Immunogenicity and Safety of the Quadrivalent Adjuvant Subunit Influenza Vaccine in Seropositive and Seronegative Healthy People and Patients with Common Variable Immunodeficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trial Design
2.2. Participants
- Healthy people aged from 18 to 52 years without chronic bronchopulmonary, cardiovascular, rheumatological diseases, hepatic or renal impairment, metabolic disorders confirmed by anamnestic data or objective clinical examination
- Signed informed consent.
- Confirmed diagnosis CVID in accordance with diagnostic criteria established by the European Society for Immunodeficiency Diseases (http://esid.org/WorkingParties/Registry/Diagnosis-criteria) and the American Academy of Allergy, Asthma and Immunology for the diagnosis and treatment of PID.
- Replacement immunotherapy with IVIG drugs no later than 28 days before vaccination and no earlier than 21 days after it, that is, a break between two subsequent administrations of immunoglobulins for at least 7 weeks
- Signed informed consent.
- A history of allergy to egg whites or any component of the studied vaccine.
- Symptoms of influenza or flu-like illness in the past 6 months.
- Vaccination against influenza within the last 12 months.
- Symptoms of acute infection or exacerbation of chronic disease at the time of vaccination or during 1 month before current vaccination.
- Glucocorticosteroid or other immunosuppressive therapy admission at the time of the study and 3 months before the start.
- Any medical interventions that have been identified as affecting the effectiveness of vaccination and receiving any type of vaccine within 1.5–2 months prior to inclusion in the study.
- Symptoms of enteropathy with protein loss in patients with CVID at the time of the study.
- Cognitive or behavioral or psychiatric disorders or alcohol abuse.
2.3. Vaccines
2.4. Immunogenicity Assessment
2.5. Safety Assessment
2.6. Statistics
3. Results
3.1. Safety of Vaccination
3.2. Assessment of Vaccine Immunogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data availability
Ethics approval and consent to participate
Abbreviations
| CVID | common variable immune deficiency |
| GMR | geometric mean rate |
| GMT | geometric mean antibody titers |
| HA | hemagglutinin |
| HI | hemagglutination inhibition assay |
| IVIG | Intravenous immunoglobulin |
| PID | primary immune deficiency |
| QIV | qudrivalent inactivated vaccine |
| aQIV | adjuvant qudrivalent inactivated vaccine |
| TIV | trivalent inactivated vaccine |
| aTIV | adjuvant trivalent inactivated vaccine |
References
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.P.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and virological characteristics of influenza B: Results of the Global Influenza B Study. Influ. Other Respir. Viruses 2015, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kharit, S.M.; Rudakova, A.M.; Uskov, A.N.; Konovalova, L.N.; Lobzin, Y.V. The averted costs due to influenza vaccination with trivalent and quadrivalent vaccines. J. Infectol. 2017, 9, 17–22. [Google Scholar] [CrossRef][Green Version]
- Ambrose, C.S.; Levin, M.J. The rationale for quadrivalent influenza vaccines. Hum. Vaccines Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef]
- Beran, J.; Wertzova, V.; Honegr, K.; Kaliskova, E.; Havlíčková, M.; Havlík, J.; Jiřincová, H.; Van Belle, P.; Jain, V.; Innis, B.L.; et al. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: A concrete example. BMC Infect. Dis. 2009, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Coelingh, K.; Ambrose, C.S.; Woo, J.C.; Wu, X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010, 28, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Pepin, S.; Donazzolo, Y.; Jambrecina, A.; Salamand, C.; Saville, M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine 2013, 31, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, D.; Sheldon, E.; Lin, W.-Y.; Yu, C.-J.; Bayas, J.M.; Gabor, J.J.; Esen, M.; Roure, J.L.F.; Perez, S.N.; Sanchez, C.A.; et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: A phase III, randomized trial in adults aged ≥18 years. BMC Infect. Dis. 2013, 13, 343. [Google Scholar] [CrossRef]
- Tinoco, J.C.; Pavia-Ruz, N.; Cruz-Valdez, A.; Doniz, C.A.; Chandrasekaran, V.; Dewé, W.; Liu, A.; Innis, B.L.; Jain, V. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: A phase III, randomized trial. Vaccine 2014, 32, 1480–1487. [Google Scholar] [CrossRef]
- Greenberg, D.P.; Robertson, C.A.; Noss, M.J.; Blatter, M.M.; Biedenbender, R.; Decker, M.D. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013, 31, 770–776. [Google Scholar] [CrossRef]
- Treanor, J.T.; Albano, F.R.; Sawlwin, D.C.; Jones, A.G.; Airey, J.; Formica, N.; Matassa, V.; Leong, J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: A phase 3, randomized noninferiority study. Vaccine 2017, 35, 1856–1864. [Google Scholar] [CrossRef]
- Cowling, B.J.; Perera, R.A.P.M.; Valkenburg, S.A.; Leung, N.H.L.; Iuliano, A.D.; Tam, Y.H.; Wong, J.H.F.; Fang, V.J.; Li, A.P.Y.; So, H.C.; et al. Comparative Immunogenicity of Several Enhanced Influenza Vaccine Options for Older Adults: A Randomized, Controlled Trial. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Essink, B.; Fierro, C.; Rosen, J.; Figueroa, A.L.; Zhang, B.; Verhoeven, C.; Edelman, J.; Smolenov, I. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine 2019, 38, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Levine, M.Z.; Olaiya, O.; Gross, F.L.; King, J.P.; Flannery, B.; McLean, H.Q. Clinical trial to assess immunogenicity of high-dose, adjuvanted, and recombinant influenza vaccines against cell-grown A(H3N2) viruses in adults 65 to 74 years, 2017–2018. Vaccine 2020, 38, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Herbinger, K.-H.; Von Sonnenburg, F.; Nothdurft, H.D.; Perona, P.; Borkowski, A.; Fragapane, E.; Nicolay, U.; Clemens, R. A phase II study of an investigational tetravalent influenza vaccine formulation combining MF59®. Hum. Vaccines Immunother. 2013, 10, 92–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Instruction for Use of the Grippol® Quadrivalent. Available online: http://petrovax.ru/upload/iblock/5b3/Grippol_-Kvadrivalent-rastvor-dlya-vnutrimyshechnogo-i-podkozhnogo-vvedeniya (accessed on 2 October 2020).
- Zverev, V.V.; Semenov, B.F.; Khaitov, R.M. (Eds.) Vaccine and Vaccination: National Guideline; GEOTAR-Media: Moscow, Russia, 2014; 640p. (In Russian) [Google Scholar]
- Kostinov, M.P.; Chuchalin, A.G. Manual on Clinical Immunology in Respiratory Medicine, 1st ed.; Atmosfera Publishing Company LLC: Moscow, Russia, 2016; 128p. (In Russian) [Google Scholar]
- Kostinov, M.P.; Tarasova, A.A. Vaccination of Pneumococcal Infection and Influenza in Autoimmune Diseases; Guide for Doctors; MDV Group: Moscow, Russia, 2009; ISBN 978-5-91629-006-6. [Google Scholar]
- Cherdantsev, A.P.; Kuselman, A.I.; Sinitsyna, M.N.; Shalyagina, M.E.; Kostinov, M.P.; Tarbaeva, D.A. A Study of the Clinical Safety of Pregnant Influenza Vaccination. Med. Alm. 2011, 4, 120–122. Available online: https://www.elibrary.ru/item.asp?id=23622202 (accessed on 2 October 2020). (In Russian).
- Kostinov, M.P.; Petrovich, C.A.; Savisko, A.A.; Alexandrovna, T.D.; Leonidovna, S.I. True and false reactions in pregnant women to introduction of influenza vaccine. Gynecol. Obstet. Perinatol. 2011, 10, 44–48. Available online: https://www.elibrary.ru/item.asp?id=17330729 (accessed on 2 October 2020). (In Russian).
- Zverev, V.V.; Kostinov, M.P.; Cherdantsev, A.P. Vaccination against Influenza in Pregnancy; Federal Clinical Guidelines; Remedium: Moscow, Russia, 2015; ISBN 978-5-906 125-13-2. (In Russian) [Google Scholar]
- Kostinov, M.P.; Cherdantsev, A.P. The clinical and immunological safett of inactivated immunologic agjuvant influenza subunit vaccine for pregnant women. Obstet. Ginecol. 2016, 2, 64–69. (In Russian) [Google Scholar]
- Kostinov, K.M.; Cherdantsev, C.A. The clinical and immunological safety of inactivated immunologic adjuvant influenza subunit vaccine for pregnant women. Akusherstvo I Ginekologiya 2016, 95, 67–71. [Google Scholar] [CrossRef]
- Kostinov, M.; Cherdantsev, A.; Semenova, S.; Tarbaeva, D.; Savisko, A.; Serova, O.; Iozefson, S.; Akhmatova, N.K.; Kostinova, T.; Praulova, D.; et al. Obstetric and perinatal outcomes among pregnant women after influenza vaccination and after transferred respiratory infection. Gynecol. 2015, 17, 43–46. [Google Scholar] [CrossRef]
- Alexia, C.; Cren, M.; Louis-Plence, P.; Vo, D.-N.; Ahmadi, Y.E.; Dufourcq-Lopez, E.; Lu, Z.-Y.; Hernandez, J.; Shamilov, F.; Chernysheva, O.; et al. Polyoxidinium® activates cytotoxic lymphocyte responses through dendritic cell maturation: Clinical effects in breast cancer. Front. Immunol. 2019, 10, 2693. [Google Scholar] [CrossRef]
- Vaccine Adjuvant «Polyoxidonium®» Strengthens the Immune Response to a Low Dose of Influenza Antigens. Available online: https://www.elibrary.ru/item.asp?id=39471103 (accessed on 2 October 2020).
- Chuchalina, A.G.; Yasnetsova, V.V. Federal Guideline on Medicine Use, 17th ed.; OOO Vidoks: Moscow, Russia, 2016; pp. 745–768. (In Russian) [Google Scholar]
- Kostinov, M.P. Vaccination of Children with Weak Health. Practical Manual for Doctors, 4th ed.; Medicine for Everybody: Moscow, Russia, 2013. (In Russian) [Google Scholar]
- Van Assen, S.; Holvast, A.; Telgt, D.S.; Benne, C.A.; De Haan, A.; Westra, J.; Kallenberg, C.G.; Bijl, M. Patients with humoral primary immunodeficiency do not develop protective anti-influenza antibody titers after vaccination with trivalent subunit influenza vaccine. Clin. Immunol. 2010, 136, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Hanitsch, L.; Löbel, M.; Mieves, J.F.; Bauer, S.; Babel, N.; Schweiger, B.; Wittke, K.; Grabowski, P.; Volk, H.-D.; Scheibenbogen, C. Cellular and humoral influenza-specific immune response upon vaccination in patients with common variable immunodeficiency and unclassified antibody deficiency. Vaccine 2016, 34, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Van Assen, S.; De Haan, A.; Holvast, A.; Horst, G.; Gorter, L.; Westra, J.; Kallenberg, C.G.M.; Telgt, D.S.; Palache, A.M.; Giezeman, K.; et al. Cell-mediated immune responses to inactivated trivalent influenza-vaccination are decreased in patients with common variable immunodeficiency. Clin. Immunol. 2011, 141, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, G.K.; Halstensen, A.; Sjursen, H.; Naess, A.; Kristoffersen, E.K.; Cox, R.J.; Næss, A. Pandemic Influenza Vaccination Elicits Influenza-Specific CD4+ Th1-cell Responses in Hypogammaglobulinaemic Patients: Four case reports. Scand. J. Immunol. 2011, 74, 210–218. [Google Scholar] [CrossRef]
- Manuel, O.; Pascual, M.; Hoschler, K.; Giulieri, S.; Alves, D.; Ellefsen, K.; Bart, P.-A.; Venetz, J.-P.; Calandra, T.; Cavassini, M. Humoral Response to the Influenza A H1N1/09 Monovalent AS03-Adjuvanted Vaccine in Immunocompromised Patients. Clin. Infect. Dis. 2010, 52, 248–256. [Google Scholar] [CrossRef]
- Khromova, E.A.; Akhmatova, E.A.; Skhodova, S.A.; Semochkin, L.A.; Khomenkov, V.G.; Akhmatova, N.K.; Kostinov, M.P. The effect of influenza vaccines on blood dendritic cell subpopulations. J. Mikrobiol. Epidemiol. Immunobiol. 2016, 5, 23–28. [Google Scholar] [CrossRef]
- Chromova, E.A.; Semochkin, L.A.; Akhmatova, E.A.; Stolpnikova, V.N.; Skhodova, S.A.; Sorokina, E.V.; Akhmatova, N.K.; Kostinov, M.P. Comparative Activity of Influenza Vaccines: Effect on Lymphocyte Subpopulation Structure. J. Mikrobiol. Epidemiol. Immunobiol. 2016, 6, 61–65. [Google Scholar] [CrossRef]
- Khromova, E.A.; Semochkin, I.A.; Akhmatova, E.A.; Stolpnikova, V.N.; Skhodova, S.A.; Sorokina, E.V.; Troynich, Y.N.; Akhmatova, N.K.; Kostinov, M.P. Change of Lymphocyte Immunophenotype under the Influence of Immunoadjuvant and Nonadjuvant Influenza Vaccines. J. Microbiol. Epidemiol. Immunobiol. 2016, 10, 503–504. Available online: https://www.elibrary.ru/item.asp?id=29124209 (accessed on 2 October 2020).
- Saxena, S.K. Influenza—Therapeutics and Challenges; IntechOpen: London, UK, 2018; Volume 1, Chapter 5; pp. 83–109. [Google Scholar]
- Alexandrovna, K.E.; Semochkin, I.A.; Akhmatova, E.A.; Stolpnikova, V.N.; Skhodova, S.A.; Sorokina, E.V.; Troynich, Y.N.; Akhmatova, N.K.; Kostinov, M.P. Influenza vaccines: Influence on TLRs expression. Russ. Immunol. J. 2016, 10, 505–507. Available online: https://www.elibrary.ru/item.asp?id=29132637 (accessed on 2 October 2020). (In Russian).
- Khromova, E.A.; Skhodova, S.A.; Stolpnikova, V.N.; Kukina, O.M.; Akhmatova, N.K.; Kostinov, M.P. Activation of Toll-like receptors with influenza vaccines (IN VITRO). Med. Immunol. 2017, 19, 71–72. Available online: https://www.elibrary.ru/item.asp?id=29758140 (accessed on 2 October 2020).
- Kharit, S.M.; Lioznov, D.A.; Ruleva, A.A.; Fridman, I.V.; Chirun, N.V.; Aprjatina, V.A. Comparative Assessment of Reactogenicity and Immunogenicity of Commercial Influenza Inactivated Vaccines: Polymer-Subunit Grippol plus, Subunit Influvac, Split Vaccine Waxigrip. Epidemiol. Vaccinal Prev. 2017, 16, 24–30. [Google Scholar] [CrossRef]
- Gardulf, A.; Abolhassani, H.; Gustafson, R.; Eriksson, L.E.; Hammarström, L. Predictive markers for humoral influenza vaccine response in patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 2018, 142, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- North, M.E.; Webster, A.D.; Farrant, J. Primary defect in CD8+ lymphocytes in the antibody deficiency diseases (common variable immunodeficiency): Abnormalities in intracellular production of interferon-gamma (INF-gamma) in CD28 (“cytotoxic”) and CD28- (“suppressor”) CD8+ subsets. Clin. Exp. Immunol. 1998, 111, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Setdikova, N. Immunomodulators in Complex Therapy of Immunocompromised Patients. Ph.D. Thesis, NRC Institute of Immunology, Moscow, Russia, 2002. (In Russian). [Google Scholar]
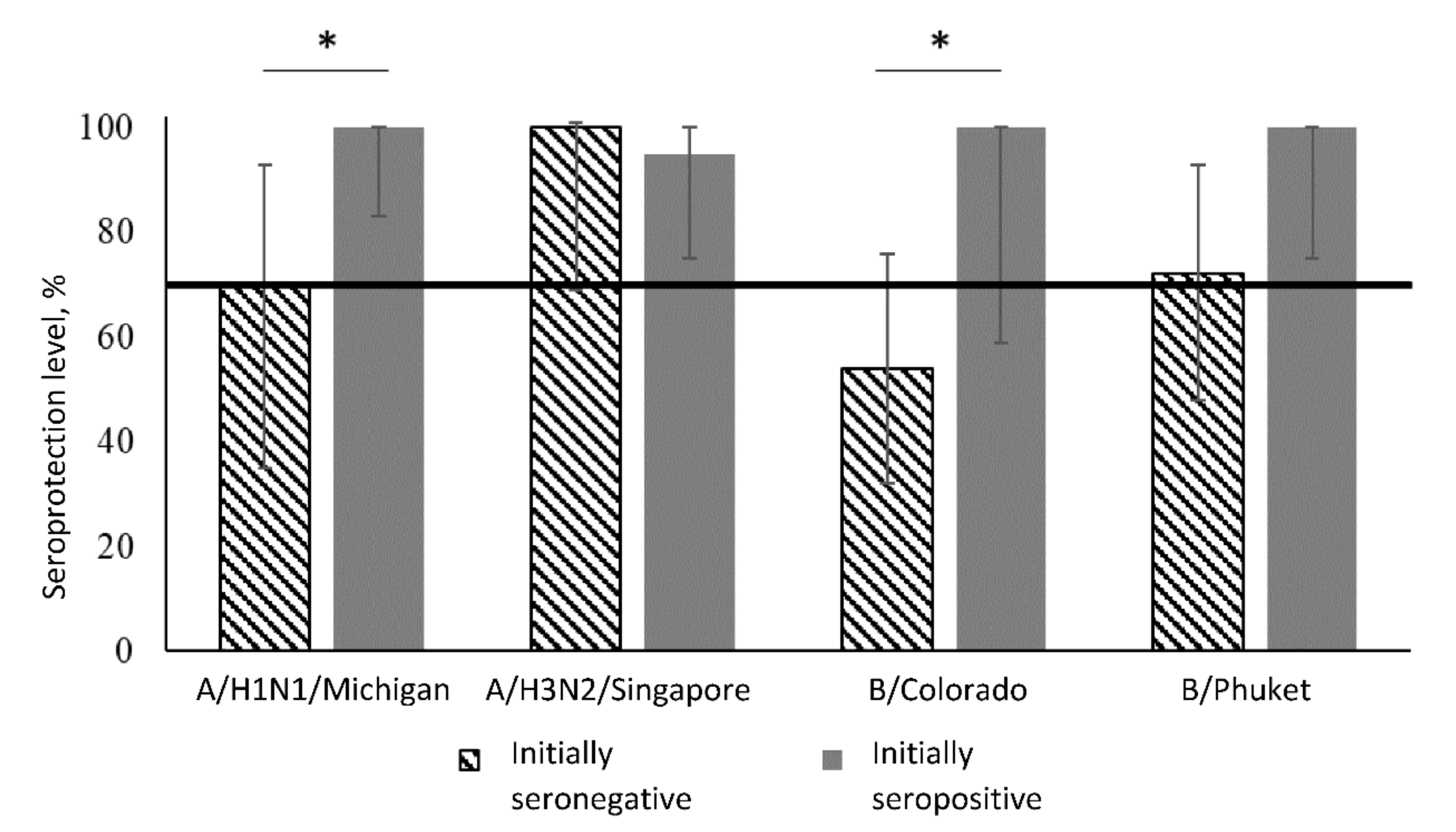
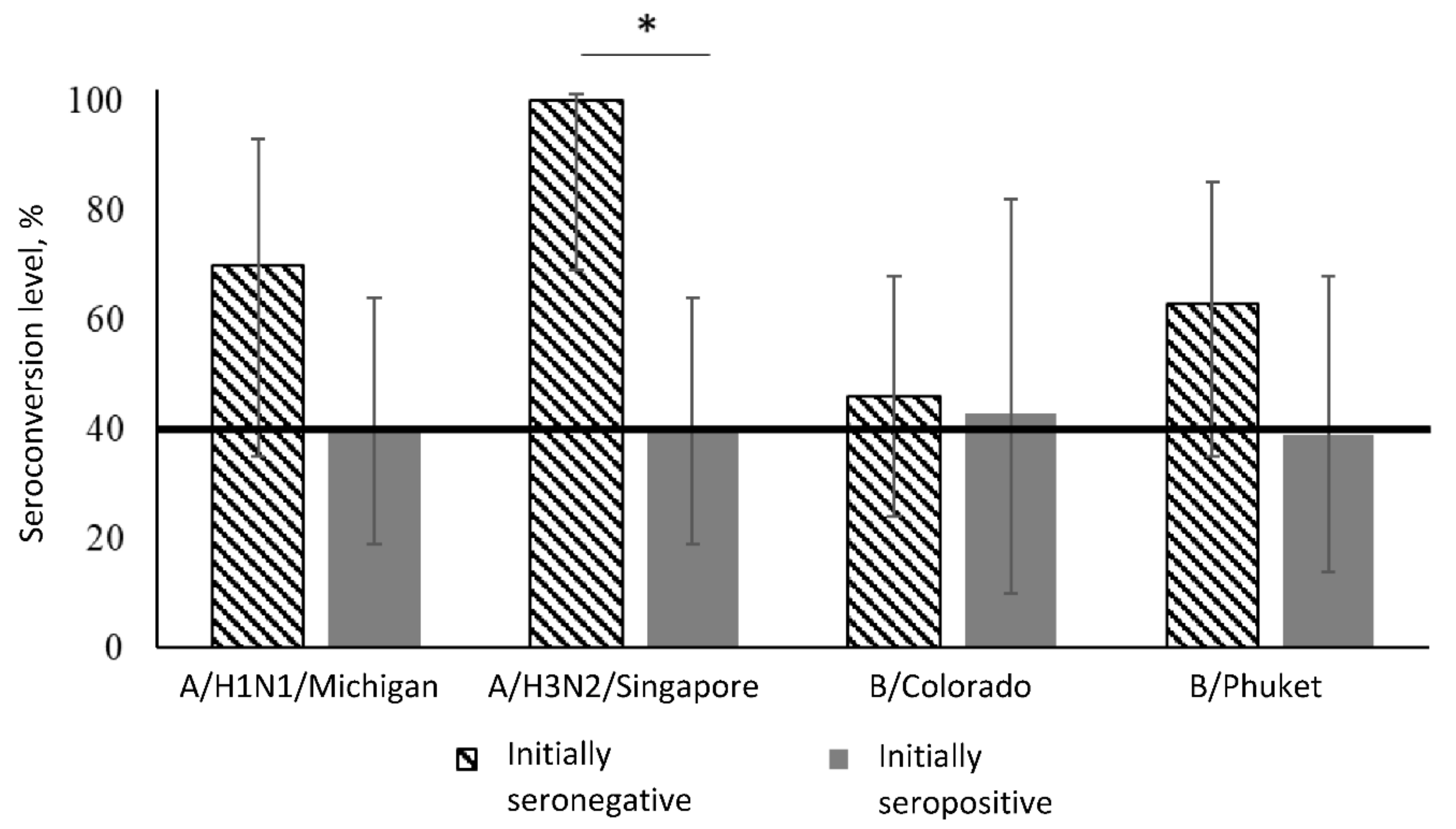
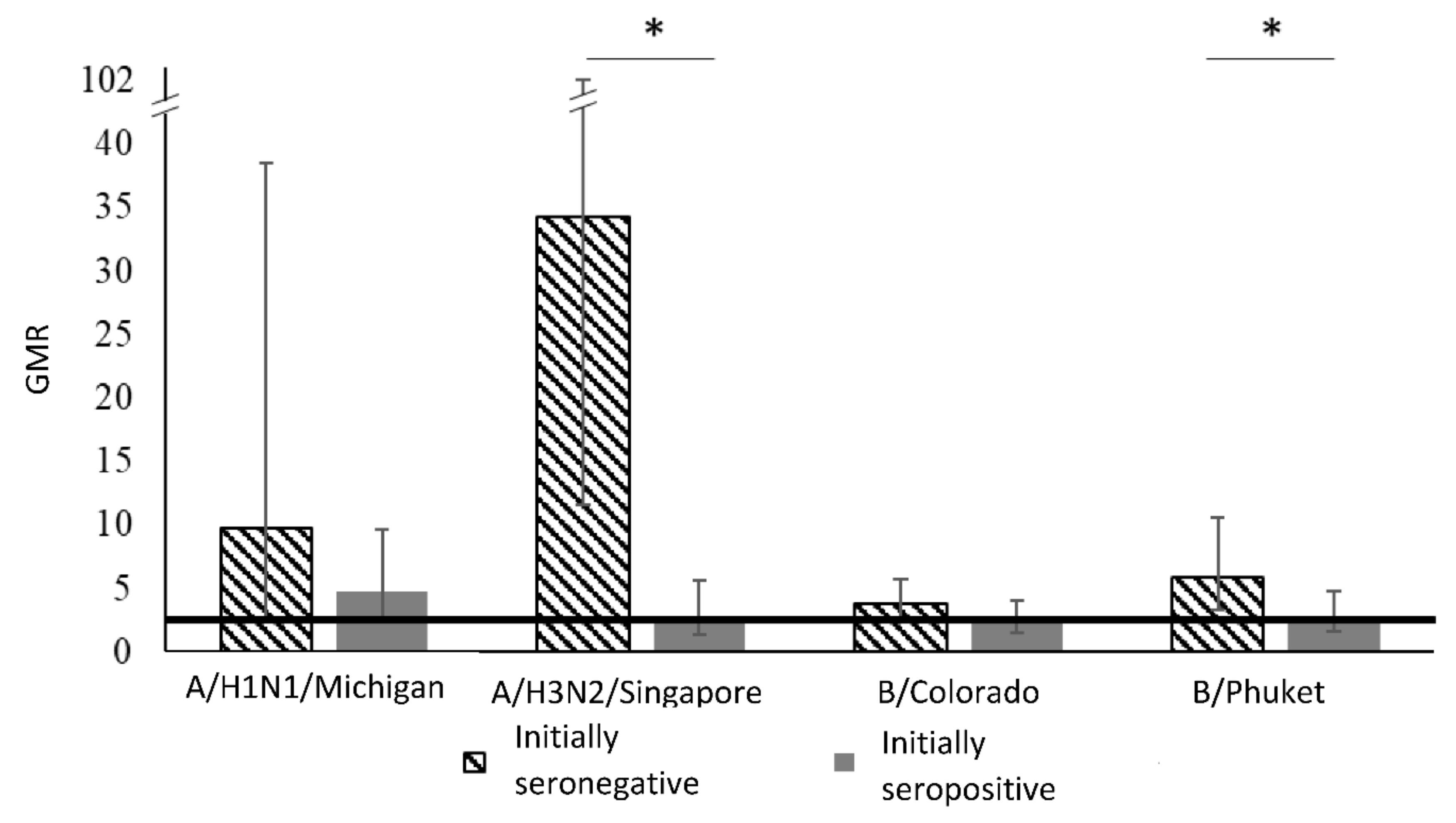
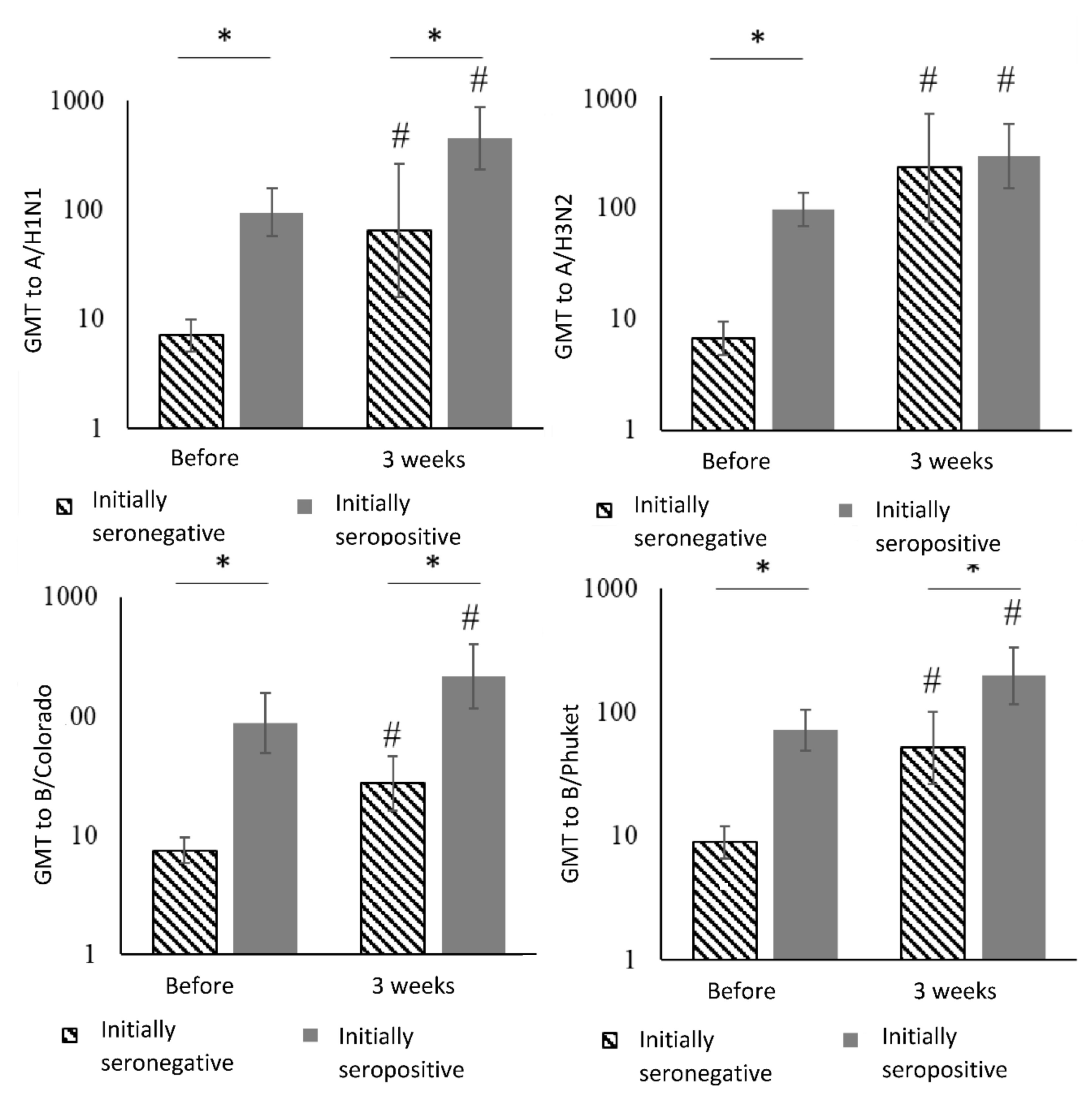
| Virus Strain | Period | Initially Seronegative | Initially Seropositive | Between Groups 1 | ||||
|---|---|---|---|---|---|---|---|---|
| People | % | 95%CI | People | % | 95%CI | |||
| A/H1N1 | Before vaccination | 0/12 | 0 | (0–26) | 20/20 | 100 | (83–100) | p < 0.001 |
| After 3 weeks | 7/10 | 70 | (35–93) | 20/20 | 100 | (83–100) | p = 0.03 | |
| Dynamics analysis 2 | p = 0.02 | p = 1.00 | – | |||||
| A/H3N2 | Before vaccination | 0/10 | 0 | (0–31) | 22/22 | 100 | (85–100) | p < 0.001 |
| After 3 weeks | 10/10 | 100 | (69–100) | 19/20 | 95 | (75–100) | p = 1.00 | |
| Dynamics analysis | p = 0.002 | p = 1.00 | – | |||||
| B/Colorado | Before vaccination | 0/24 | 0 | (0–14) | 7/7 | 100 | (59–100) | p < 0.001 |
| After 3 weeks | 12/22 | 54 | (32–76) | 7/7 | 100 | (59–100) | p = 0.03 | |
| Dynamics analysis | p < 0.001 | p = 1.00 | – | |||||
| B/Phuket | Before vaccination | 0/18 | 0 | (0–19) | 13/13 | 100 | (75–100) | p < 0.001 |
| After 3 weeks | 12/16 | 72 | (48–93) | 13/13 | 100 | (75–100) | p = 0.08 | |
| Dynamics analysis | p < 0.001 | p = 1.00 | – | |||||
| Groups | Virus Strains | ||||
|---|---|---|---|---|---|
| A/H1N1 | A/H3N2 | B/Colorado | B/Phuket | ||
| Initially seronegative | People | 7/10 | 10/10 | 10/22 | 10/16 |
| % | 70 | 100 | 46 | 63 | |
| 95% CI | (35–93) | (69–100) | (24–68) | (35–85) | |
| Initially seropositive | People | 8/20 | 8/20 | 3/7 | 5/13 |
| % | 40 | 40 | 43 | 39 | |
| 95% CI | (19–64) | (19–64) | (10–82) | (14–68) | |
| Comparison between groups 1 | p = 0.12 | p = 0.002 | p = 1.00 | p = 0.20 | |
| Groups | Virus Strains | ||||
|---|---|---|---|---|---|
| A/H1N1 | A/H3N2 | B/Colorado | B/Phuket | ||
| Initially seronegative | GMR | 9.8 | 34.3 | 3.8 | 5.9 |
| 95%CI | (2.5–38.5) | (11.6–101.3) | (2.4–5.8) | (3,3–10.6) | |
| Initially seropositive | GMR | 4,8 | 2,8 | 2,5 | 2,8 |
| 95%CI | (2.4–9.6) | (1.4–5.6) | (1,5–4.0) | (1.6–4.8) | |
| Comparison between groups 1 | 0.30 | <0.001 | 0.31 | 0.05 | |
| Virus strains | Period | Initially Seronegative | Initially Seropositive | Between Groups 1 | ||
|---|---|---|---|---|---|---|
| GMT | 95%CI | GMT | 95%CI | |||
| A/H1N1 | Before vaccination | 7.1 | (5.0–10.0) | 95.1 | (57.5–157.4) | p < 0.001 |
| After 3 weeks | 65.0 | (16.0–264.4) | 452.5 | (235.5–869.5) | p = 0.004 | |
| Dynamics analysis 2,3 | p = 0.004 | p < 0.001 | – | |||
| A/H3N2 | Before vaccination | 6.6 | (4.7–9.3) | 96.6 | (68.5–136.4) | p < 0.001 |
| After 3 weeks | 226.3 | (75.1–681.5) | 288.4 | (147.9–562.4) | p = 0.67 | |
| Dynamics analysis | p < 0.001 | p = 0.005 | – | |||
| B/Colorado | Before vaccination | 7.5 | (5.9–9.6) | 88.3 | (49.6–157.3) | p < 0.001 |
| After 3 weeks | 27.4 | (16.3–46.1) | 214.9 | (115.6–403.4) | p < 0.001 | |
| Dynamics analysis | p < 0.001 | p = 0.004 | – | |||
| B/Phuket | Before vaccination | 8.9 | (6.6–12.0) | 71.9 | (49.4–104.8) | p < 0.001 |
| After 3 weeks | 51.9 | (26.5–101.6) | 198.0 | (117.3–334.4) | p = 0.003 | |
| Dynamics analysis | p < 0.001 | p = 0.002 | – | |||
| Virus Strains | Period | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|---|
| A/H1N1 | Before vaccination | 40 | 20 | 20 | 20 | 20 | 80 |
| After 3 weeks After 3 months | 40 40 | 20 40 | 20 20 | 20 20 | 10 10 | 40 40 | |
| A/H3N2 | Before vaccination | 40 | 20 | 5 | 10 | 10 | 80 |
| After 3 weeks After 3 months | 40 40 | 20 40 | 5 20 | 80 40 | 5 5 | 80 40 | |
| B/Colorado | Before vaccination | 10 | 5 | 5 | 5 | 5 | 20 |
| After 3 weeks After 3 months | 10 20 | 5 10 | 5 5 | 10 20 | 5 5 | 10 10 | |
| B/Phuket | Before vaccination | 20 | 10 | 10 | 5 | 10 | 20 |
| After 3 weeks After 3 months | 10 10 | 10 10 | 10 10 | 5 10 | 5 5 | 20 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostinov, M.P.; Latysheva, E.A.; Kostinova, A.M.; Akhmatova, N.K.; Latysheva, T.V.; Vlasenko, A.E.; Dagil, Y.A.; Khromova, E.A.; Polichshuk, V.B. Immunogenicity and Safety of the Quadrivalent Adjuvant Subunit Influenza Vaccine in Seropositive and Seronegative Healthy People and Patients with Common Variable Immunodeficiency. Vaccines 2020, 8, 640. https://doi.org/10.3390/vaccines8040640
Kostinov MP, Latysheva EA, Kostinova AM, Akhmatova NK, Latysheva TV, Vlasenko AE, Dagil YA, Khromova EA, Polichshuk VB. Immunogenicity and Safety of the Quadrivalent Adjuvant Subunit Influenza Vaccine in Seropositive and Seronegative Healthy People and Patients with Common Variable Immunodeficiency. Vaccines. 2020; 8(4):640. https://doi.org/10.3390/vaccines8040640
Chicago/Turabian StyleKostinov, Mikhail P., Elena A. Latysheva, Aristitsa M. Kostinova, Nelly K. Akhmatova, Tatyana V. Latysheva, Anna E. Vlasenko, Yulia A. Dagil, Ekaterina A. Khromova, and Valentina B. Polichshuk. 2020. "Immunogenicity and Safety of the Quadrivalent Adjuvant Subunit Influenza Vaccine in Seropositive and Seronegative Healthy People and Patients with Common Variable Immunodeficiency" Vaccines 8, no. 4: 640. https://doi.org/10.3390/vaccines8040640
APA StyleKostinov, M. P., Latysheva, E. A., Kostinova, A. M., Akhmatova, N. K., Latysheva, T. V., Vlasenko, A. E., Dagil, Y. A., Khromova, E. A., & Polichshuk, V. B. (2020). Immunogenicity and Safety of the Quadrivalent Adjuvant Subunit Influenza Vaccine in Seropositive and Seronegative Healthy People and Patients with Common Variable Immunodeficiency. Vaccines, 8(4), 640. https://doi.org/10.3390/vaccines8040640





