Abstract
With a death toll of over one million worldwide, the COVID-19 pandemic caused by SARS-CoV-2 has become the most devastating humanitarian catastrophe in recent decades. The fear of acquiring infection and spreading to vulnerable people has severely impacted society’s socio-economic status. To put an end to this growing number of infections and deaths as well as to switch from restricted to everyday living, an effective vaccine is desperately needed. As a result, enormous efforts have been made globally to develop numerous vaccine candidates in a matter of months. Currently, over 30 vaccine candidates are under assessment in clinical trials, with several undergoing preclinical studies. Here, we reviewed the major vaccine candidates based on the specific vaccine platform utilized to develop them. We also discussed the immune responses generated by these candidates in humans and preclinical models to determine vaccine safety, immunogenicity, and efficacy. Finally, immune responses induced in recovered COVID-19 patients and their possible vaccine development implications were also briefly reviewed.
1. Introduction
Coronavirus disease 2019 (COVID-19) pandemic has resulted in nearly 50 million infections, claiming more than one million human lives globally (https://coronavirus.jhu.edu). COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a beta-coronavirus that emerged from bats and then transferred to humans through intermediate hosts [1,2,3]. SARS-CoV-2 transmits from human to human via respiratory droplets leading to a respiratory tract infection that can progress to severe pneumonia, multiple organ involvement, and fatal outcomes [4,5,6]. The SARS-CoV-2 infection can be asymptomatic or symptomatic with mild to life-threatening symptoms [6,7,8,9,10]. Irrespective of the severity of symptoms, an infected individual is very likely to spread the infection and especially pose a greater risk to the vulnerable population [11,12,13].
A vaccine is urgently needed to control the current exploding global pandemic of COVID-19 and prevent recurrent epidemics. COVID-19 combined with the seasonal Flu epidemic is expected to further aggravate the situation in terms of diagnosis, co-infections and severity of the disease. An effective vaccine would put a check on the ongoing health or medical crisis and improve the socio-economic status of the society that has severely been impacted due to COVID-19 pandemic [14,15]. Additionally, a return to normalcy with no social distancing or masks can be achieved once all are vaccinated and immune to COVID-19. Hence, eradicating SARS-CoV-2 is very much needed through an effective vaccine, especially in the absence of specific licensed drugs to treat COVID-19.
Since SARS-CoV-2 is closely related to severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and other coronaviruses, vaccine design greatly relies on the existing preventive strategies that have been tested for these viruses at the preclinical or clinical level [16,17,18,19]. While virus-neutralizing antibody responses will always be the hallmark for all anti-viral vaccines, some vaccines have also shown the potential to induce protective T-cell responses [16,20,21,22,23]. It is currently not entirely known as to what exactly will prove to be a correlate of protection against this novel coronavirus.
SARS-CoV-2 is an enveloped single-stranded RNA virus. The viral envelope is embedded with spike (S) glycoprotein, the matrix (M) protein, and envelope (E) protein. Encased into this envelope is a positive-sense single-stranded RNA as a viral genome bound to helical nucleocapsid (N) protein (Figure 1) [24,25]. The viral spike protein that mediates entry into the host cell has been identified as one of the preferred vaccine targets. SARS-CoV-2 targets the ACE2 receptor on the host cell via its spike Protein (S), composed of S1 and S2 subunits [24,26]. The S1 subunit contains the receptor-binding domain (RBD) that interacts with the ACE2 receptor, thereby inducing a series of conformational changes facilitating membrane fusion and entry [26]. Due to the critical role played by the spike protein in mediating viral attachment and entry, nearly all SARS-CoV-2 vaccines currently in development are predominantly focused on eliciting protective immune responses targeting the viral spike [21,23,25,26,27,28,29,30]. Although the spike protein is the major component of most vaccines, the technical platform determines how different platforms can modulate the immune responses. Therefore, safety, immunogenicity, and efficacy will majorly depend on the vaccine approach or delivery platforms. In general, vaccine platforms are broadly categorized into six types: live attenuated viral vaccine, inactivated virus vaccine, recombinant viral-vectored vaccines, protein subunit vaccines, virus-like particles (VLPs), and nucleic acid-based (DNA or mRNA) vaccines (https://www.vaccines.gov/basics/types). The relative progress of the vaccine candidates towards different stages of clinical development is shown in Figure 2.
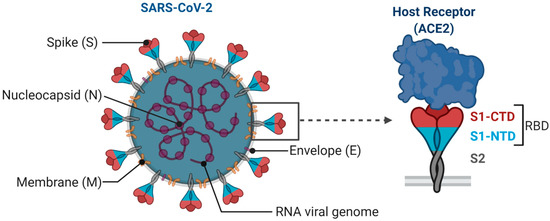
Figure 1.
Structural features of SARS-CoV-2. Spike (S) glycoprotein, the membrane (M) protein, and envelope (E) protein are embedded in the viral envelope. The RNA genome is complexed with the nucleocapsid (N) protein. Virus spike trimer is enlarged to depict its key subunits (S1 and S2) and N-terminal domains (NTD) and C-terminal domains (CTD) in the S1 subunit encompassing receptor-binding domain (RBD). S protein targets the host cell receptor, ACE2, through RBD in S1 subunit (Created with Biorender.com).
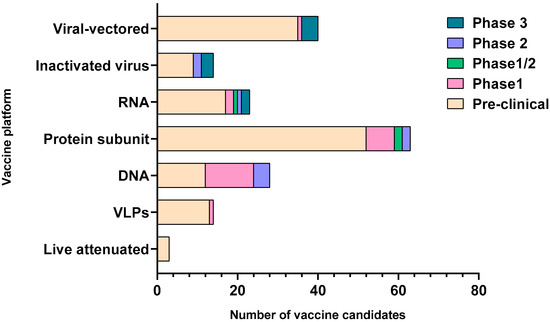
Figure 2.
Graphical representation of the vaccine candidates with respect to their clinical stages of development. The number of candidates on the x axis are compared for each of the major vaccine platforms shown on the y axis. The clinical stage for the vaccine candidates in each platform is depicted by color-coded legends on the right of the graph. The graph is constructed based on the data obtained from the World Health Organization; draft landscape of COVID-19 candidate vaccines and Coronavirus Vaccine tracker. VLPs-virus-like particles, RNA-ribonucleic acid, DNA-deoxyribonucleic acid.
Here, we will discuss the predicted and observed immune responses for major vaccine candidates depicting each platform that are currently in preclinical or clinical phases of development to prevent the SARS-CoV-2 infection. Furthermore, we discussed the immune responses elicited in recovered COVID-19 patients that can provide useful vaccine design insights.
2. Major COVID-19 Vaccine Candidates and Their Responses
Vaccine safety and efficacy vary for a protein/DNA/RNA vaccine or with the type of adjuvant/vector used in the vaccine formulation, and even with the route of administration. Similarly, whether the SARS-CoV-2 spike is made to express endogenously in the vaccines as part of the nucleic acid (DNA/mRNA) vaccine approach or administered as a recombinant protein antigen for immunization can induce considerable variations in vaccine responses that can subsequently influence the vaccine efficacy. Different types of vaccine platforms currently in trials for COVID-19 are shown in Figure 3. While the vaccine efficacy is majorly assessed through adaptive immunity components, such as the induction of robust virus-neutralizing antibody responses, the innate arm of the immune defense plays a critical role in resulting in effective adaptive responses [31,32]. The adjuvants present in the vaccine formulation primarily activate innate responses and enhance the adaptive immune responses governing the effectiveness of the vaccine [33]. Mechanistically, adjuvants act as ligands for TLRs (Toll-like receptors) or PRRs (pattern recognition receptors), and the specific interaction of each adjuvant with the respective receptors determines the outcome of response [33]. A well-known adjuvant, alum, for instance, is known to activate Nalp3 inflammasome and activate Th2 T-cell response [34,35]. The adjuvant effect can also be seen for some vaccines that lack any added adjuvants. Killed or inactivated vaccines, live-attenuated vaccines, and viral-vectored vaccines contain their own PAMPs (pathogen associated molecular patterns) that can serve as built-in adjuvants [36]. Discussed below are the major COVID-19 vaccine candidates under different vaccine technology platforms with their composition, respective responses, and development stage, along with the information summarized in Table 1.
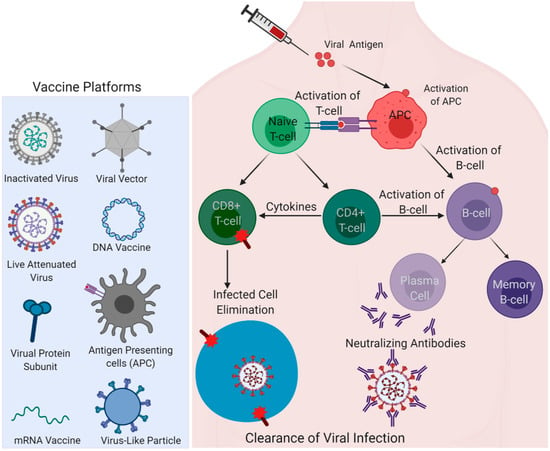
Figure 3.
Different vaccine platforms for the COVID-19 vaccine. Left; viral and non-viral vaccine delivery platforms are shown. Right; antigen induced activation of antigen-presenting cells (APCs); a nexus of innate and adaptive immune arms activating T-cell and B-cell immunity by fundamental immunological pathways are represented to show how vaccine-elicited immune responses lead to the clearance of infection. (Created with Biorender.com).

Table 1.
Various types of vaccines, their composition, developer, and stage of development.
2.1. Inactivated/Live Attenuated Virus Vaccine
Vaccine development using the weakened or inactivated virus has been a traditional approach for decades, including measles and the first iteration of the Salk and Sabin polio vaccines [37,38]. A live attenuated virus vaccine is weakened either by serial passaging in animal/human cells or by altering the viral genetic code to dampen the virus [39,40]. A virus can also be inactivated using chemicals such as formaldehyde and heat [41,42]. Codagenix (New York) partnered with Serum Institute of India, and Indian immunologicals Ltd. and Griffith University, and Mehmet Ali Aydinlar University have generated a genetically altered weakened form of SARS-CoV-2 as a live attenuated vaccine, currently under preclinical evaluation (draft landscape of COVID-19 candidate vaccines). Live virus vaccines are usually highly immunogenic; therefore, one dose is often enough to produce a substantial immune response; however, there is always a high risk of the reversion of attenuated strain to a pathogenic one.
Sinovac Biotech in Beijing, China, has been testing an inactivated form of SARS-CoV-2 in preclinical studies [43]. Sinovac’s SARS-CoV-2 virus vaccine candidate (PiCoVacc) has been produced in Vero cells and inactivated using β-propiolactone. This vaccine was tested in mice, rats, and non-human primates (NHPs) in two doses (3 and 6 µg). PiCoVacc reportedly induced antibodies in these preclinical animal models capable of neutralizing 10 representative strains of SARS-CoV-2. Furthermore, partial-to-complete protection was observed in macaques after three immunizations with PiCoVacc against the SARS-CoV-2 challenge [43]. Notably, the histopathological assessment showed no pathological changes in vaccinated macaques’ vital organs [43]. Furthermore, in contrast to a live virus, the inactivated virus vaccine did not result in a percent change in lymphocytes (CD3+, CD4+, or CD8+) or a cytokine storm that is a leading cause of death in SARS-CoV-2 infected individuals [5,6,8,43]. Another inactivated vaccine candidate, BBIBP-CorV, developed by Sinopharm, China, was also assessed for efficacy in multiple animal models, including NHPs [44]. This vaccine elicited robust neutralizing antibody titers even with the lowest dose (2 μg) tested. Additionally, BBIBP-CorV was able to confer protection in macaques without any antibody-dependent enhancement [45]. Both Sinovac (NCT04582344) and Sinopharm (NCT04560881) vaccine candidates are currently under Phase 3 study. Although it is encouraging that these inactivated vaccines could elicit desirable humoral responses in preclinical models (draft landscape of COVID-19 candidate vaccines), the complete inactivation of the virus would be critical for the safety of the vaccines in humans.
2.2. Viral Vectored Vaccine
Recombinant viral vectored vaccines are among the most common candidates leading in the race of SARS-CoV-2 vaccines, with four of them in the clinical phase and several in the preclinical development stages. Adenovirus type-5 (Ad5), Ad26, and vesicular stomatitis virus (VSV) are commonly used viral vectors for this vaccine platform. Developed by CanSino Biologics, China, a recombinant Ad5 vectored COVID-19 vaccine expressing the spike glycoprotein of a SARS-CoV-2 has been assessed recently in Phase 1 non-randomized study for safety and immunogenicity (NCT04568811). A total of 108 healthy participants were enrolled in Wuhan, China, in the age group of 18–60 years for this trial [21]. Due to adenovirus-based vaccines’ undesirable immunogenicity risks, the vaccine was tested in low, medium and high doses. All vaccine recipients had induced anti-RBD antibodies irrespective of the dose after 14 days, that peaked 28 days post-vaccination. Though not examined, antibodies against epitopes on the spike other than RBD are also expected to be induced through this vaccine approach. These responses would also govern the extent of the vaccine’s success in the subsequent phases of the clinical trials. Additionally, the Ad5-vectored vaccine generated antibodies also demonstrated in vitro neutralization of the SARS-CoV-2 virus [21].
Additionally, T-cell responses in the form of the release of IFNγ, TNFα, and IL-2 were detected from CD4+ and CD8+ T cells that peaked at day 14 post-vaccination in all dose groups [21]. However, both humoral and cell-mediated responses were significantly higher in the high dose group compared to the middle and low dose groups. Notably, antigen-specific antibodies and T-cell responses were partially reduced in the recipients with pre-existing immunity against adenovirus [21]. Most of the participants also suffered from mild to moderate adverse reactions, such as pain at the injection site, fever, fatigue, headache, and muscle pain post-vaccination. However, no severe adverse reactions were reported at least until 28 days post-vaccination [21]. Since the responses were observed only for 28 days, a follow-up study would be required to evaluate the immune response’s durability. Ad26-vectored COVID-19 vaccine is another candidate developed by Johnson & Johnson that demonstrated protection in NHPs with the advantage of being less immunogenic than Ad5 [46]. ChAdOx1-nCoV-19 vaccine vectored with chimpanzee adenovirus has also been developed by the University of Oxford and AstraZeneca [47,48]. The same platform was employed for MERS and tuberculosis (TB) with promising results in human clinical trials [17,49]. Unlike Ad5/Ad26 vectors, ChAdOx1 has far less pre-existing immunity in humans, which is a critical determinant of this platform’s efficacy. ChAdOx1-nCoV-19, indeed, has shown a robust induction of neutralizing antibody and T-cell responses, in conjunction with a reduction in viral titers in rhesus macaques. Importantly, the Phase 1/2 study has shown ChAdOx1-nCoV-19 to be safe and also effective in producing cellular and humoral responses [17,48,50]. Ad5-vectored (NCT04540419), Ad26-vectored (NCT04505722) and ChAdOx1-nCoV-19 (NCT04540393) vaccine are all currently in Phase 3 clinical trials. Other vaccines based on VSV and modified vaccinia virus Ankara (MVA) viral vectors have also shown promising results in preclinical animal models (draft landscape of COVID-19 candidate vaccines).
2.3. mRNA Vaccine
Like the DNA vaccine, no mRNA vaccine has been approved for human use. However, preclinical studies conducted for mRNA-based influenza and zika virus vaccine have demonstrated the induction of protective responses [51,52]. An mRNA vaccine, mRNA-1273 for SARS-CoV-2 encoding a prefusion stabilized form of its Spike (S) protein, has been co-developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID) and at Moderna (Cambridge, MA) [28,53]. This is the first mRNA vaccine to go into clinical trials for the safety and immunogenicity assessment. The mRNA vaccine concept is supported by the principle that SARS-CoV-2 itself is a (+) ss-RNA virus. In order to block initial virus interactions and spike mediated viral entry into the host cell, spike-specific mRNA was utilized as a vaccine target and delivered by encapsulating into lipid nanoparticles (LNPs). As a general principle, the mRNA vaccine upon delivery is expected to enter cells and translate or encode the target protein in the cell cytoplasm [53]. After translation, this foreign protein is released from the cells and encountered by APCs, and results in processing and major histocompatibility complex I (MHC I) subsequent MHC II-based presentation of the target protein. This cascade of events leads to the engagement and activation of B-cells and T-cells to orchestrate both humoral and cell-mediated antigen-specific responses. While MHC I presentation causes the activation of antigen-specific CD8+ T cells, MHC II presentation facilitates CD4+ T cell and B-cell activation followed by mounting of an antibody response [53,54,55].
Additionally, macrophages’ uptake of secreted target antigen results in the secretion of pro-inflammatory cytokines and chemokines, activating the innate arm of immune defense [53]. Results from the Phase 1 study for this novel vaccine unveiled that mRNA-based vaccines can safely induce binding and virus-neutralizing antibodies against the spike protein in all the vaccine recipients after two doses. Th1-based CD4+ T-cell responses, and to a lesser extent, CD8+ T-cell responses, were also observed in vaccine recipients. No severe side effects of the vaccine were reported [28]. Though efficacy evaluation and correlates of protection are not currently known, preclinical studies performed to evaluate mRNA-1273 vaccine responses in mice demonstrated the induction of neutralizing responses post-vaccination and even protection after challenge with SARS-CoV-2 [56]. The safety and immunogenicity data have recently been published for the phase 1 trial of mRNA-1273 in both young and older adults, assessing a dose range from 25 to 100 μg (NCT04405076). A large phase 3 efficacy trial (NCT04470427) evaluating a 100 μg dose has already begun to further assess the mRNA-1273 vaccine in approximately 30,000 adult volunteers [28,56]. Alternatively, Pfizer and BioNTech vaccine candidates based on mRNA encoding SARS-CoV-2 RBD complexed with lipid nanoparticles are also under Phase 3 study (NCT04368728). Other mRNA candidates developed by CureVac (NCT04515147) and Arcturus/Duke-NUS (National University of Singapore) (NCT04480957) are also rapidly progressing through Phase 2 trials for assessment.
2.4. DNA Vaccine
Although there are no approved licensed DNA vaccines for humans, many DNA vaccine candidates are in preclinical and even clinical trials [57,58]. Once expressed, the protective protein antigen can be processed endogenously and presented by an antigen-presenting cell (APC) complexed with MHC. DNA delivered via different viral and non-viral vaccine platforms enter the cell via endocytosis and trigger an innate immune response through innate immune-system receptors, such as Toll-Like Receptor 9 (TLR9) present in endosomes [59,60]. MERS-CoV vaccine (INO-4700) and zika vaccine candidate (GLS-5700) are the DNA vaccines that are currently in clinical testing [19,61,62]. In recipients of INO-4700, durable neutralizing antibodies (nAbs) and T cell immune responses were observed with a seroconversion rate of 96% [61]. SARS-CoV-2 spike protein-coding DNA vaccine, INO-4800, has recently been developed and evaluated for immunogenicity in mice and guinea pigs. In this preclinical testing, INO-4800 induced immunoglobulin G (IgG) responses against spike protein just after a single dose. Additionally, virus-neutralizing antibodies were observed in these immunized animals with a demonstrated potential to compete with ACE2 binding to the SARS-CoV-2 Spike protein [63]. INO-4800 (NCT04336410), along with three other DNA vaccines, are currently under Phase 1/2 study with several in the preclinical studies (draft landscape of COVID-19 candidate vaccines).
By virtue of highly flexible and cost-effective features of the DNA vaccine platform, a series of prototypic DNA vaccine candidates based on differing lengths of the SARS-CoV-2 spike encoding gene have also been evaluated for immunogenicity and efficacy in rhesus macaques via the intramuscular route without an adjuvant [23]. These candidates included six variants of the SARS-CoV-2 S protein based on the site of truncation; full-length (S), cytoplasmic tail deletion mutant (S.dCT), transmembrane domain and cytoplasmic tail deletion mutant (S.dTM), S1 subunit (S1), receptor-binding domain (RBD), and a prefusion stabilized ectodomain with two proline mutations (S.dTM.PP). Spike-specific binding and virus-neutralizing antibody responses exhibiting various subclasses and effector functions were observed after boost immunization. Higher antibody-dependent complement deposition (ADCD) responses were also observed in the S and S.dCT groups, whereas higher natural killer (NK) cell activation was observed in the RBD and S.dTM.PP groups. Cellular immune responses targeted to a pool of S peptides were also detected in the majority of the vaccinated macaques shown by IFN-γ enzyme-linked immune absorbent spot (ELISPOT) assays after the booster dose. Intracellular cytokine staining assays showed an induction of S-specific IFN-γ+ CD4+ and CD8+ T cell responses, with relatively reduced responses observed in the shorter S1 and RBD immunogen groups. Moderate S-specific IL-4+ CD4+ and CD8+ T cell responses were observed, indicating a bias towards Th1 over Th2 cellular immune responses. The vaccinated animals challenged with SARS-CoV-2 showed a significant reduction of viral RNA, demonstrating the protective efficacy. However, a minimal level of protection was seen in the S.dTM group highlighting the importance of prefusion ectodomain stabilization for an effective vaccine [23]. More optimal protection was collectively achieved with the full-length S immunogen than soluble S immunogens and smaller fragments.
While the DNA vaccine platform might have the potential to generate protective responses, the risk of random insertion mutagenesis resulting from integration in the host genome, anti-DNA antibody generation, and auto-immune diseases will remain [64].
2.5. Recombinant Protein-Based Vaccine
Protein components of the targeted pathogen that can stimulate protective responses are considered useful for the subunit vaccines [65,66]. Typically, subunit vaccines constitute surface or structural proteins. For viral vaccine candidates, the spike protein required for virus attachment and entry into the host cell is often targeted with a rationale to elicit responses that can block viral entry [67,68,69]. SARS-CoV-2 spike (S) protein is composed of the S1 subunit that encompasses- N-Terminal Domain (NTD), RBD, and C-Terminal Domain (CTD), and S2 subunit that contains fusion peptide, the transmembrane domain, and the cytoplasmic tail [24,70]. Spike protein S assembles as a homotrimer and is heavily glycosylated [24,70]. Variants of SARS-CoV-2 spike antigens have been analyzed in a preclinical study in rabbits by Ravichandran et al. [71]. In this study, rabbits were immunized with spike-ectodomain (S1 + S2), S1 domain, receptor-binding domain (RBD), and S2 domain. They observed that all but the S2 group could induce strong nAb responses. Higher titers of high-affinity nAbs were observed in the RBD immunized group, supporting it as a promising vaccine candidate [71]. Similar RBD-induced potent responses were also observed in the preclinical studies of SARS, MERS, and other coronaviruses [72,73]. Both full-length S-trimer and RBD-based vaccine candidates such as that developed by Novavax (2020-004123-16) has entered Phase 3 study, followed by candidates developed by Sanofi Pasteur (NCT04537208), Clover Biopharmaceuticals/GSK (GlaxoSmithKline plc) (NCT04405908), Vaxine Pty Ltd. (NCT04453852), etc. in Phase 1/2 and Phase 1 trials, especially with different adjuvants (draft landscape of COVID-19 candidate vaccines).
Additionally, virus-like nanoparticles (VLP) mimicking the viral structural features but devoid of the genome are also planned to be evaluated in clinical trials. Medicago (NCT04450004) SARS-CoV-2 VLP (CoVLPs) vaccine adjuvanted with GSK proprietary adjuvant system is under Phase 1 study (draft landscape of COVID-19 candidate vaccines) [74]. Similarly, many other VLP-based vaccines are in preclinical trials. A non-invasive oral vaccine for SARS-CoV-2 designed by Vaxart, aimed at eliciting mucosal immune responses, is an additional novel vaccine candidate in the pipeline (https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-fda-clearance-ind-application-oral-covid-19). Adjuvants are commonly used in protein-based vaccine formulations that might govern the outcome of responses as well as the protective efficacy of a vaccine.
Exploring the intra-cutaneous route of administration for COVID-19 vaccine, a minimally-invasive microneedle array (MNA) vaccine delivery platform is also under development. Since the skin is abundantly rich with immune cells, specifically Langerhans cells, it is a robust target for immunization to generate potent immune responses [75,76]. SARS-CoV-2 spike protein fused to a trimerization motif-foldon (derived from phage T4 fibritin) was embedded into MNA for the SARS-CoV-2 vaccine [27]. A similar strategy was also applied previously for the MERS vaccine using its spike protein [27]. Furthermore, virus-specific nAbs were detected for the MERS-MNA vaccine in mice, while the neutralizing responses are yet to be determined for the SARS-CoV-2-MNA vaccine [27]. Though MNA-mediated immunization demonstrated potent adaptive responses based on the preclinical study in mice, the actual efficacy and protection will be obtained from future human clinical trials. By targeting the skin microenvironment through microneedle array, this platform utilizes physical adjuvant to generate antigen-specific responses with relatively low doses. Further studies to determine the potency of adaptive/innate responses in SARS-CoV-2-MNA vaccine recipients in clinical stages would be highly sought.
3. Insights from Immune Responses Elicited in the Recovered Patients for Vaccine Development
A more in-depth understanding of the immune responses that are elicited in recovering COVID-19 patients might provide useful insights into vaccine design. As a hallmark for an effective vaccine, the induction of virus-neutralizing antibody responses is often considered inevitable. With the recent approval of convalescent plasma therapy, the U.S. FDA ignited great interest in its therapeutic potential. However, several independent studies performed at multiple locations globally have shown that nAbs titers in the plasma of mildly symptomatic patients recovering from COVID-19 are highly variable [77,78,79]. A similar study, based on 68 convalescent SARS-CoV-2 patients, by Robbiani et al. showed that on average, the nAb titers remained low in these patients, even undetectable in 18% of them, while only 3% had high nAb titers [80]. Variability in nAbs titers was also reported by Wu et al., based on a cohort study of 175 convalescent COVID-19 patients [81]. Additionally, they observed higher nAb titers in older rather than younger people. Both studies also confirmed the presence of spike and RBD-specific antibodies, titers of which directly correlated with virus neutralization. Interestingly, RBD-specific antibodies were found to be effective even at much lower titers when tested for virus neutralization in in vitro assays.
Furthermore, RBD-specific B-cell precursors were identified to be commonly prevalent in patients based on antibody sequencing data [80,82,83]. Studies have also shown that several epitopes were targeted exclusively in the RBD region for antibody generation in natural infections, and the majority of such antibodies proved potent in virus neutralization [80]. Other than spike-directed responses, antibodies targeting the nucleoprotein (NP) of SARS-CoV-2 were also observed with the potential of virus neutralization in the COVID-19 infected patients [84]. However, expected variability in immune responses is due to many factors such as age, sex, geographical location, and prevalent strain of the virus, as reviewed above. Considering wide variations in nAbs titers in the convalescent patients and a lack of correlation with the disease and the recovery’s mild outcome, it is difficult to say if vaccine-elicited nAbs would be enough for adequate protection against SARS-CoV-2.
In order to mount a robust immune response against an invading pathogen, both adaptive and innate arms of the immune system work in conjunction. Though antibodies are traditionally considered as necessary molecules of immune defense, their generation relies on effective cross-talk with the T-cells. Griffoni et al. detected SARS-CoV-2-specific CD4+ and CD8+ T-cells in convalescent patients based on predicted T-cell epitopes spanning the whole viral genome. Spike-specific CD4+ T-cell responses were exceptionally prevalent in all infected individuals and were notably correlated with the anti-spike RBD antibody responses [85]. Unlike SARS infections where T-cell responses were predominantly directed to the viral spike, in SARS-CoV-2 infections, M and N proteins along with a spike were targeted to elicit T-cell responses.
Furthermore, CD4+ T-cells responses were also directed towards the non-structural antigens such as; nsp4, ORF3s, ORF7a, nsp12, and ORF8 [85]. This suggests that although spike/RBD is a prime vaccine target for all current vaccine development approaches, the inclusion of other structural and non-structural viral antigens might better recapitulate a scenario occurring in the convalescent patients after natural infection. Overall, T-cell immunity has been positively correlated with improved recovery in infected patients, and SARS-CoV-2 infected individuals with severe disease have been shown to undergo T-cell lymphopenia [86]. Some studies have also predicted the occurrence of T-cell exhaustion in COVID-19 patients [87]. Although virus targeted T-cell immune responses might be beneficial to consider for vaccine development, T-cell immunopathologies should be monitored in vaccine recipients, as these undesirable responses have previously been observed for SARS vaccine candidates.
Developing a vaccine that can cross-protect against similar coronaviruses would be an ideal consideration for the future. SARS shares about 80% of the sequence homology with SARS-CoV-2 at the genomic level, with both viruses utilizing the ACE2 receptor for the host cell entry [24,88,89]. While these commonalities have shown cross-reactive responses as observed by many studies, cross-protection could not become evident. This cross-reactivity is majorly attributed to the conserved viral antigenic epitopes and would be worth considering while designing a broadly cross-protective vaccine against related coronaviruses. However, such attempts should be made cautiously as the presence of cross-reactive antibodies has also been previously observed to enhance the infection through antibody-dependent enhancement (ADE) in case of other viral infections, including SARS [18,90]. Thus, despite the urgency of a SARS-CoV-2 vaccine, both the safety and efficacy should be critically evaluated before licensing a vaccine.
4. Rapid Nature of the Vaccine Development and Its Drawbacks
Owing to the advancements in vaccine development in recent decades, the time-frame for bringing a vaccine from bench to bedside has considerably shortened. Ebola and zika vaccine development exemplify this rapid clinical translation [91]. While it is very much possible to develop any vaccine with a targeted approach in months to years’ time window, rigorous evaluation in large scale human studies with extended follow-up studies are essential to determine the durability of responses and long-term vaccine efficacy. Additionally, many vaccine studies enroll young, healthy volunteers to assess vaccine efficacy and rapidly progress through vaccine development stages. Due to the urgent need for the COVID-19 vaccine, if a vaccine is licensed based on healthy people’s safety and efficacy, then the response of high-risk people (elderly individuals, children, pregnant women, nursing mothers, etc.) to the vaccine will remain unknown. COVID-19-related immunopathologies observed in severe cases of SARS-CoV-2 infected individuals pose the greatest risk for the safety of a vaccine [6,8]. Further determination of risk due to interaction between the vaccine and virus-induced responses after natural infection in vaccinated individuals will remain a critical focus.
5. Conclusions
We are in the very initial stages of understanding the interaction between the immune system and SARS-CoV-2 vaccines to mediate protection and/or susceptibility to COVID-19. However, basic viral immunology knowledge can serve to design a vaccine. Currently, hundreds of COVID-19 vaccine candidates are in development, and success is unknown. However, owing to the concerted efforts made around the globe in a short period to end this pandemic, the likelihood of finding a successful candidate/s is relatively high, especially with the use of a variety of vaccine platforms. Hopefully, the critical evaluation of vaccine candidates for their safety, efficacy, long-term immunity, and protection in widespread population groups will soon bring the COVID-19 pandemic to an end.
Author Contributions
S.C. conceived the idea. S.J., H.B., and P.Y. wrote the manuscript. S.C. proofread the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SARS | Severe Acute Respiratory Syndrome |
| nAbs | Neutralizing antibodies |
| MERS | Middle East respiratory syndrome |
| VLPs | Virus-like particles |
| ACE2 | Angiotensin I Converting Enzyme 2 |
| APC | Antigen-presenting cell |
| Ad5 | Adenovirus type-5 |
| VSV | Vesicular Stomatitis Virus |
| RBD | Receptor-Binding Domain |
| ELISPOT | Enzyme-linked immune absorbent spot |
| TLRs | Toll-like receptors |
| PRRs | Pattern recognition receptors |
| PAMPs | Pathogen associated molecular patterns |
| NIAID | National Institute of Allergy and Infectious Diseases |
| ADCD | Antibody-dependent complement deposition |
| MNA | Microneedle array |
| MVA | Modified vaccinia virus Ankara |
| ADE | Antibody-dependent enhancement |
References
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar]
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.-J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nat. Cell Biol. 2020, 583, 286–289. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary Trajectory for the Emergence of Novel Coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; Van De Veen, W.; Brüggen, M.; O’Mahony, L.; Gao, Y.; Nadeau, K.; A Akdis, C. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lin, J.; Zhang, Z.; Xiao, L.; Jiang, Z.; Chen, J.; Hu, C.; Luo, S. Alert for non-respiratory symptoms of coronavirus disease 2019 patients in epidemic period: A case report of familial cluster with three asymptomatic COVID-19 patients. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A. Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19). Travel Med. Infect. Dis. 2020, 35, 101608. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, H.; Liu, X.; Tong, Z. Asymptomatic cases with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Rabenau, H.F.; Adams, O.; Oberle, D.; Funk, M.B.; Keller-Stanislawski, B.; Timm, J.; Drosten, C.; Ciesek, S. SARS-CoV -2 asymptomatic and symptomatic patients and risk for transfusion transmission. Transfusion 2020, 60, 1119–1122. [Google Scholar] [CrossRef]
- Lee, S.; Meyler, P.; Mozel, M.; Tauh, T.; Merchant, R. Asymptomatic carriage and transmission of SARS-CoV-2: What do we know? Can. J. Anesth. 2020, 67, 1424–1430. [Google Scholar] [CrossRef]
- Gao, M.; Yang, L.; Chen, X.; Deng, Y.; Yang, S.; Xu, H.; Chen, Z.; Gao, X. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir. Med. 2020, 169, 106026. [Google Scholar] [CrossRef]
- Pfefferbaum, B.; North, C.S. Mental Health and the Covid-19 Pandemic. N. Engl. J. Med. 2020, 383, 510–512. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Shao, Y.; Wang, X.; Zhang, H.; Shuai, L.; Ge, J.; Wen, Z.; Bu, Z. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antivir. Res. 2018, 150, 30–38. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: A dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 816–826. [Google Scholar] [CrossRef]
- Wang, S.-F.; Tseng, S.-P.; Yen, C.-H.; Yang, J.-Y.; Tsao, C.-H.; Shen, C.-W.; Chen, K.-H.; Liu, F.-T.; Liu, W.-T.; Chen, Y.-M.A.; et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014, 451, 208–214. [Google Scholar] [CrossRef]
- Muthumani, K.; Falzarano, D.; Reuschel, E.L.; Tingey, C.; Flingai, S.; Villarreal, D.O.; Wise, M.C.; Patel, A.; Izmirly, A.; Aljuaid, A.; et al. A synthetic consensus anti–spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015, 7, 301ra132. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; O’Connor, M.A.; Walls, A.C.; Hemann, E.A.; Murapa, P.; Archer, J.; Leventhal, S.; Fuller, J.T.; Lewis, T.B.; et al. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; Mcmahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Cao, H.; Liu, C. SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Zhao, P.; Ke, J.-S.; Qin, Z.-L.; Ren, H.; Zhao, L.-J.; Yu, J.-G.; Gao, J.; Zhu, S.-Y.; Qi, Z.-T. DNA Vaccine of SARS-Cov S Gene Induces Antibody Response in Mice. Acta Biochim. Biophys. Sin. 2004, 36, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Clark, R.; Kupper, T. Old Meets New: The Interaction Between Innate and Adaptive Immunity. J. Investig. Dermatol. 2005, 125, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nat. Cell Biol. 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Kool, M.; Pétrilli, V.; De Smedt, T.; Rolaz, A.; Hammad, H.; Van Nimwegen, M.; Bergen, I.M.; Castillo, R.; Lambrecht, B.N.; Tschopp, J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. J. Immunol. 2008, 181, 3755–3759. [Google Scholar] [CrossRef] [PubMed]
- Jiskoot, W.; Kersten, G.F.A.; Mastrobattista, E.; Slütter, B. Vaccines. In Pharmaceutical Biotechnology: Fundamentals and Applications; Crommelin, D.J.A., Sindelar, R.D., Meibohm, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 281–304. [Google Scholar]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. Vaccine Anal. Strat. Princip. Control. 2014, 2014, 45–80. [Google Scholar] [CrossRef]
- Pearce, J.M.S. Salk and Sabin: Poliomyelitis immunisation. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1552. [Google Scholar] [CrossRef] [PubMed]
- Badgett, M.R.; Auer, A.; Carmichael, L.E.; Parrish, C.R.; Bull, J.J. Evolutionary Dynamics of Viral Attenuation. J. Virol. 2002, 76, 10524–10529. [Google Scholar] [CrossRef]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 480, 379–392. [Google Scholar] [CrossRef]
- Poon, B.; Hsu, J.F.; Gudeman, V.; Chen, I.S.Y.; Grovit-Ferbas, K. Formaldehyde-Treated, Heat-Inactivated Virions with Increased Human Immunodeficiency Virus Type 1 Env Can Be Used To Induce High-Titer Neutralizing Antibody Responses. J. Virol. 2005, 79, 10210–10217. [Google Scholar] [CrossRef][Green Version]
- Cryz, S.J.; Fürer, E.; Germanier, R. Effect of chemical and heat inactivation on the antigenicity and immunogenicity of Vibrio cholerae. Infect. Immun. 1982, 38, 21–26. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; Mcmahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 1–11. [Google Scholar] [CrossRef]
- Ewer, K.; Sebastian, S.; Spencer, A.J.; Gilbert, S.; Hill, A.V.; Lambe, T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum. Vaccines Immunother. 2017, 13, 3020–3032. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lambe, T.; Spencer, A.J.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 1–8. [Google Scholar] [CrossRef]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.-R.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime—MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Chahal, J.S.; Fang, T.; Woodham, A.W.; Khan, O.F.; Ling, J.; Anderson, D.G.; Ploegh, H.L. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Wang, F.; Kream, R.M.; Stefano, G.B. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med. Sci. Monit. 2020, 26, e924700–e924701. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness. bioRxiv 2020. [Google Scholar] [CrossRef]
- Liu, M.A.; Ulmer, J.B. Human Clinical Trials of Plasmid DNA Vaccines. Adv. Genetics 2005, 55, 25–40. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Spies, B.; Hochrein, H.; Vabulas, M.; Huster, K.; Busch, D.H.; Schmitz, F.; Heit, A.; Wagner, H. Vaccination with Plasmid DNA Activates Dendritic Cells via Toll-Like Receptor 9 (TLR9) but Functions in TLR9-Deficient Mice. J. Immunol. 2003, 171, 5908–5912. [Google Scholar] [CrossRef]
- Kono, D.H.; Haraldsson, M.K.; Lawson, B.R.; Pollard, K.M.; Koh, Y.T.; Du, X.; Arnold, C.N.; Baccala, R.; Silverman, G.J.; Beutler, B.A.; et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc. Natl. Acad. Sci. USA 2009, 106, 12061–12066. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.-D.; et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019, 19, 1013–1022. [Google Scholar] [CrossRef]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and Immunogenicity of an Anti–Zika Virus DNA Vaccine—Preliminary Report. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Górecki, D.C.; Simons, J.P. The dangers of DNA vaccination. Nat. Med. 1999, 5, 126. [Google Scholar] [CrossRef]
- Cunningham, A.L. The herpes zoster subunit vaccine. Expert Opin. Biol. Ther. 2016, 16, 265–271. [Google Scholar] [CrossRef]
- Inoue, N.; Abe, M.; Kobayashi, R.; Yamada, S. Vaccine Development for Cytomegalovirus. Adv. Exp. Med. Biol. 2018, 1045, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wong, G.; Lu, G.; Yan, J.; Gao, G.F. MERS-CoV spike protein: Targets for vaccines and therapeutics. Antivir. Res. 2016, 133, 165–177. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Genet. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Pantophlet, R.; Burton, D.R. GP120: Target for Neutralizing HIV-1 Antibodies. Annu. Rev. Immunol. 2006, 24, 739–769. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.-C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef]
- Ravichandran, S.; Coyle, E.M.; Klenow, L.; Tang, J.; Grubbs, G.; Liu, S.; Wang, T.; Golding, H.; Khurana, S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci. Transl. Med. 2020, 12, eabc3539. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hotez, P.J.; Bottazzi, M.E. Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Hum. Vaccines Immunother. 2020, 16, 1239–1242. [Google Scholar] [CrossRef]
- Qi, X.; Ke, B.; Feng, Q.; Yang, D.; Lian, Q.Q.; Li, Z.Z.; Lu, L.; Ke, C.; Liu, Z.; Liao, G. Construction and immunogenic studies of a mFc fusion receptor binding domain (RBD) of spike protein as a subunit vaccine against SARS-CoV-2 infection. Chem. Commun. 2020, 56, 8683–8686. [Google Scholar] [CrossRef]
- Roy, S.; Ghani, K.; De Campos-Lima, P.O.; Caruso, M. Efficient production of Moloney murine leukemia virus-like particles pseudotyped with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike protein. bioRxiv 2020, 2020. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2018, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Haniffa, M.; Kaplan, D.H. Antigen-Presenting Cells in the Skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 vaccines: Neutralizing antibodies and the alum advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef]
- Kwong, P.D.; Mascola, J.R.; Nabel, G.J. Rational Design of Vaccines to Elicit Broadly Neutralizing Antibodies to HIV-1. Cold Spring Harb. Perspect. Med. 2011, 1, a007278. [Google Scholar] [CrossRef]
- Messer, R.J.; Dittmer, U.; Peterson, K.E.; Hasenkrug, K.J. Essential role for virus-neutralizing antibodies in sterilizing immunity against Friend retrovirus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 12260–12265. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nat. Cell Biol. 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L.; et al. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. SSRN Electron. J. 2020, 2020. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84. [Google Scholar] [CrossRef]
- Liu, A.; Li, Y.; Peng, J.; Huang, Y.; Xu, D. Antibody responses against SARS-CoV-2 in COVID-19 patients. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Yin, S.; Tong, X.; Huang, A.; Shen, H.; Li, Y.; Liu, Y.; Wu, C.; Huang, R.; Chen, Y. Longitudinal anti-SARS-CoV-2 antibody profile and neutralization activity of a COVID-19 patient. J. Infect. 2020, 81, e31–e32. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, W.; Wan, L.; Xiang, T.; Zhang, W. Correlation Between Relative Nasopharyngeal Virus RNA Load and Lymphocyte Count Disease Severity in Patients with COVID-19. Viral Immunol. 2020. [Google Scholar] [CrossRef]
- Drewry, A.M.; Hotchkiss, R.; Kulstad, E. Response to “COVID-19: Room for treating T cell exhaustion? ” Crit. Care 2020, 24, 345. [Google Scholar] [CrossRef]
- Jaimes, J.A.; André, N.M.; Chappie, J.S.; Millet, J.K.; Whittaker, G.R. Phylogenetic Analysis and Structural Modeling of SARS-CoV-2 Spike Protein Reveals an Evolutionary Distinct and Proteolytically Sensitive Activation Loop. J. Mol. Biol. 2020, 432, 3309–3325. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Ulrich, H.; Pillat, M.M.; Tárnok, A. Dengue Fever, COVID-19 (SARS-CoV-2), and Antibody-Dependent Enhancement (ADE): A Perspective. Cytometry A 2020, 97, 662–667. [Google Scholar]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).