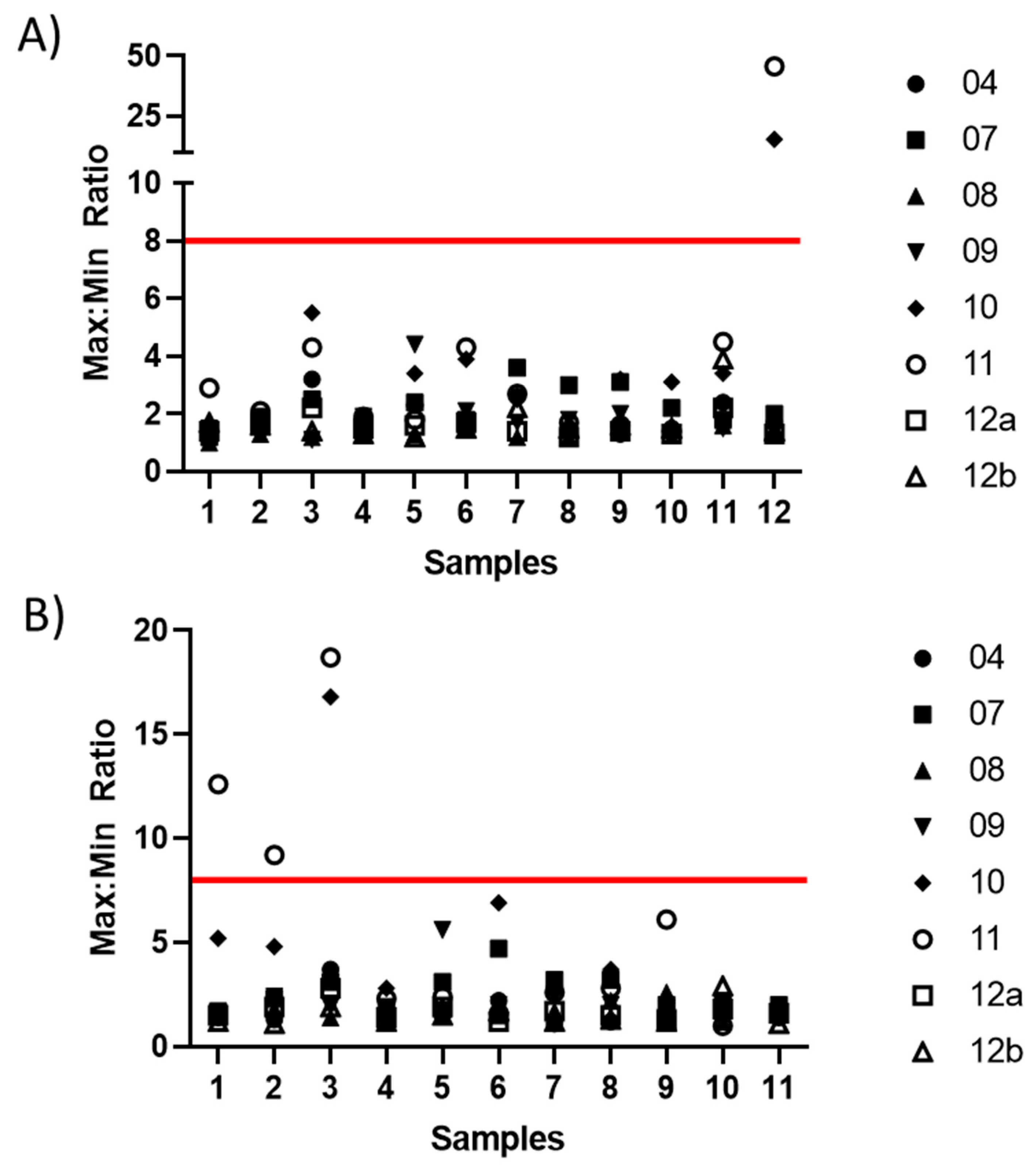
3.3. Testing of the Candidate Serum Standard in a Collaborative Study
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Palese, P. Universal Influenza Virus Vaccines That Target the Conserved Hemagglutinin Stalk and Conserved Sites in the Head Domain. J. Infect. Dis. 2019, 219 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Sachs, D.; Chen, C.J.; Hai, R.; Palese, P. Genome-Wide Mutagenesis of Influenza Virus Reveals Unique Plasticity of the Hemagglutinin and Ns1 Proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20248–20253. [Google Scholar] [CrossRef]
- Krystal, M.; Elliott, R.M.; Benz, E.W., Jr.; Young, J.F.; Palese, P. Evolution of Influenza a and B Viruses: Conservation of Structural Features in the Hemagglutinin Genes. Proc. Natl. Acad. Sci. USA 1982, 79, 4800–4804. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody Recognition of a Highly Conserved Influenza Virus Epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A Neutralizing Antibody Selected from Plasma Cells That Binds to Group 1 and Group 2 Influenza a Hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Highly Conserved Protective Epitopes on Influenza B Viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef]
- Temoltzin-Palacios, F.; Thomas, D.B. Modulation of Immunodominant Sites in Influenza Hemagglutinin Compromise Antigenic Variation and Select Receptor-Binding Variant Viruses. J. Exp. Med. 1994, 179, 1719–1724. [Google Scholar] [CrossRef]
- Kirkpatrick, E.; Qiu, X.; Wilson, P.C.; Bahl, J.; Krammer, F. The Influenza Virus Hemagglutinin Head Evolves Faster Than the Stalk Domain. Sci. Rep. 2018, 8, 10432. [Google Scholar] [CrossRef]
- Krammer, F. The Human Antibody Response to Influenza a Virus Infection and Vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Okuno, Y.; Isegawa, Y.; Sasao, F.; Ueda, S. A Common Neutralizing Epitope Conserved between the Hemagglutinins of Influenza a Virus H1 and H2 Strains. J. Virol. 1993, 67, 2552–2558. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Wilson, I.A. Broadly Neutralizing Antibodies against Influenza Virus and Prospects for Universal Therapies. Curr. Opin. Virol. 2012, 2, 134–141. [Google Scholar] [CrossRef]
- Brandenburg, B.; Koudstaal, W.; Goudsmit, J.; Klaren, V.; Tang, C.; Bujny, M.V.; Korse, H.J.; Kwaks, T.; Otterstrom, J.J.; Juraszek, J.; et al. Mechanisms of Hemagglutinin Targeted Influenza Virus Neutralization. PLoS ONE 2013, 8, e80034. [Google Scholar] [CrossRef]
- Tan, G.S.; Lee, P.S.; Hoffman, R.M.; Mazel-Sanchez, B.; Krammer, F.; Leon, P.E.; Ward, A.B.; Wilson, I.A.; Palese, P. Characterization of a Broadly Neutralizing Monoclonal Antibody That Targets the Fusion Domain of Group 2 Influenza a Virus Hemagglutinin. J. Virol. 2014, 88, 13580–13592. [Google Scholar] [CrossRef]
- Terajima, M.; Cruz, J.; Co, M.D.; Lee, J.H.; Kaur, K.; Wrammert, J.; Wilson, P.C.; Ennis, F.A. Complement-Dependent Lysis of Influenza a Virus-Infected Cells by Broadly Cross-Reactive Human Monoclonal Antibodies. J. Virol. 2011, 85, 13463–13467. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Tan, G.S.; Palese, P.; Ravetch, J.V. Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Require Fcgammar Interactions for Protection against Influenza Virus in Vivo. Nat. Med. 2014, 20, 143–151. [Google Scholar] [CrossRef]
- Asthagiri Arunkumar, G.; Ioannou, A.; Wohlbold, T.J.; Meade, P.; Aslam, S.; Amanat, F.; Ayllon, J.; Garcia-Sastre, A.; Krammer, F. Broadly Cross-Reactive, Nonneutralizing Antibodies against Influenza B Virus Hemagglutinin Demonstrate Effector Function-Dependent Protection against Lethal Viral Challenge in Mice. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.; Van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A Stable Trimeric Influenza Hemagglutinin Stem as a Broadly Protective Immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-Stem Nanoparticles Generate Heterosubtypic Influenza Protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Sutton, T.C.; Chakraborty, S.; Mallajosyula, V.V.A.; Lamirande, E.W.; Ganti, K.; Bock, K.W.; Moore, I.N.; Varadarajan, R.; Subbarao, K. Protective Efficacy of Influenza Group 2 Hemagglutinin Stem-Fragment Immunogen Vaccines. NPJ Vaccines 2017, 2, 35. [Google Scholar] [CrossRef]
- Choi, A.; Bouzya, B.; Franco, K.D.C.; Stadlbauer, D.; Rajabhathor, A.; Rouxel, R.N.; Mainil, R.; Van der Wielen, M.; Palese, P.; Garcia-Sastre, A.; et al. Chimeric Hemagglutinin-Based Influenza Virus Vaccines Induce Protective Stalk-Specific Humoral Immunity and Cellular Responses in Mice. Immunohorizons 2019, 3, 133–148. [Google Scholar] [CrossRef]
- Park, J.K.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Rosas, L.A.; Cervantes-Medina, A.; Taubenberger, J.K.; Memoli, M.J. Evaluation of Preexisting Anti-Hemagglutinin Stalk Antibody as a Correlate of Protection in a Healthy Volunteer Challenge with Influenza a/H1n1pdm Virus. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.R.; Toulmin, S.A.; Griesman, T.; Lamerato, L.E.; Petrie, J.G.; Martin, E.T.; Monto, A.S.; Hensley, S.E. Assessing the Protective Potential of H1n1 Influenza Virus Hemagglutinin Head and Stalk Antibodies in Humans. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Dhar, N.; Kwatra, G.; Nunes, M.C.; Cutland, C.; Izu, A.; Nachbagauer, R.; Krammer, F.; Madhi, S.A. Hemagglutinin Stalk Antibody Responses Following Trivalent Inactivated Influenza Vaccine Immunization of Pregnant Women and Association with Protection from Influenza Virus Illness. Clin. Infect. Dis. 2020, 71, 1072–1079. [Google Scholar] [CrossRef]
- Ng, S.; Nachbagauer, R.; Balmaseda, A.; Stadlbauer, D.; Ojeda, S.; Patel, M.; Rajabhathor, A.; Lopez, R.; Guglia, A.F.; Sanchez, N.; et al. Novel Correlates of Protection against Pandemic H1n1 Influenza a Virus Infection. Nat. Med. 2019, 25, 962–967. [Google Scholar] [CrossRef]
- Krammer, F.; Garcia-Sastre, A.; Palese, P. Is It Possible to Develop a “ Universal” Influenza Virus Vaccine? Potential Target Antigens and Critical Aspects for a Universal Influenza Vaccine. Cold Spring Harb. Perspect. Biol. 2018, 10, a028845. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Guptill, J.; Naficy, A.; Nachbagauer, R.; Berlanda-Scorza, F.; Feser, J.; Wilson, P.C.; Solorzano, A.; Van der Wielen, M.; Walter, E.B.; et al. Immunogenicity of Chimeric Haemagglutinin-Based, Universal Influenza Virus Vaccine Candidates: Interim Results of a Randomised, Placebo-Controlled, Phase 1 Clinical Trial. Lancet Infect. Dis. 2020, 20, 80–91. [Google Scholar] [CrossRef]
- WHO. Recommendations for the Preparation, Characterization and Establishment of International and Other Biological Reference Standards (Revised 2004); WHO Technical Report Series; No. 932; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Blood Products and Related Biologicals WHO. Available online: https://www.who.int/bloodproducts/catalogue/en (accessed on 20 April 2020).
- Wood, J.; Stephenson, I.; Heath, A. Report of a Who Collaborative Study to Assess the Suitability of a Candidate International Standard for Antibody to Influenza H5n1 Virus; WHO/BS/08.2085; Expert Committee on Biological Standardization; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Major, D.; Heath, A.; Wood, J. Report of a Who Collaborative Study to Assess the Suitability of a Candidate Replacement International Standard for Antibody to Pandemic H1n1 Influenza Virus; WHO/BS/2012.2190; Expert Committee on Biological Standardization; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Nachbagauer, R.; Choi, A.; Hirsh, A.; Margine, I.; Iida, S.; Barrera, A.; Ferres, M.; Albrecht, R.A.; Garcia-Sastre, A.; Bouvier, N.M.; et al. Defining the Antibody Cross-Reactome Directed against the Influenza Virus Surface Glycoproteins. Nat. Immunol. 2017, 18, 464–473. [Google Scholar] [CrossRef]
- Chromikova, V.; Tan, J.; Aslam, S.; Rajabhathor, A.; Bermudez-Gonzalez, M.; Ayllon, J.; Simon, V.; Garcia-Sastre, A.; Salaun, B.; Nachbagauer, R.; et al. Activity of Human Serum Antibodies in an Influenza Virus Hemagglutinin Stalk-Based Adcc Reporter Assay Correlates with Activity in a Cd107a Degranulation Assay. Vaccine 2020, 38, 1953–1961. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Salaun, B.; Stadlbauer, D.; Behzadi, M.A.; Friel, D.; Rajabhathor, A.; Choi, A.; Albrecht, R.A.; Debois, M.; Garcia-Sastre, A.; et al. Pandemic Influenza Virus Vaccines Boost Hemagglutinin Stalk-Specific Antibody Responses in Primed Adult and Pediatric Cohorts. NPJ Vaccines 2019, 4, 51. [Google Scholar] [CrossRef]
- Jacobsen, H.; Rajendran, M.; Choi, A.; Sjursen, H.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F.; Nachbagauer, R. Influenza Virus Hemagglutinin Stalk-Specific Antibodies in Human Serum Are a Surrogate Marker for in Vivo Protection in a Serum Transfer Mouse Challenge Model. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Miller, M.S.; Gardner, T.J.; Krammer, F.; Aguado, L.C.; Tortorella, D.; Basler, C.F.; Palese, P. Neutralizing Antibodies against Previously Encountered Influenza Virus Strains Increase over Time: A Longitudinal Analysis. Sci. Transl. Med. 2013, 5, 198ra07. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Choi, A.; Izikson, R.; Cox, M.M.; Palese, P.; Krammer, F. Age Dependence and Isotype Specificity of Influenza Virus Hemagglutinin Stalk-Reactive Antibodies in Humans. mBio 2016, 7, e01996-15. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Hai, R.; Krammer, F.; Wang, T.T.; Maamary, J.; Eggink, D.; Tan, G.S.; Krause, J.C.; Moran, T.; Stein, C.R.; et al. Hemagglutinin Stalk Antibodies Elicited by the 2009 Pandemic Influenza Virus as a Mechanism for the Extinction of Seasonal H1n1 Viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Yoshida, R.; Ekiert, D.C.; Sakai, N.; Suzuki, Y.; Takada, A.; Wilson, I.A. Heterosubtypic Antibody Recognition of the Influenza Virus Hemagglutinin Receptor Binding Site Enhanced by Avidity. Proc. Natl. Acad. Sci. USA 2012, 109, 17040–17045. [Google Scholar] [CrossRef] [PubMed]
- Ellebedy, A.H.; Krammer, F.; Li, G.M.; Miller, M.S.; Chiu, C.; Wrammert, J.; Chang, C.Y.; Davis, C.W.; McCausland, M.; Elbein, R.; et al. Induction of Broadly Cross-Reactive Antibody Responses to the Influenza Ha Stem Region Following H5n1 Vaccination in Humans. Proc. Natl. Acad. Sci. USA 2014, 111, 13133–13138. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Wohlbold, T.J.; Hirsh, A.; Hai, R.; Sjursen, H.; Palese, P.; Cox, R.J.; Krammer, F. Induction of Broadly Reactive Anti-Hemagglutinin Stalk Antibodies by an H5n1 Vaccine in Humans. J. Virol. 2014, 88, 13260–13268. [Google Scholar] [CrossRef]
- He, W.; Mullarkey, C.E.; Duty, J.A.; Moran, T.M.; Palese, P.; Miller, M.S. Broadly Neutralizing Anti-Influenza Virus Antibodies: Enhancement of Neutralizing Potency in Polyclonal Mixtures and Iga Backbones. J. Virol. 2015, 89, 3610–3618. [Google Scholar] [CrossRef]
- Rajendran, M.; Sun, W.; Comella, P.; Nachbagauer, R.; Wohlbold, T.J.; Amanat, F.; Kirkpatrick, E.; Palese, P.; Krammer, F. An Immuno-Assay to Quantify Influenza Virus Hemagglutinin with Correctly Folded Stalk Domains in Vaccine Preparations. PLoS ONE 2018, 13, e0194830. [Google Scholar] [CrossRef]
- Af Geijersstam, V.; Kibur, M.; Wang, Z.; Koskela, P.; Pukkala, E.; Schiller, J.; Lehtinen, M.; Dillner, J. Stability over Time of Serum Antibody Levels to Human Papillomavirus Type 16. J. Infect. Dis. 1998, 177, 1710–1714. [Google Scholar] [CrossRef]
- McDonald, J.U.; Rigsby, P.; Dougall, T.; Engelhardt, O.G. Establishment of the First Who International Standard for Antiserum to Respiratory Syncytial Virus: Report of an International Collaborative Study. Vaccine 2018, 36, 7641–7649. [Google Scholar] [CrossRef]
- Stephenson, I.; Heath, A.; Major, D.; Newman, R.W.; Hoschler, K.; Junzi, W.; Katz, J.M.; Weir, J.P.; Zambon, M.C.; Wood, J.M. Reproducibility of Serologic Assays for Influenza Virus a (H5n1). Emerg. Infect. Dis. 2009, 15, 1252–1259. [Google Scholar] [CrossRef]
- Wagner, R.; Gopfert, C.; Hammann, J.; Neumann, B.; Wood, J.; Newman, R.; Wallis, C.; Alex, N.; Pfleiderer, M. Enhancing the Reproducibility of Serological Methods Used to Evaluate Immunogenicity of Pandemic H1n1 Influenza Vaccines-an Effective Eu Regulatory Approach. Vaccine 2012, 30, 4113–4122. [Google Scholar] [CrossRef]
- Hosken, N.; Plikaytis, B.; Trujillo, C.; Mahmood, K.; Higgins, D. A Multi-Laboratory Study of Diverse Rsv Neutralization Assays Indicates Feasibility for Harmonization with an International Standard. Vaccine 2017, 35, 3082–3088. [Google Scholar] [CrossRef]

| Institution | Name | Country |
|---|---|---|
| Janssen Vaccines & Prevention | Boerries Brandenburg | The Netherlands |
| Centers for Disease Control and Prevention | Min Levine | United States |
| University of Bergen | Fan Zhou | Norway |
| Icahn School of Medicine at Mount Sinai | Adolfo García-Sastre & Teresa Aydillo-Gomez | United States |
| Vismederi Research Srl. | Alessandro Manenti | Italy |
| National Institutes of Health | Barney Graham | United States |
| University of Hong Kong | Sophie Valkenburg | China SAR |
| National Institute of Biological Standards & Control | Lethia Charles & Othmar Engelhardt | United Kingdom |
| Sample | Laboratory | |||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 7 | 8 | 9 | 10 | 11 | 12a | 12b | |
| 1 | 1.39 | 1.22 | 2.22 | 10.46 | 2.70 | 40.18 | 12.30 | 1.29 |
| 2 | 2.60 | 2.02 | 2.81 | 9.93 | 1.47 | 4.78 | 9.96 | 1.88 |
| 4 | 1.41 | 1.92 | 1.87 | 5.65 | 1.70 | 1.74 | 18.06 | 2.80 |
| 5 | 1.18 | 1.21 | 5.55 | 10.32 | 10.08 | 11.73 | 5.90 | 1.87 |
| 7 | 1.30 | 1.71 | 1.63 | 9.54 | 3.75 | 1.86 | 5.84 | 1.42 |
| 8 | 1.02 | 1.72 | 2.57 | 20.47 | 1.02 | 4.78 | 5.76 | 1.17 |
| 9 | 3.31 | 5.24 | 1.69 | 13.16 | 1.94 | 2.49 | 14.52 | 3.23 |
| 10 | 1.39 | 1.02 | 1.42 | 9.85 | 3.21 | 1.86 | 9.18 | 1.02 |
| 12 | 1.43 | 1.30 | 7.88 | 8.11 | N/A | N/A | 24.64 | 1.77 |
| % < 2 | 78% | 78% | 44% | 0% | 50% | 34% | 0% | 78% |
| % < 4 | 100% | 89% | 78% | 0% | 88% | 50% | 0% | 100% |
| % < 8 | 100% | 100% | 100% | 11% | 88% | 75% | 25% | 100% |
 : X < 2;
: X < 2;  : 2 < X < 4;
: 2 < X < 4;  : 4 < X < 8;
: 4 < X < 8;  : X > 8.
: X > 8.| Sample | Laboratory | |||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 7 | 8 | 9 | 10 | 11 | 12a | 12b | |
| 1 | 1.53 | 1.14 | 1.32 | 1.09 | 1.08 | 1.21 | 1.75 | 1.43 |
| 2 | 1.70 | 1.28 | 2.82 | 1.47 | 7.23 | 4.13 | 1.19 | * 1.23 |
| 4 | 2.61 | 1.01 | 2.26 | 1.01 | 3.50 | 9.39 | 1.26 | * 1.52 |
| 5 | 1.28 | 1.09 | 3.94 | 1.08 | 2.03 | 2.38 | 1.42 | * 1.73 |
| 7 | 1.46 | 1.48 | 1.20 | 1.04 | 1.04 | 14.49 | 1.50 | * 1.27 |
| 8 | 1.16 | 1.98 | 1.42 | 1.68 | 2.67 | 7.48 | 1.14 | 1.39 |
| 9 | 1.39 | 2.44 | 2.63 | 3.02 | 14.83 | 5.12 | 1.27 | * 1.36 |
| 10 | 1.31 | 1.07 | 1.01 | 1.01 | 1.07 | 15.34 | 1.12 | 1.08 |
| 12 | 1.06 | 1.80 | 6.96 | 1.06 | N/A | N/A | 2.36 | * 1.32 |
| % < 2 | 89% | 89% | 44% | 89% | 34% | 13% | 89% | 100% |
| % < 4 | 100% | 100% | 89% | 100% | 75% | 25% | 100% | 100% |
| % < 8 | 100% | 100% | 100% | 100% | 88% | 63% | 100% | 100% |
 : X < 2;
: X < 2;  : 2 < X < 4;
: 2 < X < 4;  : 4 < X < 8;
: 4 < X < 8;  : X > 8. * max:min ratio reduced when potency expressed relative to sample 6.
: X > 8. * max:min ratio reduced when potency expressed relative to sample 6.| Sample | Laboratory | GM | Ratio | Max:Min Ratio | |
|---|---|---|---|---|---|
| 04 | 10 | ||||
| 1 | 84.8 | 67.3 | 75.5 | 1.26 | 1.26 |
| 2 | 95.3 | 40 | 61.7 | 2.38 | 2.38 |
| 3 | 80 | 33.6 | 51.9 | 2.38 | 2.38 |
| 4 | 80 | 67.3 | 73.4 | 1.19 | 1.19 |
| 5 | 40 | 40 | 40 | 1.00 | 1.00 |
| 7 | 160 | 80 | 113.1 | 2.00 | 2.00 |
| 8 | 89.9 | 28.3 | 50.4 | 3.18 | 3.18 |
| 9 | 80 | 28.3 | 47.6 | 2.83 | 2.83 |
| 10 | 160 | 47.6 | 87.2 | 3.36 | 3.36 |
| 11 | 20 | 10 | 14.1 | 2.00 | 2.00 |
| 12 | 80 | 40 | 56.6 | 2.00 | 2.00 |
| Sample | Laboratory | GM | Ratio | Max:Min Ratio | |
|---|---|---|---|---|---|
| 04 | 10 | ||||
| 1 | 0.53 | 0.59 | 0.56 | 0.89 | * 1.11 |
| 2 | 0.60 | 0.35 | 0.46 | 1.68 | * 1.71 |
| 3 | 0.50 | 0.30 | 0.39 | 1.68 | * 1.67 |
| 4 | 0.50 | 0.59 | 0.55 | 0.84 | * 1.18 |
| 5 | 0.25 | 0.35 | 0.30 | 0.71 | 1.40 |
| 7 | 1.00 | 0.71 | 0.84 | 1.41 | * 1.41 |
| 8 | 0.56 | 0.25 | 0.37 | 2.25 | * 2.24 |
| 9 | 0.50 | 0.25 | 0.35 | 2.00 | * 2.00 |
| 10 | 1.00 | 0.42 | 0.65 | 2.38 | * 2.38 |
| 11 | 0.13 | 0.08 | 0.10 | 1.59 | * 1.63 |
| 12 | 0.50 | 0.35 | 0.42 | 1.41 | * 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreño, J.M.; McDonald, J.U.; Hurst, T.; Rigsby, P.; Atkinson, E.; Charles, L.; Nachbagauer, R.; Behzadi, M.A.; Strohmeier, S.; Coughlan, L.; et al. Development and Assessment of a Pooled Serum as Candidate Standard to Measure Influenza A Virus Group 1 Hemagglutinin Stalk-Reactive Antibodies. Vaccines 2020, 8, 666. https://doi.org/10.3390/vaccines8040666
Carreño JM, McDonald JU, Hurst T, Rigsby P, Atkinson E, Charles L, Nachbagauer R, Behzadi MA, Strohmeier S, Coughlan L, et al. Development and Assessment of a Pooled Serum as Candidate Standard to Measure Influenza A Virus Group 1 Hemagglutinin Stalk-Reactive Antibodies. Vaccines. 2020; 8(4):666. https://doi.org/10.3390/vaccines8040666
Chicago/Turabian StyleCarreño, Juan Manuel, Jacqueline U. McDonald, Tara Hurst, Peter Rigsby, Eleanor Atkinson, Lethia Charles, Raffael Nachbagauer, Mohammad Amin Behzadi, Shirin Strohmeier, Lynda Coughlan, and et al. 2020. "Development and Assessment of a Pooled Serum as Candidate Standard to Measure Influenza A Virus Group 1 Hemagglutinin Stalk-Reactive Antibodies" Vaccines 8, no. 4: 666. https://doi.org/10.3390/vaccines8040666
APA StyleCarreño, J. M., McDonald, J. U., Hurst, T., Rigsby, P., Atkinson, E., Charles, L., Nachbagauer, R., Behzadi, M. A., Strohmeier, S., Coughlan, L., Aydillo, T., Brandenburg, B., García-Sastre, A., Kaszas, K., Levine, M. Z., Manenti, A., McDermott, A. B., Montomoli, E., Muchene, L., ... Krammer, F. (2020). Development and Assessment of a Pooled Serum as Candidate Standard to Measure Influenza A Virus Group 1 Hemagglutinin Stalk-Reactive Antibodies. Vaccines, 8(4), 666. https://doi.org/10.3390/vaccines8040666








