Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunization of Laying Hens
2.2. Isolation and Purification of Yolk IgY
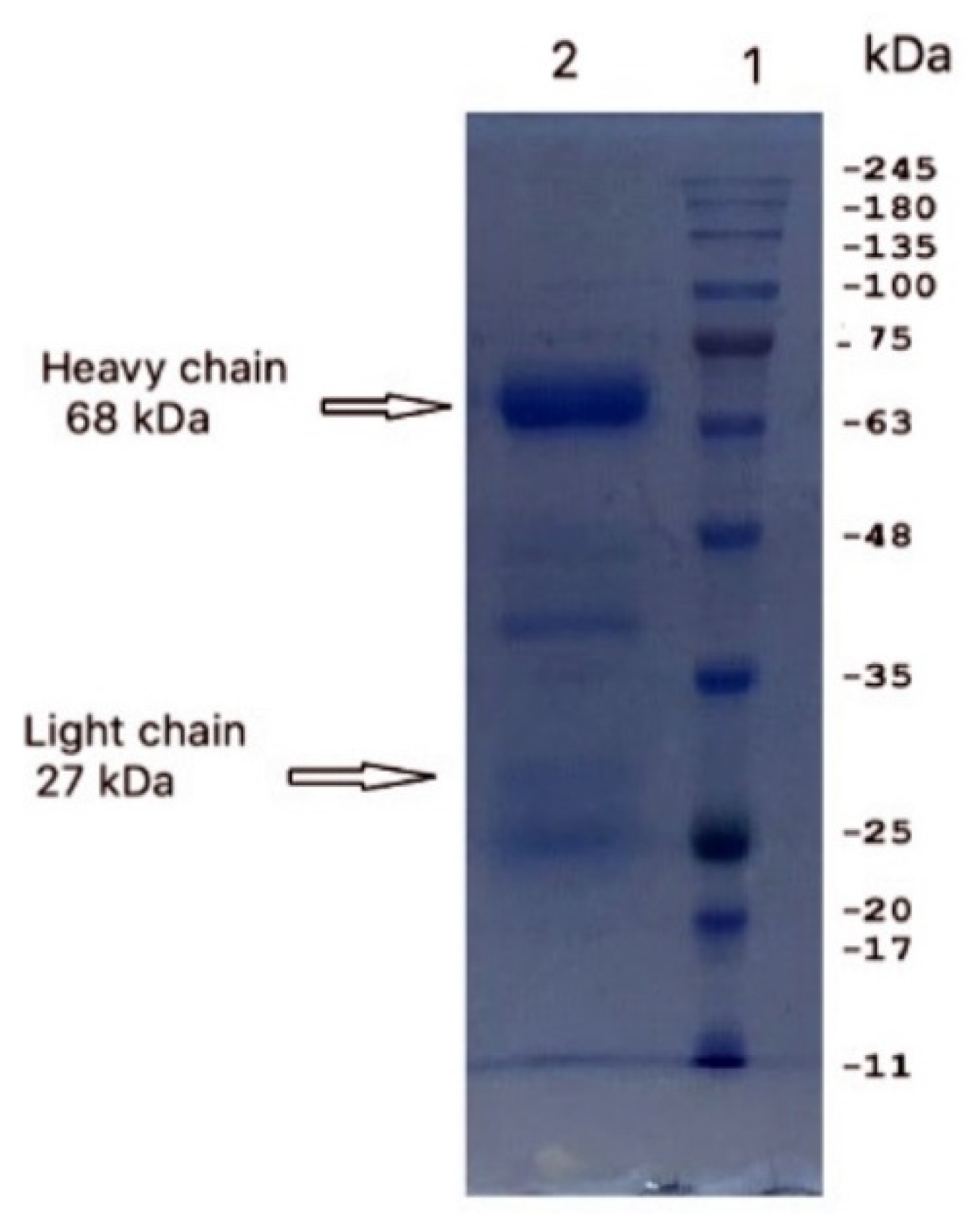
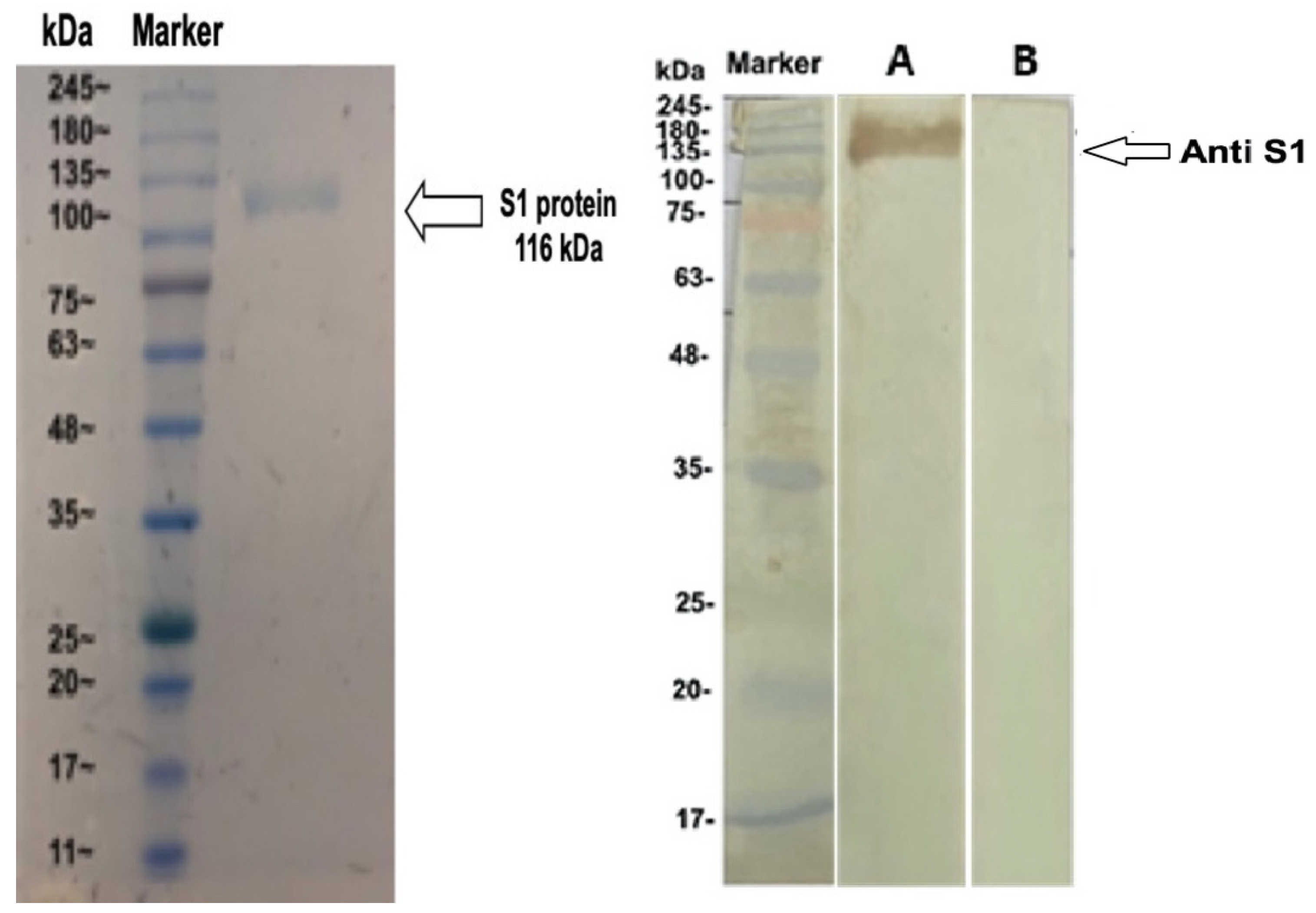
2.3. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
2.4. Reactivity of Anti-S1 IgY Antibodies
2.5. Western Blot Assay
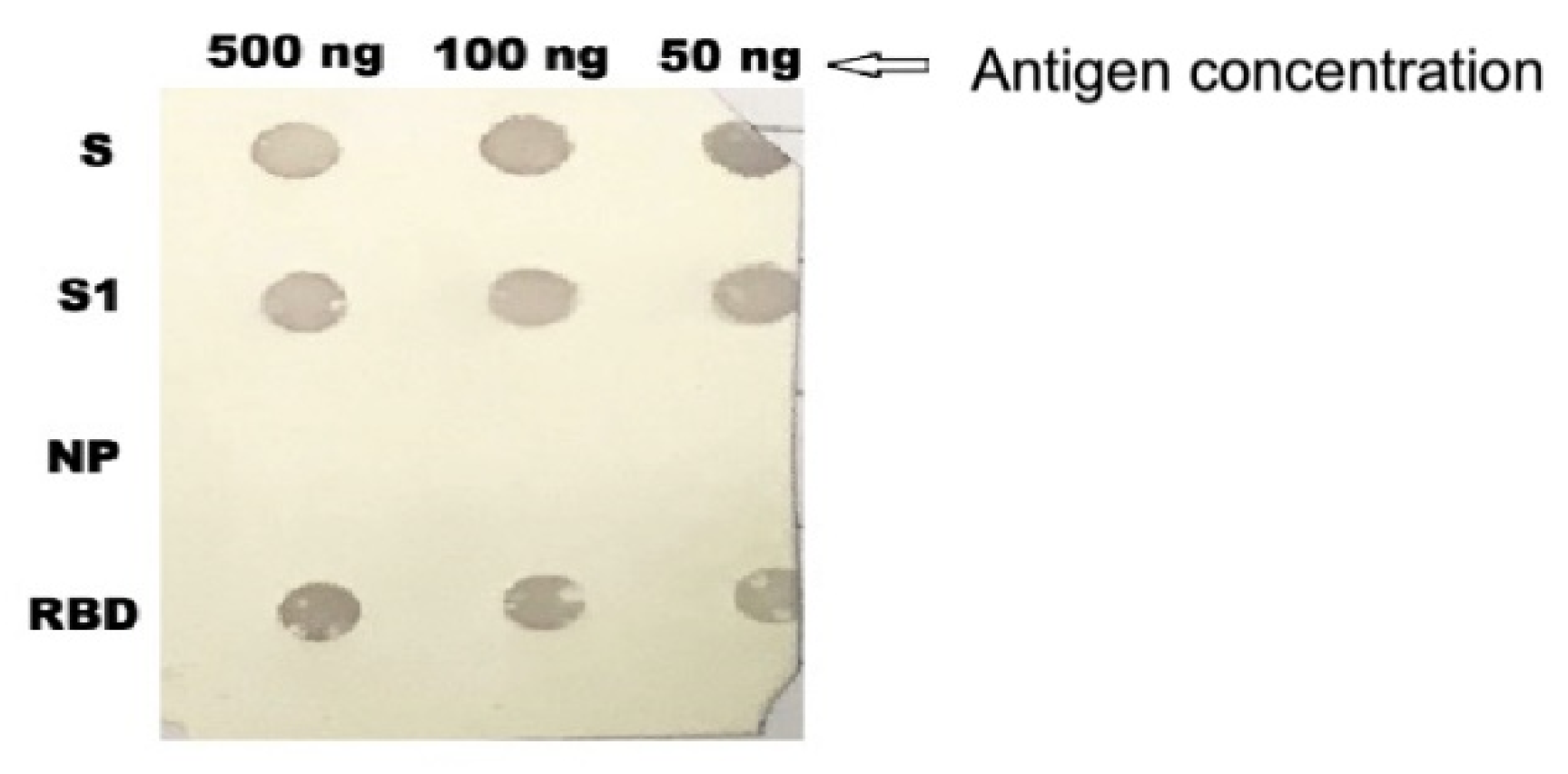
2.6. Immuno-Dot-Blot Assay
2.7. Immunofluorescence Assay
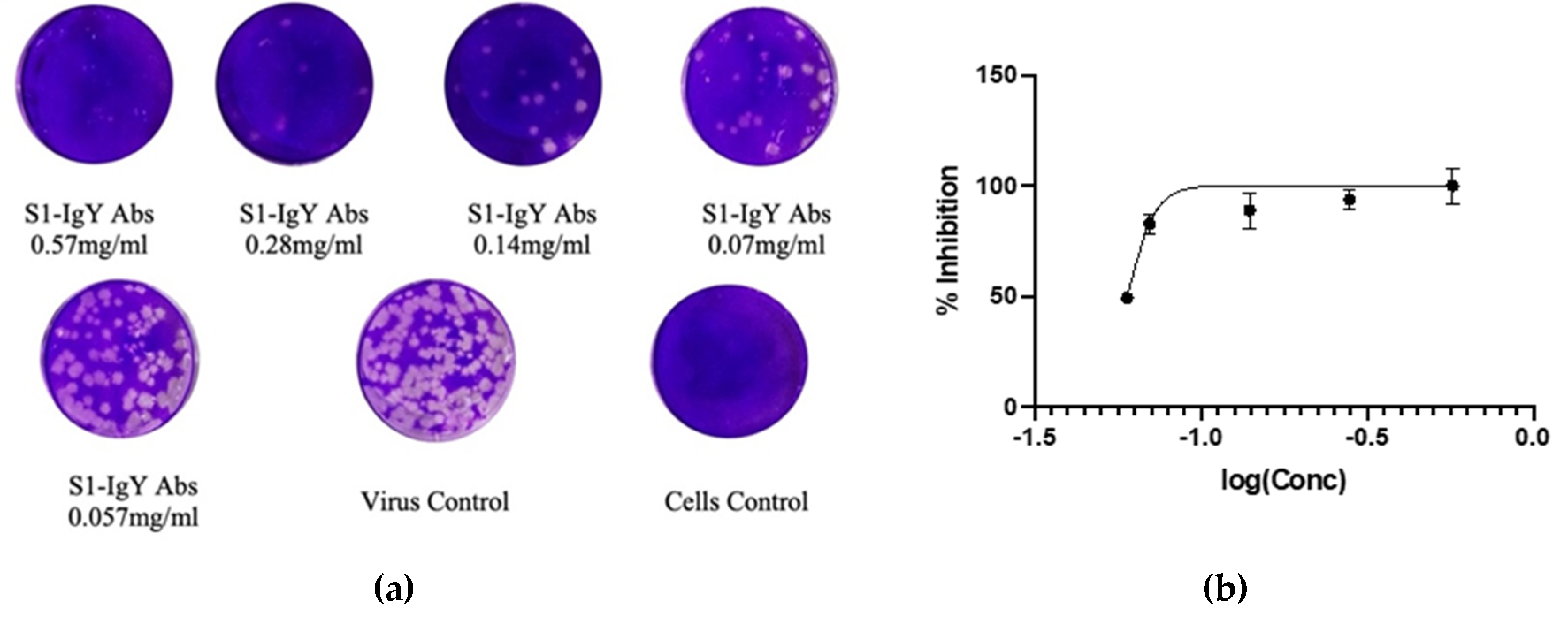
2.8. Neutralization Assay
2.9. Plaque Reduction Neutralization Test
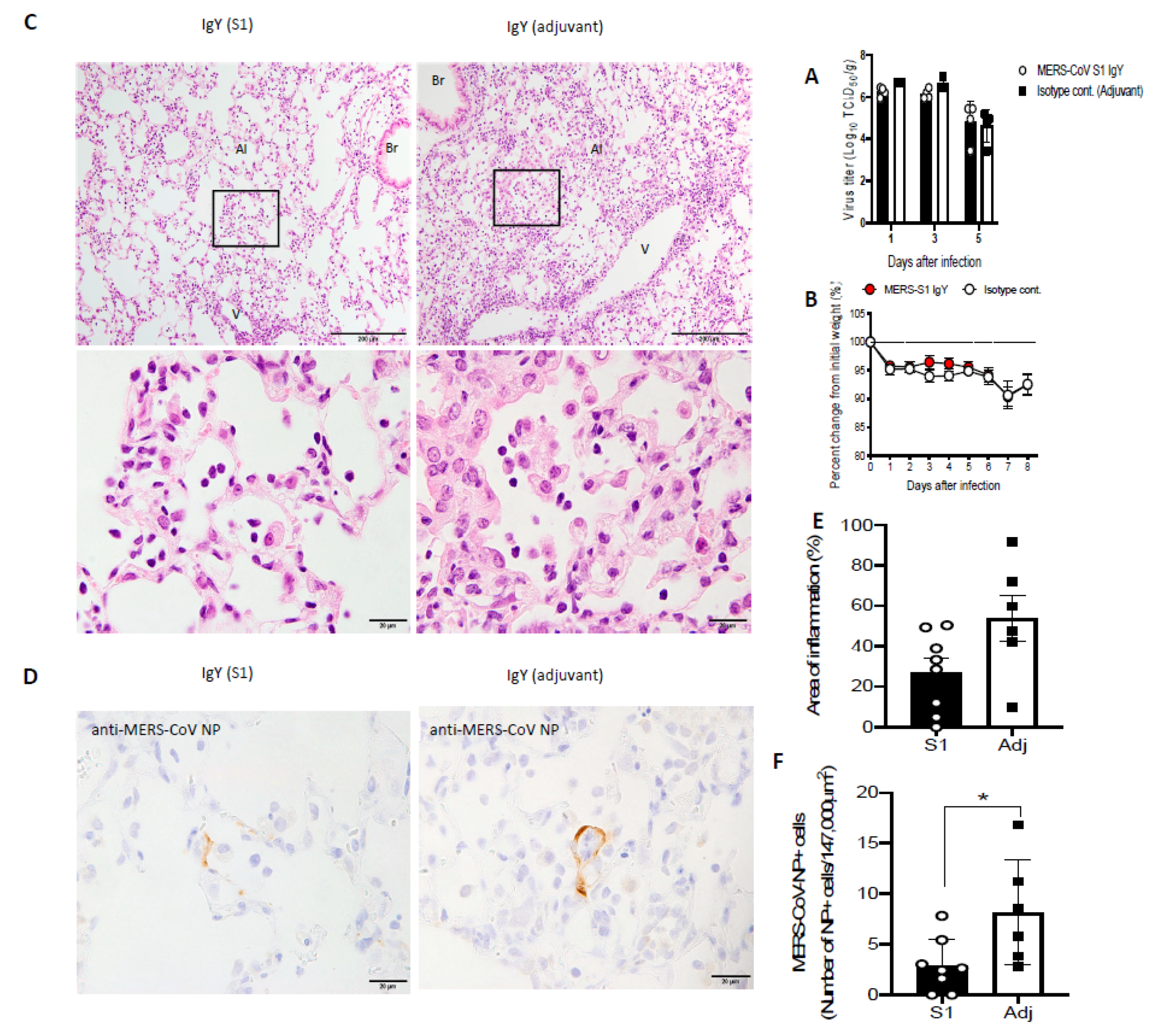
2.10. Effect of Anti-S1 IgY Antibodies in Transgenic Mice after MERS-CoV Infection
2.11. Histopathology and Immunohistochemistry
2.12. Quantitative Analysis of Inflammation and Viral Antigen Positive Cells
2.13. Statistical Analysis
2.14. Ethics Statement
3. Results
3.1. Isolation and Purification of IgY
3.2. Dynamics of Anti-S IgY Antibodies in Chickens’ Sera and Egg Yolks
3.3. Immunoreactivity of Anti-S1 IgY of the MERS-COV
3.4. Dot Blotting
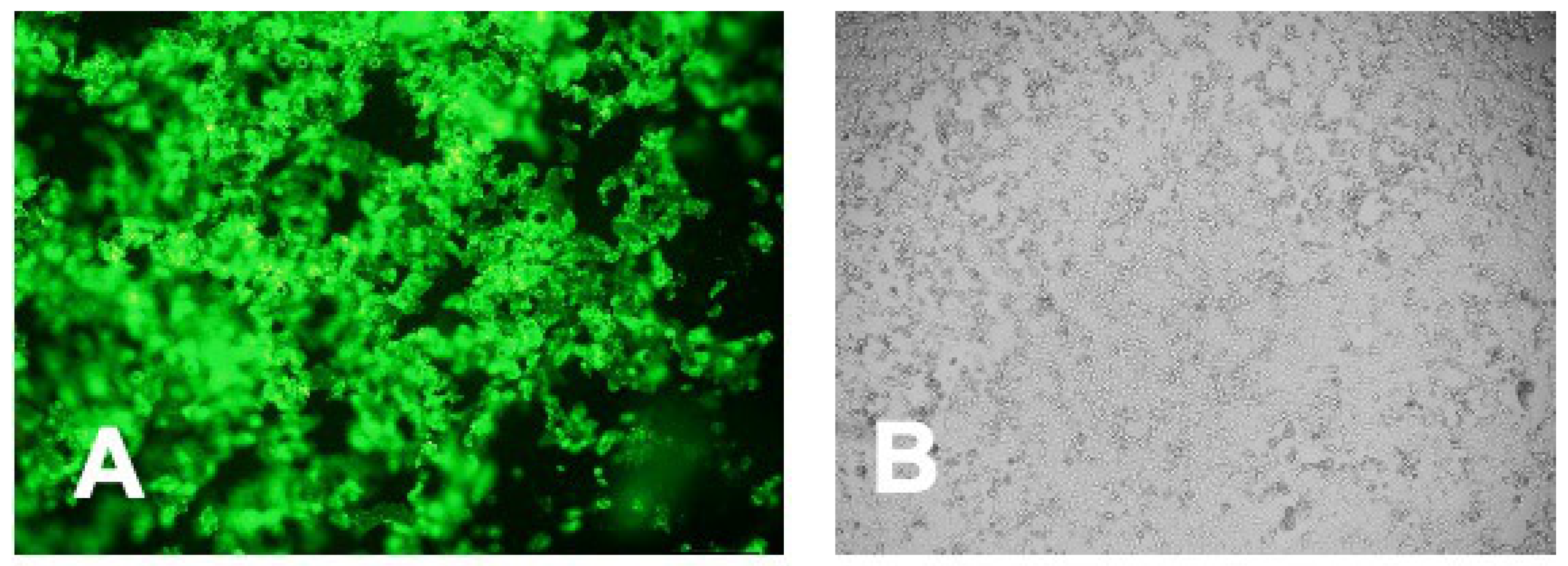
3.5. Intracellular Immunofluorescent Detection of IgY Antibodies Binding to MERS-CoV
3.6. Anti-S1 IgY Neutralizes MERS-CoV Infection
3.7. IgY Inhibits Virus Replication In Vitro
3.8. IgY Confers In Vivo Protection in Virus-Challenged Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, I.; Wang, C.; van Haperen, R.; Gutierrez-Alvarez, J.; van Dieren, B.; Okba, N.M.A.; Raj, V.S.; Li, W.; Fernandez-Delgado, R.; Grosveld, F.; et al. Towards a solution to MERS: Protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg. Microbes Infect. 2019, 8, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef]
- World Health Organization. Mers Situation Update. Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (accessed on 30 October 2020).
- Oh, M.D.; Park, W.B.; Park, S.W.; Choe, P.G.; Bang, J.H.; Song, K.H.; Kim, E.S.; Kim, H.B.; Kim, N.J. Middle East respiratory syndrome: What we learned from the 2015 outbreak in the Republic of Korea. Korean J. Intern. Med. 2018, 33, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Shehata, M.M.; Gomaa, M.R.; Kandeil, A.; El-Shesheny, R.; Kayed, A.S.; El-Taweel, A.N.; Atea, M.; Hassan, N.; Bagato, O.; et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microbes Infect. 2017, 6, e1. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, D.; Kamissoko, B.; de Wit, E.; Maiga, O.; Cronin, J.; Samake, K.; Traore, A.; Milne-Price, S.; Munster, V.J.; Sogoba, N.; et al. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health 2017, 3, 41–43. [Google Scholar] [CrossRef]
- Ali, M.; El-Shesheny, R.; Kandeil, A.; Shehata, M.; Elsokary, B.; Gomaa, M.; Hassan, N.; El Sayed, A.; El-Taweel, A.; Sobhy, H.; et al. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- El-Kafrawy, S.A.; Corman, V.M.; Tolah, A.M.; Al Masaudi, S.B.; Hassan, A.M.; Muller, M.A.; Bleicker, T.; Harakeh, S.M.; Alzahrani, A.A.; Alsaaidi, G.A.; et al. Enzootic patterns of Middle East respiratory syndrome coronavirus in imported African and local Arabian dromedary camels: A prospective genomic study. Lancet Planet. Health 2019, 3, e521–e528. [Google Scholar] [CrossRef]
- Sikkema, R.S.; Farag, E.; Himatt, S.; Ibrahim, A.K.; Al-Romaihi, H.; Al-Marri, S.A.; Al-Thani, M.; El-Sayed, A.M.; Al-Hajri, M.; Haagmans, B.L.; et al. Risk Factors for Primary Middle East Respiratory Syndrome Coronavirus Infection in Camel Workers in Qatar During 2013–2014: A Case-Control Study. J. Infect. Dis. 2017, 215, 1702–1705. [Google Scholar] [CrossRef]
- Alhakeem, R.F.; Midgley, C.M.; Assiri, A.M.; Alessa, M.; Al Hawaj, H.; Saeed, A.B.; Almasri, M.M.; Lu, X.; Abedi, G.R.; Abdalla, O.; et al. Exposures among MERS Case-Patients, Saudi Arabia, January-February 2016. Emerg. Infect. Dis. 2016, 22, 2020–2022. [Google Scholar] [CrossRef] [PubMed]
- Yusof, M.F.; Queen, K.; Eltahir, Y.M.; Paden, C.R.; Al Hammadi, Z.; Tao, Y.; Li, Y.; Khalafalla, A.I.; Shi, M.; Zhang, J.; et al. Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg. Microbes Infect. 2017, 6, e101. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.A.; Azhar, E.I.; Hashem, A.M. Evidence for camel-to-human transmission of MERS coronavirus. New Engl. J. Med. 2014, 371, 1360. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.; Sims, A.; Leist, S.; Schäfer, A.; Won, J.; Brown, A.; Montgomery, S.; Hogg, A.; Babusis, D.; Clarke, M. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Qasim, I.; Almasoud, A.; Aljami, H.A.; Alenazi, M.W.; Alhafufi, A.; Aldibasi, O.S.; Hashem, A.M.; Kasem, S.; Albrahim, R.; et al. Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels. Sci. Rep. 2019, 9, 16292. [Google Scholar] [CrossRef]
- Kleine-Weber, H.; Elzayat, M.T.; Hoffmann, M.; Pohlmann, S. Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein. Sci. Rep. 2018, 8, 16597. [Google Scholar] [CrossRef]
- Corti, D.; Zhao, J.; Pedotti, M.; Simonelli, L.; Agnihothram, S.; Fett, C.; Fernandez-Rodriguez, B.; Foglierini, M.; Agatic, G.; Vanzetta, F.; et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. USA 2015, 112, 10473–10478. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Liu, P.; Zhao, J.; Lu, G.; Qi, J.; Wang, Q.; Lu, X.; Wu, Y.; Liu, W.; et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015, 25, 1237–1249. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Wohlford-Lenane, C.; Agnihothram, S.S.; Fett, C.; Zhao, J.; Gale, M.J., Jr.; Baric, R.S.; Enjuanes, L.; Gallagher, T.; et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4970–4975. [Google Scholar] [CrossRef]
- Sabir, J.S.; Lam, T.T.; Ahmed, M.M.; Li, L.; Shen, Y.; Abo-Aba, S.E.; Qureshi, M.I.; Abu-Zeid, M.; Zhang, Y.; Khiyami, M.A.; et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science 2016, 351, 81–84. [Google Scholar] [CrossRef]
- Zhao, J.; Perera, R.A.; Kayali, G.; Meyerholz, D.; Perlman, S.; Peiris, M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J. Virol. 2015, 89, 6117–6120. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Qiu, B.; Li, C.; Wang, H.; Jin, H.; Gai, W.; Zheng, X.; Wang, T.; Sun, W.; et al. Passive immunotherapy for Middle East Respiratory Syndrome coronavirus infection with equine immunoglobulin or immunoglobulin fragments in a mouse model. Antivir. Res. 2017, 137, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, M.; Thommes, P.; Weiser, T.; Hubscher, U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990, 4, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Faith, R.E.; Clem, L.W. Passive cutaneous anaphylaxis in the chicken. Biological fractionation of the mediating antibody population. Immunology 1973, 25, 151–164. [Google Scholar] [PubMed]
- Yi, L.; Qin, Z.; Lin, H.; Zhou, Y.; Li, J.; Xu, Z.; Babu, V.S.; Lin, L. Features of chicken egg yolk immunoglobulin (IgY) against the infection of red-spotted grouper nervous necrosis virus. Fish Shellfish. Immunol. 2018, 80, 534–539. [Google Scholar] [CrossRef]
- Carlander, D.; Stalberg, J.; Larsson, A. Chicken antibodies: A clinical chemistry perspective. Upsala J. Med. Sci. 1999, 104, 179–189. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Jin, L.; Zhen, Y.; Lu, Y.; Li, S.; You, J.; Wang, L. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol. Adv. 2011, 29, 860–868. [Google Scholar] [CrossRef]
- Pauly, D.; Dorner, M.; Zhang, X.; Hlinak, A.; Dorner, B.; Schade, R. Monitoring of laying capacity, immunoglobulin Y concentration, and antibody titer development in chickens immunized with ricin and botulinum toxins over a two-year period. Poult. Sci. 2009, 88, 281–290. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: A review. J. Anim. Sci. Biotechnol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Gadde, U.; Rathinam, T.; Lillehoj, H.S. Passive immunization with hyperimmune egg-yolk IgY as prophylaxis and therapy for poultry diseases–A review. Anim. Health Res. Rev. 2015, 16, 163–176. [Google Scholar] [CrossRef]
- Sharma, J.M. Introduction to poultry vaccines and immunity. Adv. Vet. Med. 1999, 41, 481–494. [Google Scholar] [PubMed]
- Ikemori, Y.; Peralta, R.C.; Kuroki, M.; Yokoyama, H.; Kodama, Y. Research note: Avidity of chicken yolk antibodies to enterotoxigenic Escherichia coli fimbriae. Poult. Sci. 1993, 72, 2361–2365. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tumpey, T.M.; Park, H.J.; Byun, Y.H.; Tran, L.D.; Nguyen, V.D.; Kilgore, P.E.; Czerkinsky, C.; Katz, J.M.; Seong, B.L.; et al. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE 2010, 5, e10152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.E.; Wen, J.L.; Zhao, S.Q.; Zhang, K.; Zhou, Y.L. Prophylaxis and therapy of pandemic H1N1 virus infection using egg yolk antibody. J. Virol. Methods 2014, 206, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wallach, M.G.; Webby, R.J.; Islam, F.; Walkden-Brown, S.; Emmoth, E.; Feinstein, R.; Gronvik, K.O. Cross-Protection of Chicken Immunoglobulin Y Antibodies against H5N1 and H1N1 Viruses Passively Administered in Mice. Clin. Vaccine Immunol. 2011, 18, 1083–1090. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Hiroi, S.; Adachi, K.; Kato, H.; Inai, M.; Konishi, I.; Tanaka, M.; Yamamoto, R.; Sawa, M.; Handharyani, E.; et al. Antibodies against swine influenza virus neutralize the pandemic influenza virus A/H1N1. Mol. Med. Rep. 2011, 4, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Zhao, S.Q.; He, D.G.; Yang, Y.N.; Li, Y.M.; Zhu, S.S. Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus. Antivir. Res. 2012, 93, 154–159. [Google Scholar] [CrossRef]
- Fu, C.Y.; Huang, H.; Wang, X.M.; Liu, Y.G.; Wang, Z.G.; Cui, S.J.; Gao, H.L.; Li, Z.; Li, J.P.; Kong, X.G. Preparation and evaluation of anti-SARS coronavirus IgY from yolks of immunized SPF chickens. J. Virol. Methods 2006, 133, 112–115. [Google Scholar] [CrossRef]
- Ferella, A.; Bellido, D.; Chacana, P.; Wigdorovitz, A.; Santos, M.J.D.; Mozgovoj, M.V. Chicken egg yolk antibodies against bovine respiratory syncytial virus neutralize the virus in vitro. Procedia Vaccinol. 2012, 6, 33–38. [Google Scholar] [CrossRef][Green Version]
- Sudjarwo, S.A.; Eraiko, K.; Sudjarwo, G.W.; Koerniasari. The potency of chicken egg yolk immunoglobulin (IgY) specific as immunotherapy to Mycobacterium tuberculosis infection. J. Adv. Pharm. Technol. Res. 2017, 8, 91–96. [Google Scholar] [CrossRef]
- Kollberg, H.; Carlander, D.; Olesen, H.; Wejaker, P.E.; Johannesson, M.; Larsson, A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: A phase I feasibility study. Pediatric Pulmonol. 2003, 35, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Owlia, P.; Rasooli, I.; Salimian, J.; Derakhshanifar, E.; Naghipour Erami, A.; Darzi Eslam, E.; Darvish Alipour Astaneh, S. Specific egg yolk antibodies (IgY) confer protection against Acinetobacter baumannii in a murine pneumonia model. J. Appl. Microbiol. 2018, 126, 624–632. [Google Scholar] [CrossRef]
- Otterbeck, A.; Hanslin, K.; Lantz, E.L.; Larsson, A.; Stalberg, J.; Lipcsey, M. Inhalation of specific anti-Pseudomonas aeruginosa IgY antibodies transiently decreases P. aeruginosa colonization of the airway in mechanically ventilated piglets. Intensiv. Care Med. Exp. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, S.; Du, L. Prospects for a MERS-CoV spike vaccine. Expert Rev. Vaccines 2018, 17, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, S.S.; Abbas, A.T.; Siddiq, L.A.; Alghamdi, A.; Sanki, M.A.; Al-Muhanna, M.K.; Alhabbab, R.Y.; Azhar, E.I.; Li, X.; Hashem, A.M. Immunogenicity of Candidate MERS-CoV DNA Vaccines Based on the Spike Protein. Sci. Rep. 2017, 7, 44875. [Google Scholar] [CrossRef]
- Chi, H.; Zheng, X.; Wang, X.; Wang, C.; Wang, H.; Gai, W.; Perlman, S.; Yang, S.; Zhao, J.; Xia, X. DNA vaccine encoding Middle East respiratory syndrome coronavirus S1 protein induces protective immune responses in mice. Vaccine 2017, 35, 2069–2075. [Google Scholar] [CrossRef]
- Thibodeau, A.; Fravalo, P.; Perron, A.; Lewandowski, S.L.; Letellier, A. Production and characterization of anti-Campylobacter jejuni IgY derived from egg yolks. Acta Vet. Scand. 2017, 59, 80. [Google Scholar] [CrossRef]
- Landry, M.L.; Stanat, S.; Biron, K.; Brambilla, D.; Britt, W.; Jokela, J.; Chou, S.; Drew, W.L.; Erice, A.; Gilliam, B. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob. Agents Chemother. 2000, 44, 688–692. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Per Cent Endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Kotani, O.; Sato, H.; Sekimukai, H.; Fukushi, S.; Suzuki, T.; Sato, Y.; Takeda, M. Acute respiratory infection in human dipeptidyl peptidase 4-transgenic mice infected with Middle East respiratory syndrome coronavirus. J. Virol. 2019, 93, e01818-18. [Google Scholar] [CrossRef]
- Rizzardini, G.; Saporito, T.; Visconti, A. What is new in infectious diseases: Nipah virus, MERS-CoV and the blueprint list of the World Health Organization. Infez. Med. 2018, 26, 195–198. [Google Scholar] [PubMed]
- Bossart, K.N.; Broder, C.C. Developments towards effective treatments for Nipah and Hendra virus infection. Expert Rev. Anti-Infect. Ther. 2006, 4, 43–55. [Google Scholar] [CrossRef]
- Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Azhar, E.I.A. IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum. Vaccines Immunother. 2019, 15, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Kostyuchenko, V.A.; Lim, E.X.; Zhang, S.; Fibriansah, G.; Ng, T.-S.; Ooi, J.S.; Shi, J.; Lok, S.-M. Structure of the thermally stable Zika virus. Nature 2016, 533, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; van Tilburg, M.; Florean, E.; Guedes, M. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Schade, R.; Bürger, W.; Schöneberg, T.; Schniering, A.; Schwarzkopf, C.; Hlinak, A.; Kobilke, H. Avian egg yolk antibodies. The egg laying capacity of hens following immunisation with antigens of different kind and origin and the efficiency of egg yolk antibodies in comparison to mammalian antibodies. Altex 1994, 11, 75–84. [Google Scholar]
- Ababneh, M.; AlRwashdeh, M.; Khalifeh, M. Recombinant adenoviral vaccine encoding the spike 1 subunit of the Middle East Respiratory Syndrome Coronavirus elicits strong humoral and cellular immune responses in mice. Vet. World 2019, 12, 1554. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Y.; Zhang, J.-C.; Feng, L.; Bao, J.-K. The receptor binding domain of MERS-CoV: The dawn of vaccine and treatment development. J. Formos. Med. Assoc. 2014, 113, 143–147. [Google Scholar] [CrossRef]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986. [Google Scholar] [CrossRef]
- Du, L.; Kou, Z.; Ma, C.; Tao, X.; Wang, L.; Zhao, G.; Chen, Y.; Yu, F.; Tseng, C.-T.K.; Zhou, Y. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: Implication for developing therapeutics and vaccines. PLoS ONE 2013, 8, e81587. [Google Scholar] [CrossRef]
- Kassim, N.; Mtenga, A.B.; Shim, W.-B.; Chung, D.-H. The in vitro and in vivo efficacy of hen IgY against Vibrio parahaemolyticus and Vibrio vulnificus. J. Microbiol. Biotechnol. 2012, 22, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Wallach, M.; Smith, N.C.; Petracca, M.; Miller, C.M.; Eckert, J.; Braun, R. Eimeria maxima gametocyte antigens: Potential use in a subunit maternal vaccine against coccidiosis in chickens. Vaccine 1995, 13, 347–354. [Google Scholar] [CrossRef]
- Widagdo, W.; Okba, N.M.; Li, W.; de Jong, A.; de Swart, R.L.; Begeman, L.; van den Brand, J.M.; Bosch, B.-J.; Haagmans, B.L. Species-Specific Colocalization of Middle East Respiratory Syndrome Coronavirus Attachment and Entry Receptors. J. Virol. 2019, 93, e00107-19. [Google Scholar] [CrossRef]
- Wirblich, C.; Coleman, C.M.; Kurup, D.; Abraham, T.S.; Bernbaum, J.G.; Jahrling, P.B.; Hensley, L.E.; Johnson, R.F.; Frieman, M.B.; Schnell, M.J. One-health: A safe, efficient, dual-use vaccine for humans and animals against Middle East respiratory syndrome coronavirus and rabies virus. J. Virol. 2017, 91, e02040-16. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chakraborti, S.; Dimitrov, A.S.; Gramatikoff, K.; Dimitrov, D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003, 312, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Conroy, P.J.; Law, R.H.; Gilgunn, S.; Hearty, S.; Caradoc-Davies, T.T.; Lloyd, G.; O’Kennedy, R.J.; Whisstock, J.C. Reconciling the structural attributes of avian antibodies. J. Biol. Chem. 2014, 289, 15384–15392. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, C.-A.; Anquez, V.; Grimal, H.; Weill, J.-C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell 1987, 48, 379–388. [Google Scholar] [CrossRef]
- McCormack, W.T.; Tjoelker, L.W.; Barth, C.F.; Carlson, L.M.; Petryniak, B.; Humphries, E.H.; Thompson, C.B. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Genes Dev. 1989, 3, 838–847. [Google Scholar] [CrossRef]
- Coleman, C.M.; Matthews, K.L.; Goicochea, L.; Frieman, M.B. Wild-type and innate immune-deficient mice are not susceptible to the Middle East respiratory syndrome coronavirus. J. Gen. Virol. 2014, 95, 408. [Google Scholar] [CrossRef]
- Qiu, H.; Sun, S.; Xiao, H.; Feng, J.; Guo, Y.; Tai, W.; Wang, Y.; Du, L.; Zhao, G.; Zhou, Y. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antivir. Res. 2016, 132, 141–148. [Google Scholar] [CrossRef]
- New, R.; Moore, B.; Butcher, W.; Mahood, R.; Lever, M.; Smither, S.; O’Brien, L.; Weller, S.; Bayliss, M.; Gibson, L. Antibody-mediated protection against MERS-CoV in the murine model. Vaccine 2019, 37, 4094–4102. [Google Scholar] [CrossRef]
- Cockrell, A.S.; Johnson, J.C.; Moore, I.N.; Liu, D.X.; Bock, K.W.; Douglas, M.G.; Graham, R.L.; Solomon, J.; Torzewski, L.; Bartos, C. A spike-modified Middle East respiratory syndrome coronavirus (MERS-CoV) infectious clone elicits mild respiratory disease in infected rhesus macaques. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Hua, X.; Vijay, R.; Channappanavar, R.; Athmer, J.; Meyerholz, D.K.; Pagedar, N.; Tilley, S.; Perlman, S. Nasal priming by a murine coronavirus provides protective immunity against lethal heterologous virus pneumonia. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wohlford-Lenane, C.; Perlman, S.; Zhao, J.; Jewell, A.K.; Reznikov, L.R.; Gibson-Corley, K.N.; Meyerholz, D.K.; McCray, P.B., Jr. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016, 213, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Yount, B.L.; Debbink, K.; Agnihothram, S.; Gralinski, L.E.; Plante, J.A.; Graham, R.L.; Scobey, T.; Ge, X.-Y.; Donaldson, E.F. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015, 21, 1508–1513. [Google Scholar] [CrossRef]
- Salje, H.; Rodríguez-Barraquer, I.; Rainwater-Lovett, K.; Nisalak, A.; Thaisomboonsuk, B.; Thomas, S.J.; Fernandez, S.; Jarman, R.G.; Yoon, I.-K.; Cummings, D.A. Variability in dengue titer estimates from plaque reduction neutralization tests poses a challenge to epidemiological studies and vaccine development. PLoS Negl. Trop. Dis. 2014, 8, e2952. [Google Scholar] [CrossRef]
- Heppner, D.G., Jr.; Kemp, T.L.; Martin, B.K.; Ramsey, W.J.; Nichols, R.; Dasen, E.J.; Link, C.J.; Das, R.; Xu, Z.J.; Sheldon, E.A. Safety and immunogenicity of the rVSV∆ G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: A phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect. Dis. 2017, 17, 854–866. [Google Scholar] [CrossRef]
- Pascal, K.E.; Coleman, C.M.; Mujica, A.O.; Kamat, V.; Badithe, A.; Fairhurst, J.; Hunt, C.; Strein, J.; Berrebi, A.; Sisk, J.M. Pre-and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2015, 112, 8738–8743. [Google Scholar] [CrossRef]
- dos Santos, G.; Parra, E.; Stegun, F.; Cirqueira, C.; Capelozzi, V. Immunohistochemical detection of virus through its nuclear cytopathic effect in idiopathic interstitial pneumonia other than acute exacerbation. Braz. J. Med Biol. Res. 2013, 46, 985–992. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Tabll, A.A.; Hassan, A.M.; Iwata-Yoshikawa, N.; Nagata, N.; Azhar, E.I. Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model. Vaccines 2020, 8, 634. https://doi.org/10.3390/vaccines8040634
Abbas AT, El-Kafrawy SA, Sohrab SS, Tabll AA, Hassan AM, Iwata-Yoshikawa N, Nagata N, Azhar EI. Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model. Vaccines. 2020; 8(4):634. https://doi.org/10.3390/vaccines8040634
Chicago/Turabian StyleAbbas, Aymn T., Sherif A. El-Kafrawy, Sayed Sartaj Sohrab, Ashraf A. Tabll, Ahmed M. Hassan, Naoko Iwata-Yoshikawa, Noriyo Nagata, and Esam I. Azhar. 2020. "Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model" Vaccines 8, no. 4: 634. https://doi.org/10.3390/vaccines8040634
APA StyleAbbas, A. T., El-Kafrawy, S. A., Sohrab, S. S., Tabll, A. A., Hassan, A. M., Iwata-Yoshikawa, N., Nagata, N., & Azhar, E. I. (2020). Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model. Vaccines, 8(4), 634. https://doi.org/10.3390/vaccines8040634






