A Polypeptide of Tumor-Associated Antigen L6 with Intrinsic Adjuvant Activity Enhances Antitumor Immunity
Abstract
1. Introduction
2. Materials and Methods
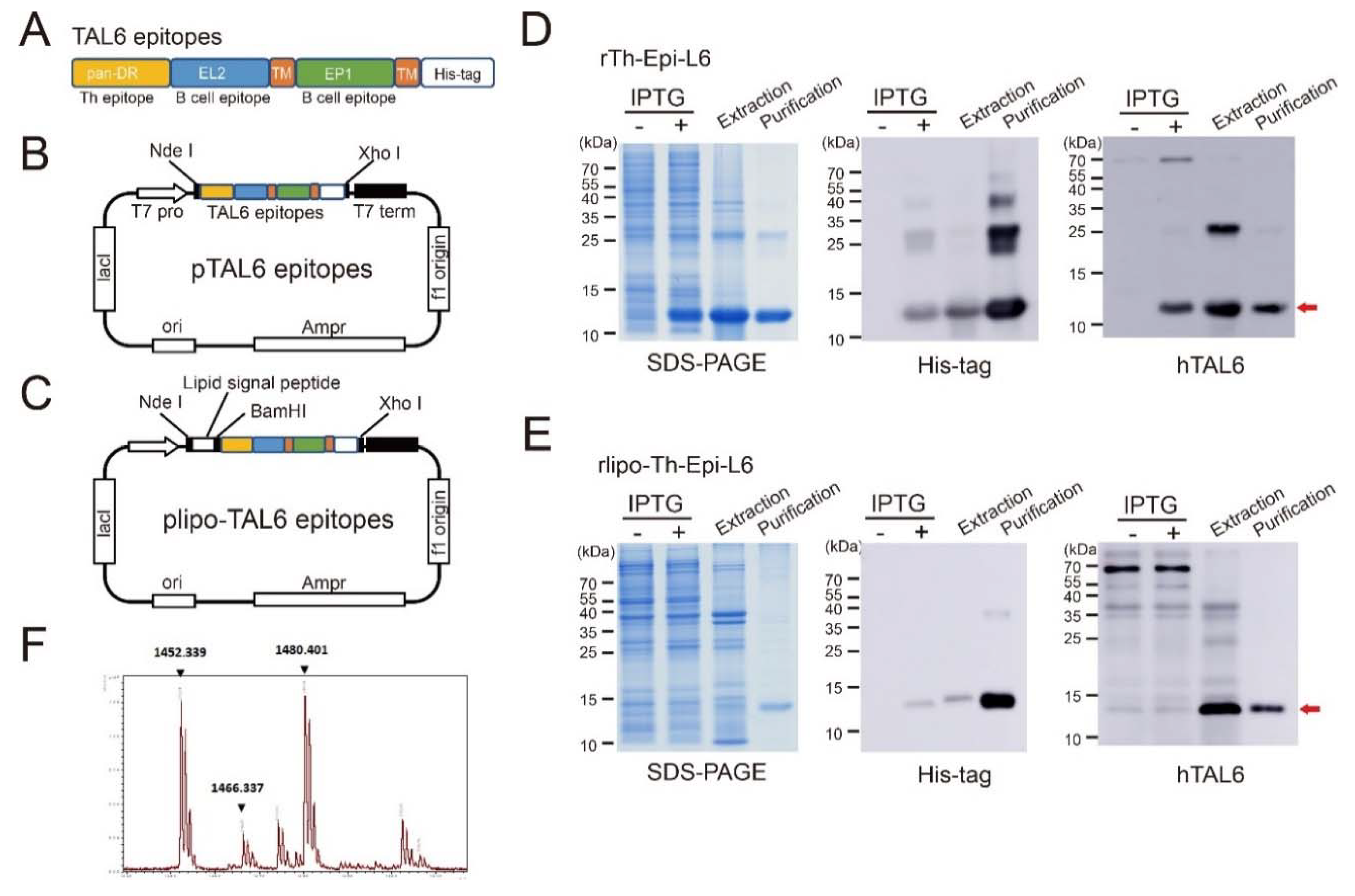
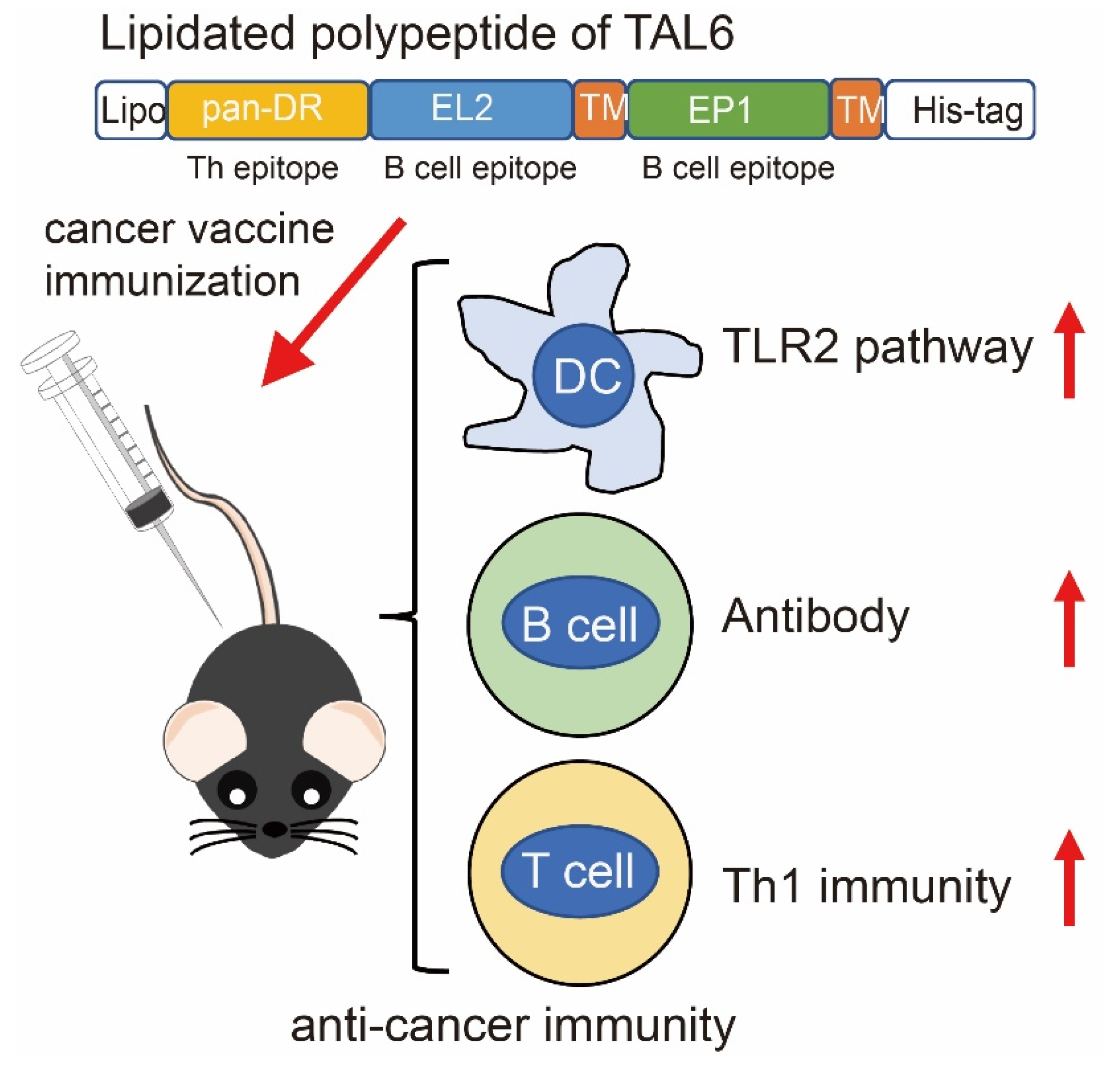
2.1. Production of Recombinant Proteins rTh-Epi-L6 and Rlipo-Th-Epi-L6
2.2. Characterization of the Recombinant Proteins
2.3. Activation of Bone Marrow-Derived Dendritic Cells (BM-DCs)
2.4. Antibody Isotypes Analysis
2.5. T Cell Response Assay
2.6. In Vivo Tumor Protection Experiments
2.7. Statistical Analysis
3. Results
3.1. Characterization of Purified Recombinant Proteins Composed with Polyepitopes of TAL6
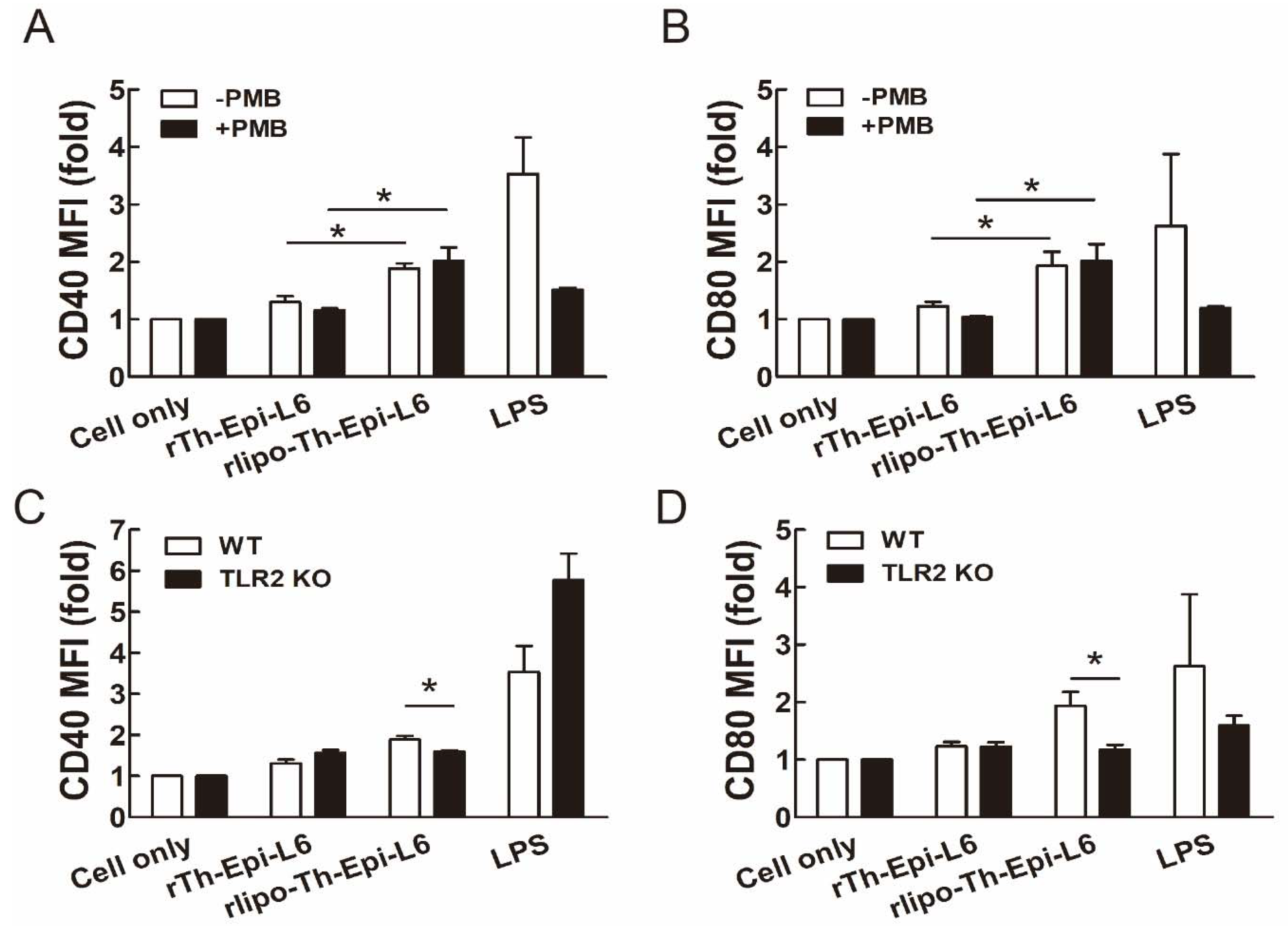
3.2. Rlipo-Th-Epi-L6 Proteins Activate BM-DCs through TLR2
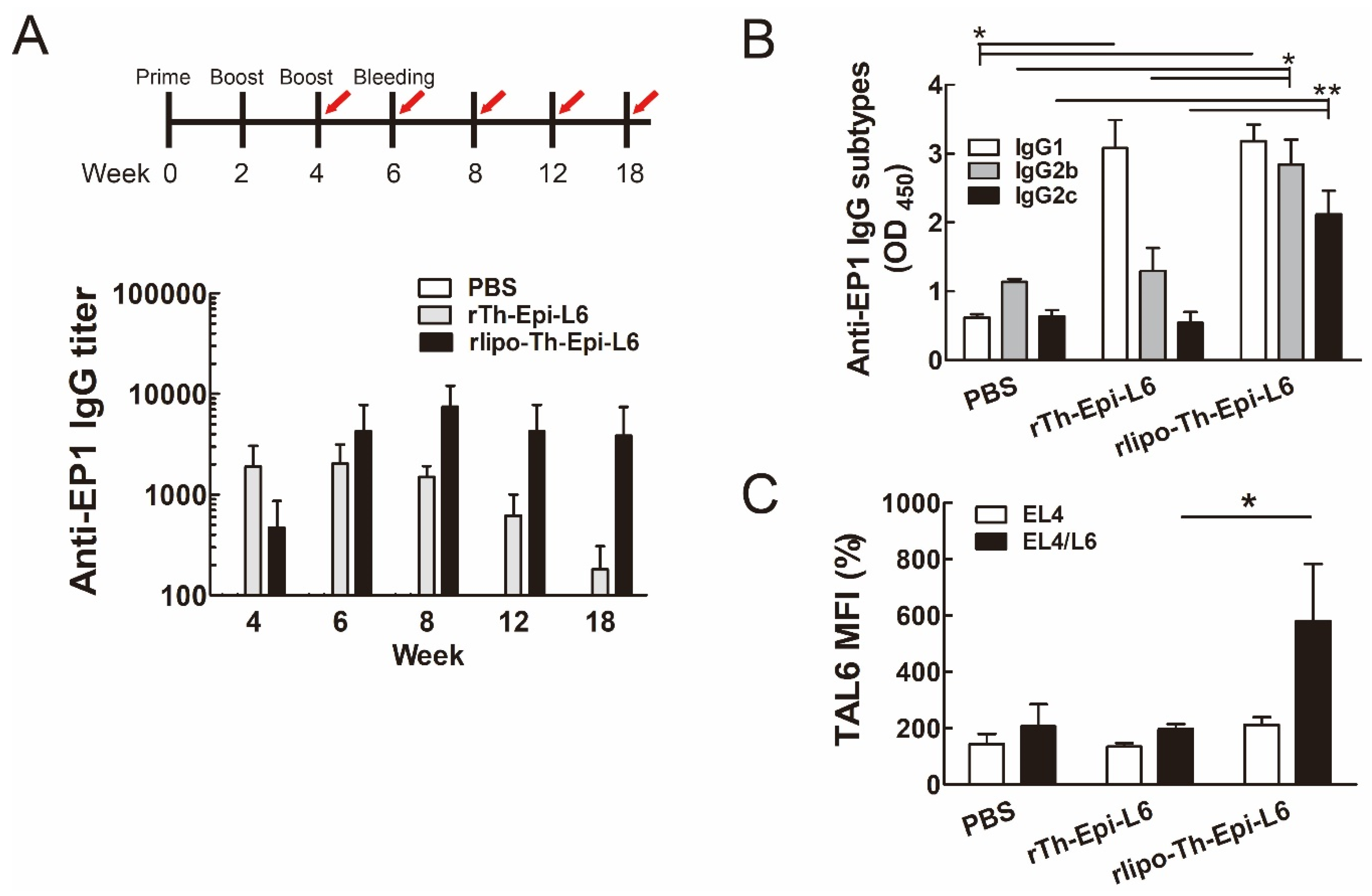
3.3. Immunization with Rlipo-Th-Epi-L6 Increases Antigen-Specific Antibody Levels and T Helper Cell Responses
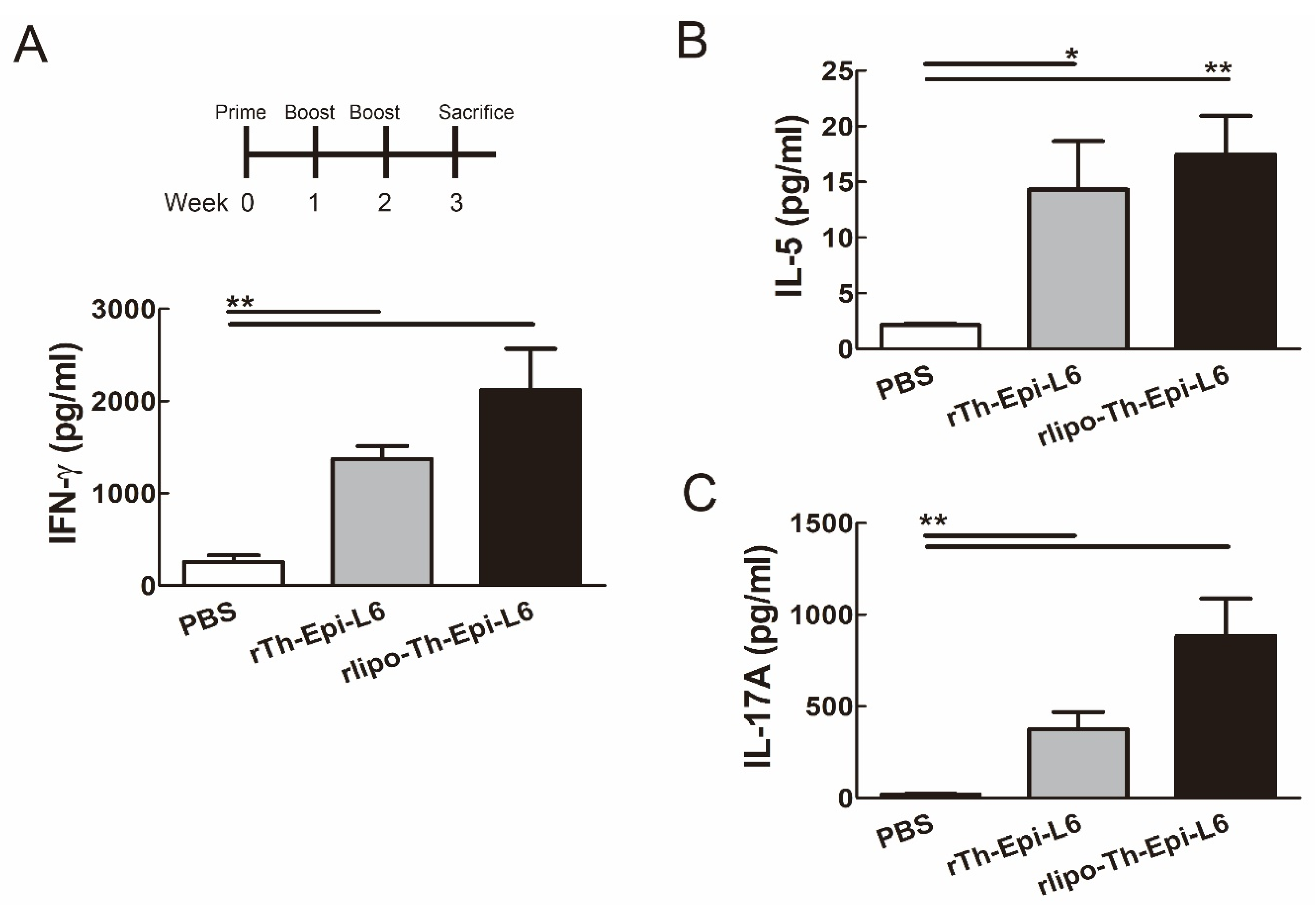
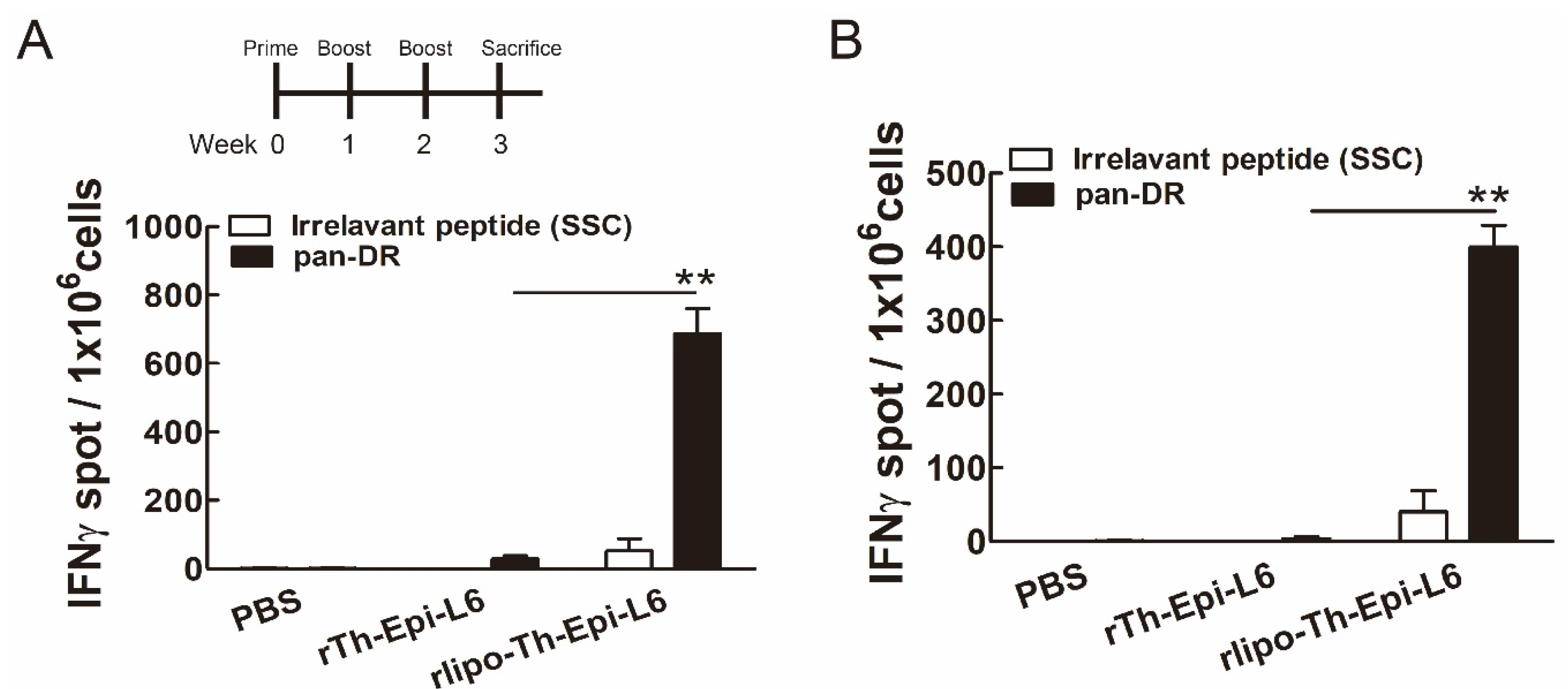
3.4. Immunization with Rlipo-Th-Epi-L6 Induces Antigen-Specific T Cell Responses
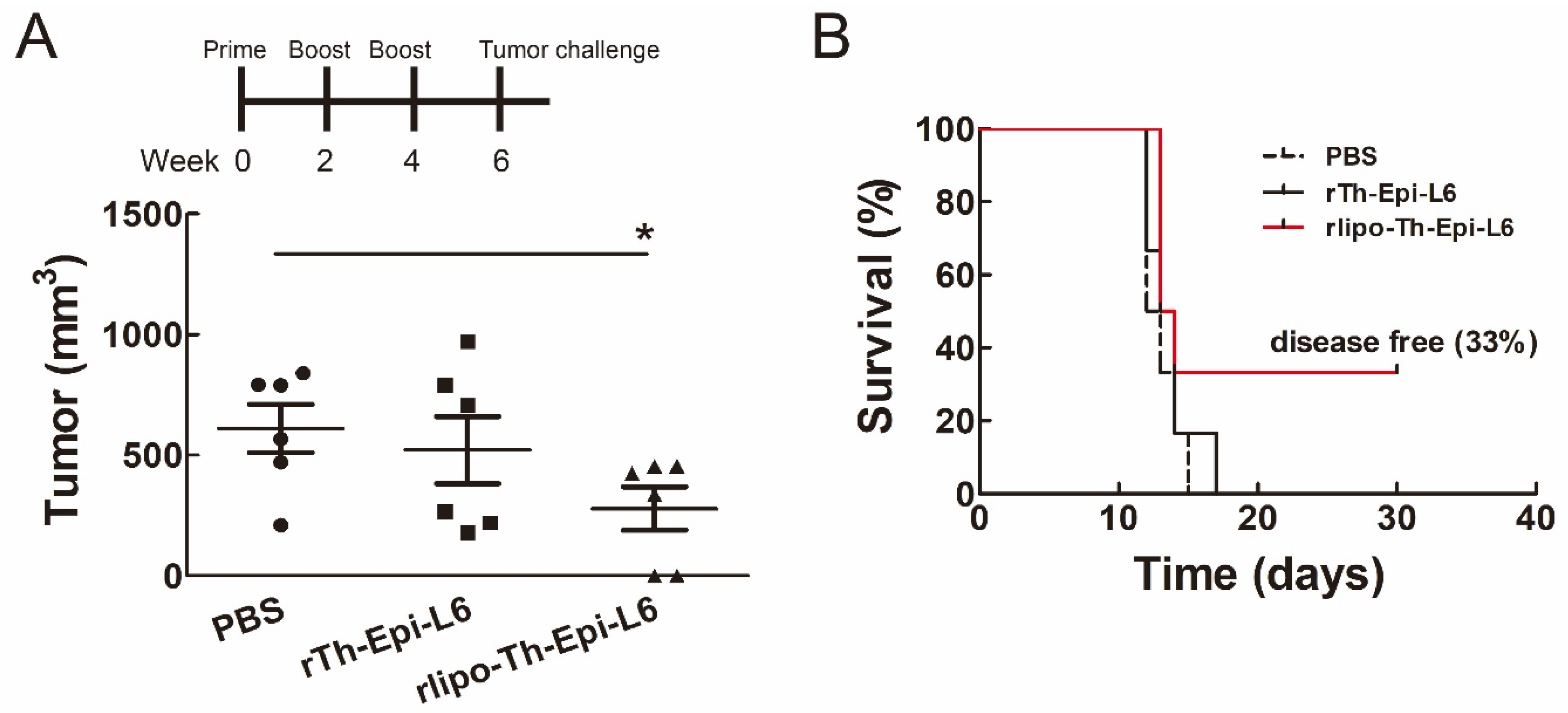
3.5. Immunization with Rlipo-Th-Epi-L6 Reduces Tumor Size and Provide the Protective Effect in the Disease-Free Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corraliza-Gorjon, I.; Somovilla-Crespo, B.; Santamaria, S.; Garcia-Sanz, J.A.; Kremer, L. New Strategies Using Antibody Combinations to Increase Cancer Treatment Effectiveness. Front. Immunol. 2017, 8, 1804. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Jazayeri, S.D.; Ramanathan, B.; Poh, C.L. Enhancement of Tetravalent Immune Responses to Highly Conserved Epitopes of a Dengue Peptide Vaccine Conjugated to Polystyrene Nanoparticles. Vaccines 2020, 8, 417. [Google Scholar] [CrossRef]
- De Leon, P.; Canas-Arranz, R.; Saez, Y.; Forner, M.; Defaus, S.; Cuadra, D.; Bustos, M.J.; Torres, E.; Andreu, D.; Blanco, E.; et al. Association of Porcine Swine Leukocyte Antigen (SLA) Haplotypes with B- and T-Cell Immune Response to Foot-and-Mouth Disease Virus (FMDV) Peptides. Vaccines 2020, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol. Exp. 2020, 68, 2. [Google Scholar] [CrossRef] [PubMed]
- Kaumaya, P.T. B-cell epitope peptide cancer vaccines: A new paradigm for combination immunotherapies with novel checkpoint peptide vaccine. Future Oncol. 2020, 16, 1767–1791. [Google Scholar] [CrossRef]
- Christodoulides, A.; Boyadjian, A.; Kelesidis, T. Spirochetal Lipoproteins and Immune Evasion. Front. Immunol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Infante-Duarte, C.; Kamradt, T. Lipopeptides of Borrelia burgdorferi outer surface proteins induce Th1 phenotype development in alphabeta T-cell receptor transgenic mice. Infect. Immun. 1997, 65, 4094–4099. [Google Scholar] [CrossRef]
- Oftung, F.; Wiker, H.G.; Deggerdal, A.; Mustafa, A.S. A novel mycobacterial antigen relevant to cellular immunity belongs to a family of secreted lipoproteins. Scand. J. Immunol. 1997, 46, 445–451. [Google Scholar] [CrossRef]
- Wright, M.D.; Ni, J.; Rudy, G.B. The L6 membrane proteins--a new four-transmembrane superfamily. Protein. Sci. 2000, 9, 1594–1600. [Google Scholar] [CrossRef]
- Marken, J.S.; Schieven, G.L.; Hellstrom, I.; Hellstrom, K.E.; Aruffo, A. Cloning and expression of the tumor-associated antigen L6. Proc. Natl. Acad. Sci. USA 1992, 89, 3503–3507. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, I.; Horn, D.; Linsley, P.; Brown, J.P.; Brankovan, V.; Hellstrom, K.E. Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res. 1986, 46, 3917–3923. [Google Scholar] [PubMed]
- Hellstrom, I.; Beaumier, P.L.; Hellstrom, K.E. Antitumor effects of L6, an IgG2a antibody that reacts with most human carcinomas. Proc. Natl. Acad. Sci. USA 1986, 83, 7059–7063. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.C.; Zukauskas, A.; Li, D.; Liu, G.; Ang, L.H.; Nagy, J.A.; Brown, L.F.; Dvorak, H.F. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009, 69, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, Y.; Xu, J.; Lu, D.; Wang, J. Role of TM4SF1 in regulating breast cancer cell migration and apoptosis through PI3K/AKT/mTOR pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 9081–9088. [Google Scholar]
- Goodman, G.E.; Hellstrom, I.; Brodzinsky, L.; Nicaise, C.; Kulander, B.; Hummel, D.; Hellstrom, K.E. Phase I trial of murine monoclonal antibody L6 in breast, colon, ovarian, and lung cancer. J. Clin. Oncol. 1990, 8, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Svensson, H.P.; Vrudhula, V.M.; Emswiler, J.E.; MacMaster, J.F.; Cosand, W.L.; Senter, P.D.; Wallace, P.M. In vitro and in vivo activities of a doxorubicin prodrug in combination with monoclonal antibody beta-lactamase conjugates. Cancer Res. 1995, 55, 2357–2365. [Google Scholar] [PubMed]
- Denardo, S.J.; Richman, C.M.; Goldstein, D.S.; Shen, S.; Salako, Q.; Kukis, D.L.; Meares, C.F.; Yuan, A.; Welborn, J.L.; Denardo, G.L. Yttrium-90/indium-111-DOTA-peptide-chimeric L6: Pharmacokinetics, dosimetry and initial results in patients with incurable breast cancer. Anticancer Res. 1997, 17, 1735–1744. [Google Scholar]
- Visintin, A.; Knowlton, K.; Tyminski, E.; Lin, C.I.; Zheng, X.; Marquette, K.; Jain, S.; Tchistiakova, L.; Li, D.; O’Donnell, C.J.; et al. Novel Anti-TM4SF1 Antibody-Drug Conjugates with Activity against Tumor Cells and Tumor Vasculature. Mol. Cancer 2015, 14, 1868–1876. [Google Scholar] [CrossRef]
- Sher, Y.P.; Lin, S.I.; Chen, I.H.; Liu, H.Y.; Lin, C.Y.; Chiang, I.P.; Roffler, S.; Chen, H.W.; Liu, S.J. A HLA-A2-restricted CTL epitope induces anti-tumor effects against human lung cancer in mouse xenograft model. Oncotarget 2016, 7, 671–683. [Google Scholar] [CrossRef][Green Version]
- Lin, S.I.; Huang, M.H.; Chang, Y.W.; Chen, I.H.; Roffler, S.; Chen, B.M.; Sher, Y.P.; Liu, S.J. Chimeric peptide containing both B and T cells epitope of tumor-associated antigen L6 enhances anti-tumor effects in HLA-A2 transgenic mice. Cancer Lett. 2016, 377, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sarramegn, V.; Muller, I.; Milon, A.; Talmont, F. Recombinant G protein-coupled receptors from expression to renaturation: A challenge towards structure. Cell Mol. Life Sci. 2006, 63, 1149–1164. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, J.S.; Lau, S.Y.; Ramirez, E.M.; De Giovanni, C.; Forni, G.; Morrison, S.L.; Penichet, M.L. Protein vaccination with the HER2/neu extracellular domain plus anti-HER2/neu antibody-cytokine fusion proteins induces a protective anti-HER2/neu immune response in mice. Vaccine 2003, 21, 1317–1326. [Google Scholar] [CrossRef]
- Chen, H.W.; Liu, S.J.; Liu, H.H.; Kwok, Y.; Lin, C.L.; Lin, L.H.; Chen, M.Y.; Tsai, J.P.; Chang, L.S.; Chiu, F.F.; et al. A novel technology for the production of a heterologous lipoprotein immunogen in high yield has implications for the field of vaccine design. Vaccine 2009, 27, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chen, J.J.; Shen, K.Y.; Chang, L.S.; Yeh, Y.C.; Chen, I.H.; Chong, P.; Liu, S.J.; Leng, C.H. Recombinant lipidated HPV E7 induces a Th-1-biased immune response and protective immunity against cervical cancer in a mouse model. PLoS ONE 2012, 7, e40970. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Singh, M. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J. Pharm. Sci. 2011, 100, 34–37. [Google Scholar] [CrossRef]
- Kipps, T.J.; Parham, P.; Punt, J.; Herzenberg, L.A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J. Exp. Med. 1985, 161, 1–17. [Google Scholar] [CrossRef]
- Clay, T.M.; Osada, T.; Hartman, Z.C.; Hobeika, A.; Devi, G.; Morse, M.A.; Lyerly, H.K. Polyclonal immune responses to antigens associated with cancer signaling pathways and new strategies to enhance cancer vaccines. Immunol. Res. 2011, 49, 235–247. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Heck, S.; Lustigman, S.; Jiang, S. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: Implication for vaccine design. J. Virol. 2006, 80, 5757–5767. [Google Scholar] [CrossRef]
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef]
- Jiang, S.; Lu, L.; Du, L.; Debnath, A.K. A predicted receptor-binding and critical neutralizing domain in S protein of the novel human coronavirus HCoV-EMC. J. Infect. 2013, 66, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.; Chionh, Y.T. Why can’t we make an effective vaccine against Helicobacter pylori? Expert Rev. Vaccines 2013, 12, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Lehours, P.; Ferrero, R.L. Review: Helicobacter: Inflammation, immunology, and vaccines. Helicobacter 2019, 24 (Suppl. S1), e12644. [Google Scholar] [CrossRef]
- Song, Z.; Li, B.; Zhang, Y.; Li, R.; Ruan, H.; Wu, J.; Liu, Q. Outer Membrane Vesicles of Helicobacter pylori 7.13 as Adjuvants Promote Protective Efficacy Against Helicobacter pylori Infection. Front. Microbiol. 2020, 11, 1340. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Sunay, M.M.E.; Martins, K.A.O.; Steffens, J.T.; Gregory, M.; Vantongeren, S.A.; Van Hoeven, N.; Garnes, P.G.; Bavari, S. Glucopyranosyl lipid adjuvant enhances immune response to Ebola virus-like particle vaccine in mice. Vaccine 2019, 37, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Wasan, E.K.; Syeda, J.; Strom, S.; Cawthray, J.; Hancock, R.E.; Wasan, K.M.; Gerdts, V. A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice. Vaccine 2019, 37, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Liu, S.J.; Hsieh, C.H.; Chen, M.Y.; Tsai, J.P.; Liu, H.H.; Chen, I.H.; Chong, P.; Leng, C.H.; Chen, H.W. Recombinant lipidated dengue-3 envelope protein domain III stimulates broad immune responses in mice. Vaccine 2016, 34, 1054–1061. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiang, C.Y.; Pan, C.H.; Chen, M.Y.; Hsieh, C.H.; Tsai, J.P.; Liu, H.H.; Liu, S.J.; Chong, P.; Leng, C.H.; Chen, H.W. Immunogenicity of a novel tetravalent vaccine formulation with four recombinant lipidated dengue envelope protein domain IIIs in mice. Sci. Rep. 2016, 6, 30648. [Google Scholar] [CrossRef]
- Chen, M.Y.; Chai, K.M.; Chiang, C.Y.; Wu, C.C.; Yu, G.Y.; Liu, S.J.; Chen, H.W. Recombinant lipidated Zika virus envelope protein domain III elicits durable neutralizing antibody responses against Zika virus in mice. J. Biomed. Sci. 2020, 27, 51. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, Y.-P.; Chai, K.M.; Chen, W.-C.; Shen, K.-Y.; Chen, I.-H.; Lee, M.-H.; Chiu, F.-F.; Liu, S.-J. A Polypeptide of Tumor-Associated Antigen L6 with Intrinsic Adjuvant Activity Enhances Antitumor Immunity. Vaccines 2020, 8, 620. https://doi.org/10.3390/vaccines8040620
Sher Y-P, Chai KM, Chen W-C, Shen K-Y, Chen I-H, Lee M-H, Chiu F-F, Liu S-J. A Polypeptide of Tumor-Associated Antigen L6 with Intrinsic Adjuvant Activity Enhances Antitumor Immunity. Vaccines. 2020; 8(4):620. https://doi.org/10.3390/vaccines8040620
Chicago/Turabian StyleSher, Yuh-Pyng, Kit Man Chai, Wen-Ching Chen, Kuan-Yin Shen, I-Hua Chen, Ming-Hui Lee, Fang-Feng Chiu, and Shih-Jen Liu. 2020. "A Polypeptide of Tumor-Associated Antigen L6 with Intrinsic Adjuvant Activity Enhances Antitumor Immunity" Vaccines 8, no. 4: 620. https://doi.org/10.3390/vaccines8040620
APA StyleSher, Y.-P., Chai, K. M., Chen, W.-C., Shen, K.-Y., Chen, I.-H., Lee, M.-H., Chiu, F.-F., & Liu, S.-J. (2020). A Polypeptide of Tumor-Associated Antigen L6 with Intrinsic Adjuvant Activity Enhances Antitumor Immunity. Vaccines, 8(4), 620. https://doi.org/10.3390/vaccines8040620





