The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines
Abstract
1. Introduction
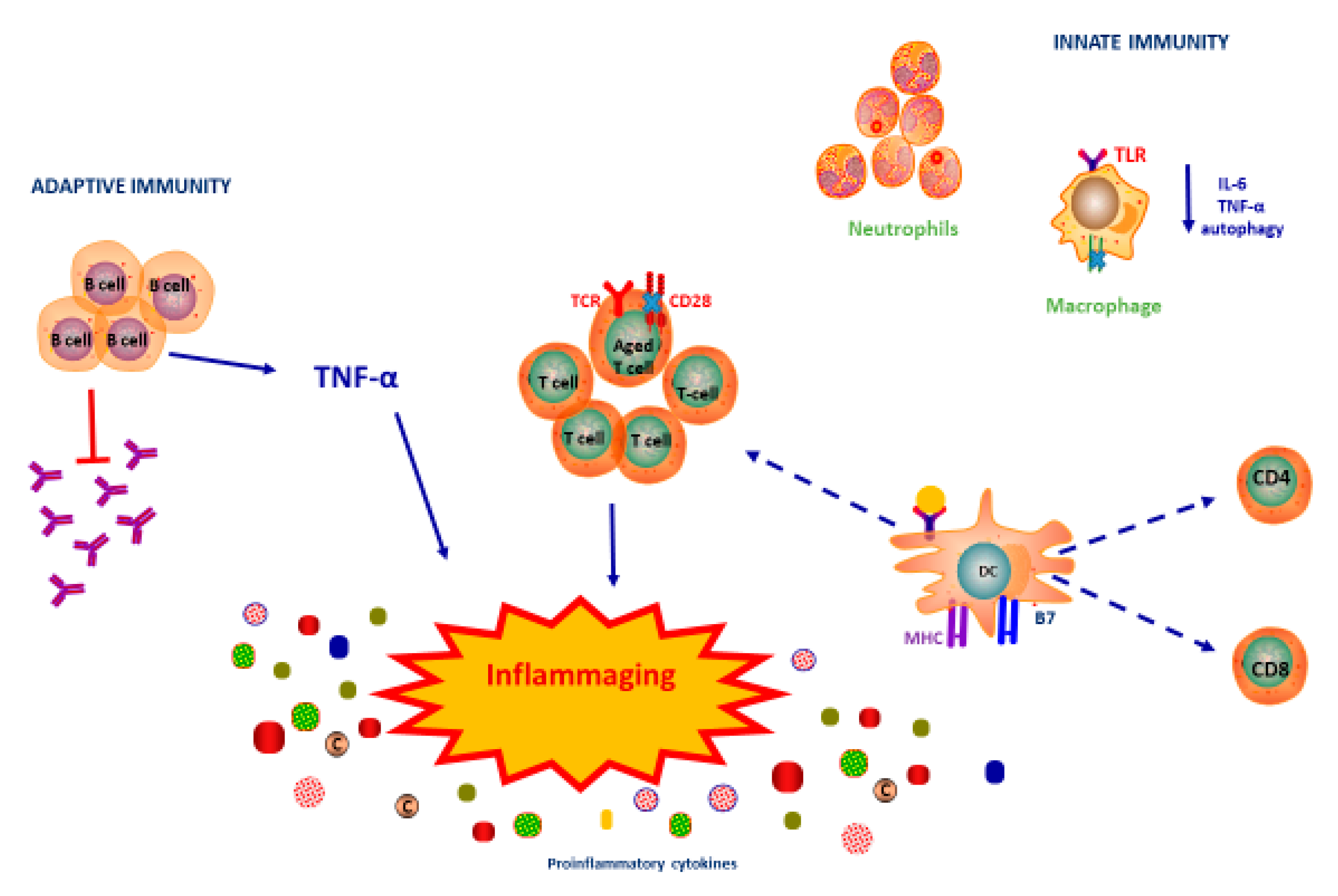
2. Immunosenescence
3. Innate Immune Response
4. Adaptive Immune Response
5. Immunosenescence and Inflammaging
6. Immunosenescence in the Context of Reduced Vaccines Response
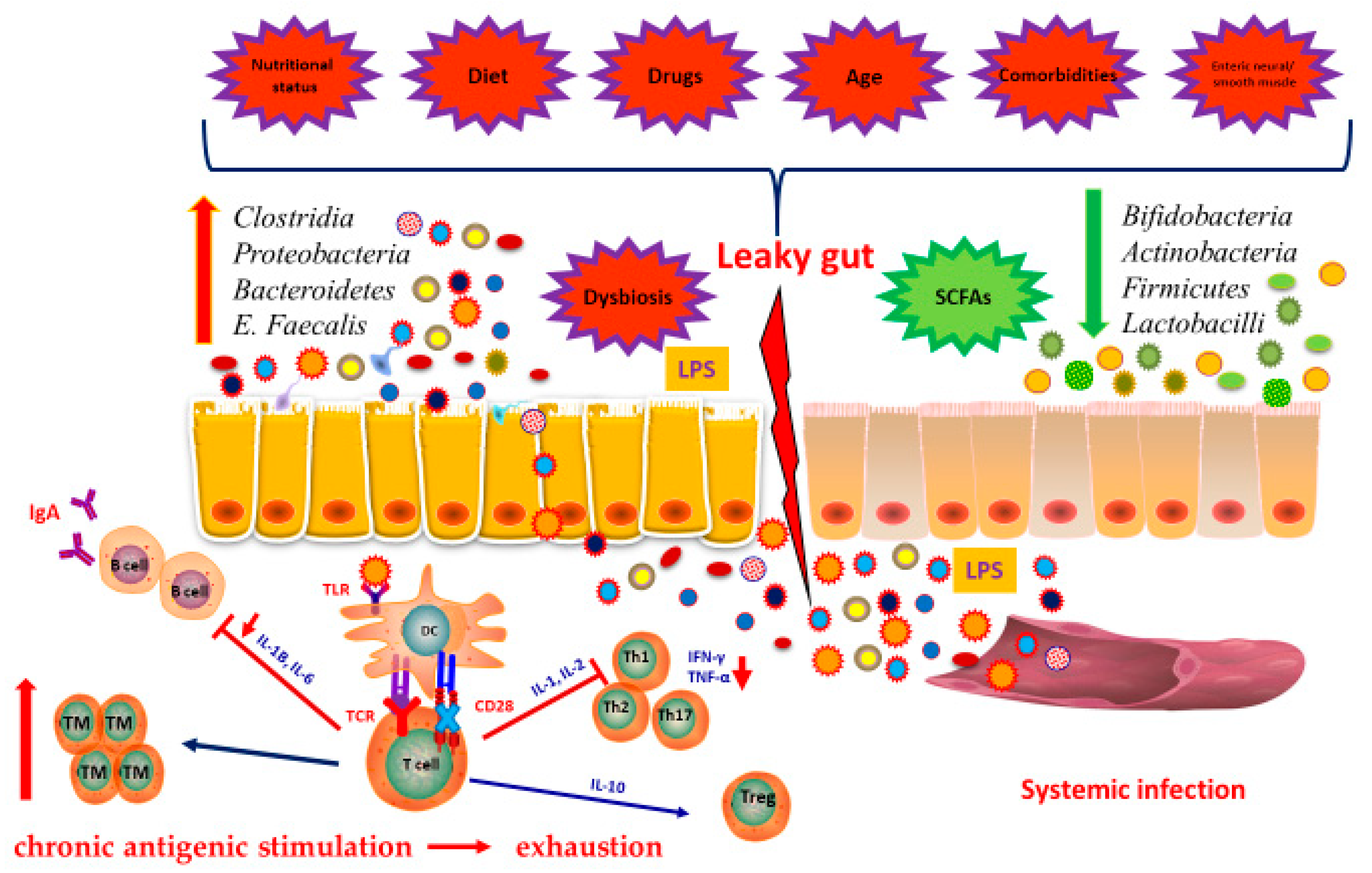
7. Immunity and Microbiota
8. Microbiota in the Elderly
9. Microbiota and Vaccinations in the Elderly, a Love-Hate Relationship
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2017, 50, 110–120. [Google Scholar] [CrossRef]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 480, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Chovancová, Z. Secondary immunodeficiency as a consequence of chronic diseases. Vnitrni Lek. 2019, 65, 117–124. [Google Scholar]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Vitlic, A.; Khanfer, R.; Lord, J.M.; Carroll, D.; Whittaker, A.C. Bereavement reduces neutrophil oxidative burst only in older adults: Role of the HPA axis and immunesenescence. Immun. Ageing 2014, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Zareian, N.; Aprile, S.; Cristaldi, L.; Ligotti, M.E.; Vasto, S.; Farzaneh, F. Triggering of Toll-like Receptors in Old Individuals. Relevance for Vaccination. Curr. Pharm. Des. 2019, 25, 4163–4167. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef]
- Simell, B.; Vuorela, A.; Ekström, N.; Palmu, A.; Reunanen, A.; Meri, S.; Käyhty, H.; Väkeväinen, M. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011, 29, 1929–1934. [Google Scholar] [CrossRef]
- Gonçalves, M.T.; Mitchell, T.J.; Lord, J.M. Immune ageing and susceptibility to Streptococcus pneumoniae. Biogerontology 2015, 17, 449–465. [Google Scholar] [CrossRef]
- Sauce, D.; Dong, Y.; Campillo-Gimenez, L.; Casulli, S.; Bayard, C.; Autran, B.; Boddaert, J.; Appay, V.; Elbim, C. Reduced Oxidative Burst by Primed Neutrophils in the Elderly Individuals Is Associated With Increased Levels of the CD16bright/CD62LdimImmunosuppressive Subset. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 72, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Smith, L.M.; Heires, A.J.; Katafiasz, D.M.; Romberger, D.J.; LeVan, T.D. Aging leads to dysfunctional innate immune responses to TLR2 and TLR4 agonists. Aging Clin. Exp. Res. 2018, 31, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Macian, F. Autophagy and the immune function in aging. Curr. Opin. Immunol. 2014, 29, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yoon, P.; Keylock, K.; Hartman, M.E.; Freund, G.; Woods, J.A. Macrophage hypo-responsiveness to interferon-γ in aged mice is associated with impaired signaling through Jak-STAT. Mech. Ageing Dev. 2004, 125, 137–143. [Google Scholar] [CrossRef]
- Herrero, C.; Marqués, L.; Lloberas, J.; Celada, A. IFN-γ–dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J. Clin. Investig. 2001, 107, 485–493. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Immunosenescence: The potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell. Mol. Life Sci. 2019, 76, 1901–1918. [Google Scholar] [CrossRef]
- Chidrawar, S.M.; Khan, N.; Chan, Y.L.T.; Nayak, L.; Moss, P.A.H. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 2006, 3, 10. [Google Scholar] [CrossRef]
- Hayhoe, R.P.; Henson, S.M.; Akbar, A.N.; Palmer, D.B. Variation of human natural killer cell phenotypes with age: Identification of a unique KLRG1-negative subset. Hum. Immunol. 2010, 71, 676–681. [Google Scholar] [CrossRef]
- Kaszubowska, L.; Foerster, J.; Schetz, D.; Kmiec, Z. CD56bright cells respond to stimulation until very advanced age revealing increased expression of cellular protective proteins SIRT1, HSP70 and SOD2. Immun. Ageing 2018, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Gupta, S. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 2011, 10, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Aydar, Y.; Balogh, P.; Tew, J.G.; Szakal, A.K. Altered Regulation of FcγRII on Aged Follicular Dendritic Cells Correlates with Immunoreceptor Tyrosine-Based Inhibition Motif Signaling in B Cells and Reduced Germinal Center Formation. J. Immunol. 2003, 171, 5975–5987. [Google Scholar] [CrossRef]
- Cancro, M.P. Age-Associated B Cells. Annu. Rev. Immunol. 2020, 38, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.P.; Sanders, V.M.; Witte, P.L. Stage-specific alterations in murine B lymphopoiesis with age. Int. Immunol. 1996, 8, 509–518. [Google Scholar] [CrossRef]
- Jin, R.; Kaneko, H.; Suzuki, H.; Arai, T.; Teramoto, T.; Fukao, T.; Kondo, N. Age-related changes in BAFF and APRIL profiles and upregulation of BAFF and APRIL expression in patients with primary antibody deficiency. Int. J. Mol. Med. 2008, 21, 233–238. [Google Scholar] [CrossRef]
- Kline, G.H.; Hayden, A.T.; Klinman, N.R. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J. Immunol. 1999, 162, 3342–3349. [Google Scholar]
- Gibson, K.L.; Wu, Y.-C.; Barnett, Y.; Duggan, O.; Vaughan, R.; Kondeatis, E.; Nilsson, B.-O.; Wikby, A.; Kipling, D.; Dunn-Walters, D.K. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009, 8, 18–25. [Google Scholar] [CrossRef]
- Frasca, D.; Landin, A.M.; Riley, R.L.; Blomberg, B.B. Mechanisms for Decreased Function of B Cells in Aged Mice and Humans. J. Immunol. 2008, 180, 2741–2746. [Google Scholar] [CrossRef]
- Pritz, T.; Lair, J.; Ban, M.; Keller, M.; Weinberger, B.; Krismer, M.; Grubeck-Loebenstein, B. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 2014, 45, 738–746. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Landin, A.M.; Blomberg, B.B. High TNF-α levels in resting B cells negatively correlate with their response. Exp. Gerontol. 2014, 54, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Britanova, O.V.; Putintseva, E.V.; Shugay, M.; Merzlyak, E.M.; Turchaninova, M.A.; Staroverov, D.B.; Bolotin, D.A.; Lukyanov, S.; Bogdanova, E.A.; Mamedov, I.Z.; et al. Age-Related Decrease in TCR Repertoire Diversity Measured with Deep and Normalized Sequence Profiling. J. Immunol. 2014, 192, 2689–2698. [Google Scholar] [CrossRef]
- Yu, Q.; Erman, B.; Bhandoola, A.; Sharrow, S.O.; Singer, A. In Vitro Evidence That Cytokine Receptor Signals Are Required for Differentiation of Double Positive Thymocytes into Functionally Mature CD8+ T Cells. J. Exp. Med. 2003, 197, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, A.-R.; Shin, E.-C. Cytomegalovirus Infection and Memory T Cell Inflation. Immune Netw. 2015, 15, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Moro-García, M.A.; Alonso-Arias, R.; Lopez-Larrea, C. When aging reaches CD4+ T-cells: Phenotypic and functional changes. Front. Immunol. 2013, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Bryl, E.; Vallejo, A.N.; Matteson, E.L.; Witkowski, J.M.; Weyand, C.M.; Goronzy, J.J. Modulation of CD28 expression with anti-tumor necrosis factor α therapy in rheumatoid arthritis. Arthritis Rheum. 2005, 52, 2996–3003. [Google Scholar] [CrossRef]
- Decman, V.; Laidlaw, B.J.; DiMenna, L.J.; Abdulla, S.; Mozdzanowska, K.; Erikson, J.; Ertl, H.C.J.; Wherry, E.J. Cell-Intrinsic Defects in the Proliferative Response of Antiviral Memory CD8 T Cells in Aged Mice upon Secondary Infection. J. Immunol. 2010, 184, 5151–5159. [Google Scholar] [CrossRef]
- Briceño, O.; Lissina, A.; Wanke, K.; Afonso, G.; Von Braun, A.; Ragon, K.; Miquel, T.; Gostick, E.; Papagno, L.; Stiasny, K.; et al. Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell 2015, 15, 14–21. [Google Scholar] [CrossRef]
- Rondy, M.M.; El Omeiri, N.; Thompson, M.M.; Levêque, A.; Moren, A.A.; Sullivan, S.S. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J. Infect. 2017, 75, 381–394. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.-M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. Organo Hospital Enferm. Nutr. 2016, 68, 84–91. [Google Scholar]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef]
- Wilkinson, K.; Wei, Y.; Szwajcer, A.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M.; Mahmud, S.M. Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine 2017, 35, 2775–2780. [Google Scholar] [CrossRef]
- Wagner, A.; Garner-Spitzer, E.; Jasinska, J.; Kollaritsch, H.; Stiasny, K.; Kundi, M.; Wiedermann, U. Age-related differences in humoral and cellular immune responses after primary immunisation: Indications for stratified vaccination schedules. Sci. Rep. 2018, 8, 9825. [Google Scholar] [CrossRef]
- Wroe, P.C.; Finkelstein, J.A.; Ray, G.T.; Linder, J.A.; Johnson, K.M.; Rifas-Shiman, S.; Moore, M.R.; Huang, S.S. Aging Population and Future Burden of Pneumococcal Pneumonia in the United States. J. Infect. Dis. 2012, 205, 1589–1592. [Google Scholar] [CrossRef]
- Gozalo, P.L.; Pop-Vicas, A.; Feng, Z.; Gravenstein, S.; Mor, V. Effect of Influenza on Functional Decline. J. Am. Geriatr. Soc. 2012, 60, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, W.; Van Buynder, P.; McNeil, S.; Osterhaus, A.D.M.E. Seasonal influenza immunisation: Strategies for older adults. Int. J. Clin. Pr. 2018, 72, e13249. [Google Scholar] [CrossRef]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef]
- Nuñez, I.A.; Carlock, M.A.; Allen, J.D.; Owino, S.O.; Moehling, K.K.; Nowalk, P.; Susick, M.; Diagle, K.; Sweeney, K.; Mundle, S.; et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza A strains. PLoS ONE 2017, 12, e0185666. [Google Scholar] [CrossRef]
- Ju, C.-H.; Blum, L.K.; Kongpachith, S.; Lingampalli, N.; Mao, R.; Brodin, P.; Dekker, C.L.; Davis, M.M.; Robinson, W.H. Plasmablast antibody repertoires in elderly influenza vaccine responders exhibit restricted diversity but increased breadth of binding across influenza strains. Clin. Immunol. 2018, 193, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yager, E.J.; Ahmed, M.; Lanzer, K.; Randall, T.D.; Woodland, D.L.; Blackman, M.A. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008, 205, 711–723. [Google Scholar] [CrossRef]
- Lefebvre, J.S.; Masters, A.R.; Hopkins, J.W.; Haynes, L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci. Rep. 2016, 6, 25051. [Google Scholar] [CrossRef]
- Saurwein-Teissl, M.; Lung, T.L.; Marx, F.; Gschösser, C.; Asch, E.; Blasko, I.; Parson, W.; Böck, G.; Schönitzer, D.; Trannoy, E.; et al. Lack of antibody production following immunization in old age: Association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002, 168, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Przemska-Kosicka, A.; Childs, C.E.; Maidens, C.; Dong, H.; Todd, S.; Gosney, M.A.; Tuohy, K.M.; Yaqoob, P. Age-Related Changes in the Natural Killer Cell Response to Seasonal Influenza Vaccination Are Not Influenced by a Synbiotic: A Randomised Controlled Trial. Front. Immunol. 2018, 9, 591. [Google Scholar] [CrossRef]
- Hagiwara, Y.; McGhee, J.R.; Fujihashi, K.; Kobayashi, R.; Yoshino, N.; Kataoka, K.; Etani, Y.; Kweon, M.-N.; Tamura, S.; Kurata, T.; et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J. Immunol. 2003, 170, 1754–1762. [Google Scholar] [CrossRef]
- Fukuyama, Y.; King, J.D.; Kataoka, K.; Kobayashi, R.; Gilbert, R.S.; Hollingshead, S.K.; Briles, D.E.; Fujihashi, K. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J. Immunol. 2011, 186, 2454–2461. [Google Scholar] [CrossRef]
- Park, S.; Nahm, M.H. Older Adults Have a Low Capacity to Opsonize Pneumococci Due to Low IgM Antibody Response to Pneumococcal Vaccinations. Infect. Immun. 2010, 79, 314–320. [Google Scholar] [CrossRef]
- Schenkein, J.G.; Park, S.; Nahm, M.H. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 2008, 26, 5521–5526. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool Microbiota and Vaccine Responses of Infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, K.E.; Parashar, U.; Victor, J.C.; Tate, J.; De Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant Correlation Between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J. Infect. Dis. 2016, 215, 34–41. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; McArthur, M.A.; Seekatz, A.M.; Drabek, E.F.; Rasko, D.A.; Sztein, M.B.; Fraser, C.M. Impact of Oral Typhoid Vaccination on the Human Gut Microbiota and Correlations with S. Typhi-Specific Immunological Responses. PLoS ONE 2013, 8, e62026. [Google Scholar] [CrossRef]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.-F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.-B. Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.-G.; Seo, S.-U.; Kim, D.-J.; Kamada, N.; Prescott, D.; Chamaillard, M.; Philpott, D.J.; Rosenstiel, P.; Inohara, N.; et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 2016, 22, 524–530. [Google Scholar] [CrossRef]
- Lynn, D.J.; Pulendran, B. The potential of the microbiota to influence vaccine responses. J. Leukoc. Biol. 2017, 103, 216R–225R. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2016, 10, 18–26. [Google Scholar] [CrossRef]
- Nalin, D. Immunosenescence and SARS-CoV-2 Vaccine Development. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of probiotics on vaccine responses—A systematic review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Church, J.A.; Parker, E.P.; Kirkpatrick, B.D.; Grassly, N.C.; Prendergast, A.J. Interventions to improve oral vaccine performance: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 203–214. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, J.; Lee, J.H.; Sim, J.H.; Cho, D.-H.; Bae, I.-H.; Lee, H.; Seol, M.A.; Shin, H.M.; Kim, T.-J.; et al. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci. Rep. 2016, 6, 39026. [Google Scholar] [CrossRef]
- De Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Tomkovich, S.; Jobin, C. Microbiota and host immune responses: A love-hate relationship. Immunology 2015, 147, 1–10. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Hand, T.W. Role of nutrition, infection, and the microbiota in the efficacy of oral vaccines. Clin. Sci. 2018, 132, 1169–1177. [Google Scholar] [CrossRef]
- Magwira, C.A.; Taylor, M.B. Composition of gut microbiota and its influence on the immunogenicity of oral rotavirus vaccines. Vaccine 2018, 36, 3427–3433. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Takanashi, S.; Miyazaki, A.; Rajashekara, G.; Saif, L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr. Opin. Virol. 2019, 37, 16–25. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- MacPherson, A.J.; De Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Himes, J.E.; Martinez, D.R.; Permar, S.R. The Impact of the Gut Microbiota on Humoral Immunity to Pathogens and Vaccination in Early Infancy. PLoS Pathog. 2016, 12, e1005997. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of Bacterial Communities in Feces from Healthy Elderly Volunteers and Hospitalized Elderly Patients by Using Real-Time PCR and Effects of Antibiotic Treatment on the Fecal Microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Zamparelli, M.S.; Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; Marrone, G.; Gasbarrini, A.; Grieco, A.; Nardone, G.; Miele, L. The Metabolic Role of Gut Microbiota in the Development of Nonalcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2016, 17, 1225. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Machado-Vieira, R.; Anderson, G.; Solmi, M.; Sanz, Y.; Berk, M.; Köhler, C.A.; Carvalho, A.F.; Machado-Veira, R. Intestinal Dysbiosis, Gut Hyperpermeability and Bacterial Translocation: Missing Links Between Depression, Obesity and Type 2 Diabetes. Curr. Pharm. Des. 2016, 22, 6087–6106. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Goel, R.; Kim, S.; Richards, E.M.; Carter, C.S.; Pepine, C.J.; Raizada, M.K.; Buford, T.W. Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. J. Am. Med Dir. Assoc. 2017, 18, 810.e1–810.e4. [Google Scholar] [CrossRef]
- Meier, J.; Sturm, A. The Intestinal Epithelial Barrier: Does It Become Impaired with Age? Dig. Dis. 2009, 27, 240–245. [Google Scholar] [CrossRef]
- Lord, J.M.; Butcher, S.; Killampali, V.; Lascelles, D.; Salmon, M. Neutrophil ageing and immunesenescence. Mech. Ageing Dev. 2001, 122, 1521–1535. [Google Scholar] [CrossRef]
- Fernandes, D.P.D.S.; Duarte, M.S.L.; Pessoa, M.C.; Franceschini, S.D.C.C.; Ribeiro, A.Q. Evaluation of diet quality of the elderly and associated factors. Arch. Gerontol. Geriatr. 2017, 72, 174–180. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2015, 65, 740–748. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.-Y.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Franza, L.; Schinzari, G.; Rossi, E.; Ianiro, G.; Tortora, G.; Gasbarrini, A.; Gambassi, G.; Cammarota, G. The Interplay between Immunity and Microbiota at Intestinal Immunological Niche: The Case of Cancer. Int. J. Mol. Sci. 2019, 20, 501. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.H.; Roelofsen, H.; Priebe, M.; Weening, D.; Dijkstra, M.; Hoek, A.; Rezaee, F.; Venema, K.; Vonk, R.J. Regulation of adipokine production in human adipose tissue by propionic acid. Eur. J. Clin. Investig. 2010, 40, 401–407. [Google Scholar] [CrossRef]
- Eisenstein, M. Towards a universal flu vaccine. Nat. Cell Biol. 2019, 573, S50–S52. [Google Scholar] [CrossRef]
- Keener, A. Tailoring vaccines for older people and the very young. Nat. Cell Biol. 2019, 575, S48–S50. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Franza, L.; Carusi, V.; Altamura, S.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Conti, P.; Pandolfi, F. Interrelationship between inflammatory cytokines (IL-1, IL-6, IL-33, IL-37) and acquired immunity. J. Biol. Regul. Homeost. Agents 2019, 33, 1321–1326. [Google Scholar]
- Pabst, O.; Hornef, M. Gut Microbiota: A Natural Adjuvant for Vaccination. Immunity 2014, 41, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-Mediated Sensing of Gut Microbiota Is Necessary for Antibody Responses to Seasonal Influenza Vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef]
- Bartley, J.M.; Zhou, X.; Kuchel, G.A.; Weinstock, G.M.; Haynes, L. Impact of Age, Caloric Restriction, and Influenza Infection on Mouse Gut Microbiome: An Exploratory Study of the Role of Age-Related Microbiome Changes on Influenza Responses. Front. Immunol. 2017, 8, 1164. [Google Scholar] [CrossRef]
- Ryan, F.J.; Drew, D.P.; Douglas, C.; Leong, L.E.X.; Moldovan, M.; Lynn, M.; Fink, N.; Sribnaia, A.; Penttila, I.; McPhee, A.J.; et al. Changes in the Composition of the Gut Microbiota and the Blood Transcriptome in Preterm Infants at Less than 29 Weeks Gestation Diagnosed with Bronchopulmonary Dysplasia. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Littman, D.R. Do the Microbiota Influence Vaccines and Protective Immunity to Pathogens? If So, is There Potential for Efficacious Microbiota-Based Vaccines? Cold Spring Harb. Perspect. Biol. 2017, 10, a029355. [Google Scholar] [CrossRef]
- Collins, N.; Belkaid, Y. Do the Microbiota Influence Vaccines and Protective Immunity to Pathogens? Engaging Our Endogenous Adjuvants. Cold Spring Harb. Perspect. Biol. 2017, 10, a028860. [Google Scholar] [CrossRef] [PubMed]
- Przemska-Kosicka, A.; Childs, C.E.; Enani, S.; Maidens, C.; Dong, H.; Bin Dayel, I.; Tuohy, K.; Todd, S.; Gosney, M.A.; Yaqoob, P. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun. Ageing 2016, 13, 6. [Google Scholar] [CrossRef]
| Microbial Species | Effect | Reference |
|---|---|---|
| Actinobacteria | Improves response to oral vaccination | [56,59] |
| Proteobacteria | Low cellular and humoral response to vaccination, very common in centenerians | [56] |
| Firmicutes | Improves response to oral vaccination; most common in the “oldest old” | [57] |
| Bacteroidetes | Low humoral response to oral vaccination | [58] |
| Enterococcus faecalis | Inflammatory effect through ROS production; increases risk of epithelial damage | [67] |
| Clostridium septicum | Inflammatory effect; increases risk of infectious complications. | [67,73] |
| B. fragilis | Stimulates T-reg differentiation | [67] |
| B. fragilis enterotoxigenic | Stimulates Th-17 differentiation | [67] |
| Bifidobacter spp. | Promotes gut homeostasis through competition with pathogens; anti-inflammatory effects. | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, R.; Franza, L.; Massaro, M.G.; Borriello, R.; De Vito, F.; Gambassi, G. The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines. Vaccines 2020, 8, 636. https://doi.org/10.3390/vaccines8040636
Cianci R, Franza L, Massaro MG, Borriello R, De Vito F, Gambassi G. The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines. Vaccines. 2020; 8(4):636. https://doi.org/10.3390/vaccines8040636
Chicago/Turabian StyleCianci, Rossella, Laura Franza, Maria Grazia Massaro, Raffaele Borriello, Francesco De Vito, and Giovanni Gambassi. 2020. "The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines" Vaccines 8, no. 4: 636. https://doi.org/10.3390/vaccines8040636
APA StyleCianci, R., Franza, L., Massaro, M. G., Borriello, R., De Vito, F., & Gambassi, G. (2020). The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines. Vaccines, 8(4), 636. https://doi.org/10.3390/vaccines8040636







