Abstract
Ever since the discovery of vaccines, many deadly diseases have been contained worldwide, ultimately culminating in the eradication of smallpox and polio, which represented significant medical achievements in human health. However, this does not account for the threat influenza poses on public health. The currently licensed seasonal influenza vaccines primarily confer excellent strain-specific protection. In addition to the seasonal influenza viruses, the emergence and spread of avian influenza pandemic viruses such as H5N1, H7N9, H7N7, and H9N2 to humans have highlighted the urgent need to adopt a new global preparedness for an influenza pandemic. It is vital to explore new strategies for the development of effective vaccines for pandemic and seasonal influenza viruses. The new vaccine approaches should provide durable and broad protection with the capability of large-scale vaccine production within a short time. The adenoviral (Ad) vector-based vaccine platform offers a robust egg-independent production system for manufacturing large numbers of influenza vaccines inexpensively in a short timeframe. In this review, we discuss the progress in the development of Ad vector-based influenza vaccines and their potential in designing a universal influenza vaccine.
1. Introduction
Adenoviruses (Ads) belong to the family Adenoviridae. They are widely prevalent in humans, other mammals, birds, reptiles, amphibians, and fish. Out of more than 100 human Ad types, several are involved in mild respiratory infections, gastroenteritis, or conjunctivitis [1,2,3]. They are nonenveloped icosahedral viruses of approximately 90 nm in diameter with a core comprising a double-stranded linear DNA genome of roughly 25–48 kilobase pairs (kbp). The Ad genome has inverted terminal repeats (ITRs) on the right and left ends. The ITRs vary in length from 30 to 371 bp. These ITRs are essential in the initiation of the Ad genome replication. A genome packaging signal (Ψ) is found next to the left ITR, which is necessary for Ad genome packaging (Figure 1). The Ad genome encodes early genes (E1A, E1B, E2–E4) and late genes (L1–L5). The early genes are essential for the modulation of the host cell genes and the initiation of the Ad genome replication. The Ad late genes are encoding the majority of structural proteins required for the Ad capsid assembly [4].
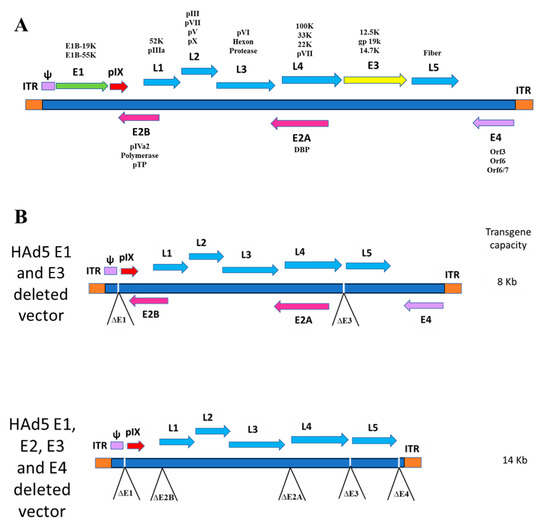
Figure 1.
(A) The transcriptional map of human adenovirus type 5 (HAd5). It is composed of early (E) region (E1–E4) genes, which are responsible for genome replication, regulation of the viral transcription, and suppression of the infected cell response to the virus. The late gene transcription units (L1–L5) are expressed late in the viral replication cycle leading to the synthesis of the majority of viral structural proteins. (B) Diagrammatic representation of HAd5 vaccine vectors. The upper panel represents the vector genome containing the E1 and E3 deletions, and the lower panel shows the vector genome organization consisting of the E1–E4 deletions to increase the insertion capacity of foreign gene cassette.
Ad-based vectors have been used as gene delivery systems for recombinant vaccines and gene therapy applications [5]. There are many critical advantages of Ad as a gene delivery system, including the simplicity of the vector development, their ability to replicate to very high titers in cell culture, the convenience of certified cell lines for large-scale production and purification, and their safety for human applications. Moreover, they induce high levels of transgene expression, as well as high levels of antigen-specific humoral and cell-mediated immune (CMI) responses, by inducing the activation of innate immunity [6,7,8]. Ad vectors have the advantage of being delivered via either the systemic or the mucosal route. Typically, the foreign gene insertion in an Ad vector can be in any early region (E), predominately in the E1 region. To increase the transgene insertion capacity, the E1–E4 region can be deleted (Figure 1). The E3 genes are not essential for virus replication, whereas the genes in the E1, E2, and E4 regions are critical for virus replication. Therefore, for growing Ad vectors having a deletion in the E1, E2, or E4 region, there is a need for a cell line that expresses these gene cassettes to complement the viral functions for vector replication [9]. Ad vectors having only the E3 deletion are replication-competent, whereas vectors with deletion of E1, E2, E4, or any combination are replication-defective.
Influenza viruses infect humans, pigs, horses, dogs, bats, ferrets, seals, and a wide variety of birds, and belong to the family Orthomyxoviridae [10,11]. Human influenza viruses are grouped into A–C types. Influenza A viruses, in particular, are essential for the periodic influenza pandemics due to their prevalence in a variety of hosts, including birds. Adaptations of an avian or swine influenza A virus in humans or an antigenic shift, due to the reassortment of influenza segmented RNA genome in a mixed infection, could generate a novel influenza A virus against which humans have little to no immunity leading to an influenza pandemic. Furthermore, both influenza A and influenza B viruses undergo antigenic drifts due to immune pressure and/or the lack of proofreading ability of influenza RNA-dependent RNA polymerase. This results in the seasonal variability of influenza viruses from year to year.
2. Current Seasonal Influenza Vaccines and Their Potential Limitations
It is estimated that around one billion cases, including 3–5 million cases of severe illness and 290,000–650,000 influenza-related deaths occur globally every year [12]. The wide prevalence of influenza infections between humans has highlighted the significance of the disease and the immediate need for developing the next generation of influenza vaccines. On the basis of extensive influenza surveillance worldwide, influenza strains for the seasonal influenza vaccine are selected every year for better protection during the influenza season, which peaks between December and February in the USA. The majority of vaccines are still based on inactivated influenza viruses grown in embryonated eggs. For the 2020–2021 season, the egg-based trivalent vaccines contain A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09-like virus, A/Hong Kong/2671/2019(H3N2)-like virus, and B/Washington/02/2019 (Victoria lineage)-like virus, while the egg-based quadrivalent vaccines contain the same three components of the trivalent vaccine plus B/Phuket/3073/2013 (Yamagata lineage)-like virus [13]. There are two types of vaccines for the elderly in the US this season, a quadrivalent high-dose vaccine and a quadrivalent vaccine adjuvanted with MF59 [14,15].
For the 2020–2021 season, the other type of influenza vaccines is the cell- or recombinant HA-based vaccines comprising A/Hawaii/70/2019(H1N1)pdm09-like virus, A/Hong Kong/45/2019 (H3N2)-like virus, B/Washington/02/2019 (Victoria lineage)-like virus, and B/Phuket/3073/2013 (Yamagata lineage)-like virus. A quadrivalent live attenuated influenza vaccine (LAIV) as a nasal spray is also available for use in 2–49-year-old healthy individuals.
The success of the currently available seasonal influenza vaccines is mainly dependent on the match between the vaccine strains and the circulating influenza viruses during the influenza season. A significant antigenic mismatch could result in a variable degree of vaccine failure and higher incidence of influenza-like illnesses [16,17,18]. The use of GSK’s AS03 [15,19], Sanofi Pasteur’s AF03 [20], MF59 of Novartis [14,15], or Vaxine’s Advax-CpG55.2 adjuvant [21] may, on the other hand, broaden the coverage of the current influenza vaccines. The diagrammatic representation of influenza virus structural components, including the immunogenic antigens that could be used for developing Ad vector-based influenza vaccines, is shown in Figure 2.
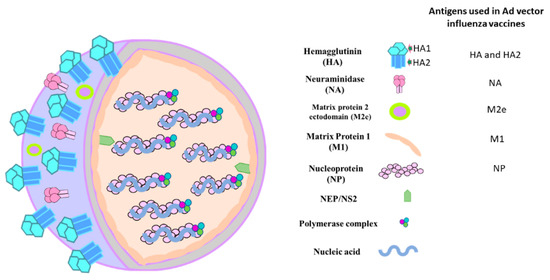
Figure 2.
Diagrammatic sketch of influenza A virus structure. The influenza A virus is composed of eight segments of negative-sense ribonucleic acid (RNA) coupled with nucleoprotein (NP). The internal proteins include polymerase complex, nuclear export protein (NEP), and matrix 1 (M1) protein. The surface proteins are hemagglutinin (HA), neuraminidase (NA), and matrix 2 (M2) protein.
4. Significance of Ad Vector Immunity for Vaccine Platform
There are high incidences of Ad infections in humans due to the prevalence of over 100 types of Ad, leading to the development of varying levels of Ad neutralizing antibodies commonly mentioned as “Ad vector immunity” or “preexisting Ad vector immunity” [41,42]. The levels of preexisting Ad vector immunity largely depend on the exposure to Ad type(s) and vary across geographic regions and age [43,44,45]. The levels of human Ad type 5 (HAd5)-neutralizing antibody titers are in the range of 18 to 4690 [46]. HAd5-neutralizing antibody titers between 256 and 512 were observed in 16% of adults in the US [47], while a median titer of 512 in sub-Saharan children was reported [43].
The neutralizing antibodies are mainly targeted against the Ad capsid proteins (hexon, fiber, and penton), and Ad-specific CMI responses are directed against the capsid, as well as internal proteins [24,48,49]. Ad-neutralizing antibodies can neutralize the vector before internalization into the cells, whereas Ad-specific CD8+ T cells can eliminate the cells expressing vector proteins [50,51]. Therefore, it is anticipated that vector immunity negatively influences the duration and expression levels of the desired immunogen, as well as the quality and quantity of immune responses to the immunogen.
The impact of vector immunity on inhibiting the development of immunogen-specific immune responses in a mouse model was investigated. There was a vector immunity titer-dependent inhibition in the levels of humoral and CMI responses by the HAd5 vector expressing the hemagglutinin (HA) and nucleoprotein (NP) of A/Vietnam/1203/ 04 (H5N1) influenza virus [46]. A virus-neutralization titer of 520 resulted in a decline in the humoral and CMI responses against HA and NP but still conferred complete protection following the influenza challenge. Furthermore, a virus-neutralization titer of approximately 1500 can even be overcome either by changing the route of immunization or by increasing the vaccine dose.
In addition to the prevalence of Ad-specific immunity due to natural exposure, immunization with the Ad vector-based vaccine elicits vector-specific neutralizing antibodies in conjunction with the immunogen-specific immune responses. The acquired vector-specific neutralizing antibodies could be vital in hampering the vaccine effectiveness with the same vector if the vector neutralization antibody titers remain high. For example, seasonal influenza vaccines are required every year due to the waning of immunity against seasonal influenza viruses. Therefore, a study was conducted to investigate the effectiveness of Ad vector-based influenza vaccine annual immunization. This study examined whether Ad-neutralizing antibody titers wane to levels that do not adversely impact yearly vaccination with the same vector. The mice were mocked or HAd-primed then inoculated intramuscularly (i.m.) with 108 plaque-forming units (PFU) of HAd-H5HA (HAd5 vector expressing H5N1 HA) at 1, 3, 6, and 10 months post HAd priming [52]. With time, there was a continual decline in HAd5 vector neutralization antibody titers with constant rises in the levels of HA-specific humoral and CMI responses, resulting in significant protection against challenge with an antigenically distinct H5N1 influenza virus at 6 months and onward [52]. These findings suggest that yearly immunization with the same vector is possible due to a significant drop in the vector immunity.
Due to the high seroprevalence of HAd5 in humans [24,41,44,48,53], which affects the potency of HAd5 vectored vaccines [46,52,54], rare human Ads such as HAd6, HAd11, HAd19a, HAd26, HAd28, HAd35, HAd48, and HAd49 [43,55,56,57,58,59,60,61] were developed as vectors for vaccine and gene therapy to circumvent the preexisting vector immunity. In addition, nonhuman Ad vectors (bovine Ad (BAd), chimpanzee Ad (chAd), porcine Ad (PAd), Canine Ad (CAd), and others) [47,53,62,63,64] demonstrated significant potential to overcome the preexisting vector immunity. Furthermore, the encapsulation of the Ad vector into microparticles [65,66], the coating of the vector with polyethylene glycol [66], and the alteration of Ad capsid proteins [67] were investigated to evade the preexisting vector immunity.
5. Ad Vector-Based Influenza Vaccines in Preclinical Trials
5.1. Human Ad (HAd) Vectors in Preclinical Trials
Several HAd vectors have been used for developing influenza vaccines, but the majority of these studies were conducted with HAd5 vectors (Table 1). Many studies were performed in mice using external (HA, NA, and M2e) and internal (NP and M1) influenza virus antigens expressed in the HAd5 vector [68,69,70,71,72,73,74,75,76,77,78,79]. One of the earliest studies illustrated the partial protection of mice by the HAd5 vector carrying the HA gene [68]. In this study, mice were challenged with A/HK/1/68(H3N2) influenza virus, while the HAd5 vector expressed HA of A/Swine/Iowa/99(H3N2) virus. In another study with the HAd5 vector expressing the full HA from the A/Hong Kong/156/97(H5N1) influenza virus, the mice were completely protected against distinct strains of H5N1 influenza viruses [70] due to the development of excellent HA-specific cell-mediated and neutralizing antibody responses. On the other hand, the intranasal (i.n.) immunization of mice with HAd5 vector expressing HA of A/HK/156/97(H5N1) resulted in complete protection from the challenge with a heterologous A/VN/1203/RG(H5N1) influenza virus [80]. Similar results were reported from studies conducted on mice and ferrets [81,82,83] using HAd5 vectors. Another interesting research assessed the protection against A/Indo/05/2005(H5N1) avian influenza virus challenge in animals immunized through oral administration of HAd5 vector expressing HA of A/Indo/05/2005(H5N1) and a double-stranded RNA adjuvant [83]. The vaccinated ferrets and mice were protected from the homologous influenza virus lethal challenge. Furthermore, cross-clade neutralizing antibodies were recognized in the immunized ferrets.

Table 1.
Preclinical trials with adenoviral vector-based influenza vaccines.
The efficacies of HAd5 vector-based influenza vaccines in protecting pigs from lethal swine influenza infections were demonstrated [84,85,86]. Immunogenicity and protection efficacy of a single i.n. inoculation of HAd5-vectored vaccine expressing HA of A/CA/04/09(H1N1) was compared with the i.m. administration of the whole virus inactivated vaccine in pigs [86]. The mucosal immunoglobulin A (IgA) was induced as a result of the homologous influenza virus, in addition to cell-mediated immunity against homologous and heterologous influenza viruses in vaccinated animals. The induced immune response resulted in complete protection against the homologous influenza virus challenge and partial protection against the heterologous challenge [86].
HAd4 was used as a replication-competent vaccine vector to construct the Ad4-H5-Vtn vaccine. It contained a deletion in the E3 region to accommodate the HA gene of A/Vietnam/1194/2004(H5N1) influenza virus. The i.n. immunized mice with the Ad4-H5-Vtn vaccine survived the challenge with a lethal A/VN/PR8/CDC-RG/H5N1 virus even in the presence of preexisting immunity to the HAd4 virus [87].
5.2. Nonhuman Ad Vectors in Preclinical Trials
Nonhuman Ad vectors have been developed to overcome the HAd preexisting immunity in the human population [45]. Replication-defective ChAd vector-based influenza vaccines were evaluated [53,88,89,90]. The ChAd (simian AdC24 or AdC7) vector expressing the nucleoprotein (NP) of A/Puerto Rico/8/34(H1N1) influenza virus was effective in protecting BALB/c mice from two H5N1 strains [53]. A single i.n. administration of a replication-defective pan Ad type 3 (PanAd3), expressing a fusion protein of conserved NP and matrix 1 (M1) consensus sequences, was able to elicit humoral and T-cell immune responses against NP and M1 proteins [88]. The vaccinated BALB/c mice were protected from the lethal challenge of the mouse-adapted A/Fort Monmouth/1/47-ma (H1N1) virus [88]. In another study, immunization of mice with a replication-deficient AdC7 expressing HA of A/chicken/Henan/12/2004(H5N1) induced both HA-specific humoral and CMI responses and conferred complete protection against 5 LD50 (median lethal dose) of A/chicken/Henan/12/2004(H5N1) [89].
The replication-defective BAd3 vector-based vaccine was evaluated against highly infectious avian influenza [54,80]. BAd3 internalization is dependent on the α (2,3) or α (2,6)-linked sialic acid receptors [91], while the CAR receptors are used by most human and chimpanzee Ad vectors [92,93,94]. This feature of the BAd3 vector is attractive for mucosal immunization. A replication-defective BAd3 vector (BAd-H5HA) expressing HA of A/HK/156(H5N1) induced high levels of humoral and CMI responses in mice leading to full protection from morbidity and mortality following a lethal challenge with A/HK/483/97(H5N1) [54]. The immunogenicity and protective efficacy of the BAd-H5HA vaccine were not impacted by the exceptionally high levels of preexisting HAd5-neutralizing antibodies [54]. A low dose of BAd-H5HA vaccine (106 PFU) when administered i.n. in mice conferred complete protection against an antigenically distinct A/VN/1203/RG/H5N1 influenza virus [80], whereas the i.m. immunization required a 3 × 107 PFU dose of BAd-H5HA vaccine for complete protection from a heterologous influenza virus challenge. In the same study, the i.m. vaccination of mice with a 3 × 108 PFU dose of HAd-H5HA failed to provide full protection following the influenza virus challenge, while i.n. immunization with a 3 × 107 PFU dose of BAd-H5HA conferred complete protection. Overall, these studies demonstrate the dose-sparing superiority of the BAd3 vector compared to the HAd5 vector, even in the presence of high levels of HAd-neutralizing antibodies.
The replication-defective PAd3 vector system was also used to develop a recombinant influenza vaccine [95]. The PAd3 vector (PAV3-HA) expressing an optimized HA antigen of A/Hanoi/30408/2005(H5N1) influenza virus generated antibodies and CMI responses in mice after i.m. inoculation. The PAV3-HA vector provided better survival and lower virus load compared to the HAd5 vector with the same HA insert at 8 days and 12 months after i.m. immunization.
6. Ad Vector-Based Influenza Vaccines in Clinical Trials
6.1. Human Ad Vectors in Clinical Trials
HAd5 vector-based influenza vaccines have been used in several clinical trials [96,97,98,99,100] (Table 2). A phase II randomized influenza A challenge study (clinical trial NCT02918006) used the oral administration of an H1N1 HA HAd5 vector vaccine and a dsRNA adjuvant (VXA-A1.1) [101]. Healthy adult volunteers with undetectable or low preexisting antibodies to the H1N1 pdm09-like virus were chosen. A single dose of VXA-A1.1, a commercial injectable quadrivalent influenza vaccine (QIV), or a placebo group was used. Ninety days after immunization, vaccinated or placebo subjects were challenged with the A/H1N1 influenza virus. The outcome of this trial demonstrated 48% protection in the orally administered individuals compared to approximately 38% for those injected with QIV.

Table 2.
Adenovirus vector-based influenza vaccines in clinical trials.
The safety and immunogenicity of an HAd5 vectored vaccine (AdhVN1203/04.H5) expressing HA of A/VN/1203/04(H5N1) were assessed in a phase I clinical trial (NCT00755703) [98]. The objective of this study was to determine the safety and immunogenicity of a two-dose i.n. vaccine at 4 weeks intervals. Three different doses (108, 109, and 1010 virus particles (VP)) were evaluated in 19–49-year-old healthy adults. The vaccine was well tolerated and induced humoral immunity as measured by hemagglutination inhibition (HI) assay [98]. Currently, a phase IIa clinical trial (NCT03232567) is ongoing with an HAd5 vector-based influenza vaccine (NasoVAX) carrying the H1HA gene as an intranasal spray [102]. The purpose of this dose-escalating study using 109, 1010, or 1011 VP is to evaluate the safety and immunogenicity of the NasoVAX intranasal spray vaccine [102]. These studies signify the implication of the mucosal route of inoculation in eliciting improved mucosal protection against influenza.
The replication-competent HAd4 expressing HA of A/VN/1194/2004/H5N1 from the E3 region (Ad4-H5-Vtn) was formulated as an enteric capsule for oral administration [103,104]. A randomized, double-blind, placebo-controlled phase I clinical trial (NCT01006798) was conducted to evaluate the safety and immunogenicity of orally administered Ad4-H5-Vtn [103]. The outcomes suggested that the priming by Ad4-H5-Vtn might enhance the immunogenicity of an egg-derived H5N1 influenza vaccine.
6.2. Nonhuman Ad Vectors in Clinical Trials
The replication-deficient ChAdOx1 NP + M1 vaccine is based on the ChAd Y25 isolate, with deletions in E1 and E3 regions. ChAdOx1 NP + M1 is expressing NP and the matrix protein 1 (M1) of A/Panama/2007/99/H3N2 [105,106]. The safety and immunogenicity of the ChAdOx1 NP + M1 vaccine in a dose-escalation study (5 × 108, 5 × 109, 2.5 × 1010, or 5 × 1010 VP) were evaluated in a phase I clinical trial (NCT01818362) [107]. The significant adverse signs from mild to moderate in severity were dose-dependent and resolved within 48 h. The noticeable symptoms included pain and warmth at the site of injection, malaise, headache, and fatigue. There were dose-dependent increases in influenza virus antigen-specific T-cell responses and virus-neutralization titers. The prime-boost regimen of ChAdOx1 NP + M1 and modified vaccinia Ankara with the same insert (MVA NP + M1) lead to increased levels of humoral and CMI responses, which persisted for at least 18 months [108], suggesting the significance of prime boost with heterologous vectors for durable immunity.
7. Potential of Ad Vectors for Developing Universal Influenza Vaccines
The prevention and control of emerging and recurring influenza viruses require effective strategies to deal with the rapid changes in the virus antigens. Influenza viruses can evolve and evade the host immune mechanisms through two powerful phenomena: antigenic shift and antigenic drift [109,110,111,112]. Influenza pandemics have been occurring at regular intervals, and the next pandemic can happen anytime in the future. The current vaccination strategies against influenza viruses are less efficient against seasonal influenza and will not be useful in a pandemic influenza situation [16,113]. Therefore, effective novel vaccines are necessary for dealing with any potential influenza pandemic situation. The World Health Organization (WHO), the National Institutes of Health (NIH), and several other international organizations are highly supportive of developing universal influenza vaccines. These broadly protective vaccines should have the ability to confer protection against currently known and potential pandemic influenza viruses.
For developing Ad vector-based broadly protective influenza vaccines, several relatively conserved influenza proteins, including NP, M1, matrix protein 2 (M2), and the stem region of the HA protein (HA2), have been considered [75,76,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128], and some are currently in clinical trials [97,99,105]. An Ad vector encoding the secreted HA2 fused with murine CD40L (rAd-SHA2FCD40L) was used for i.n. immunization of mice [117]. The vaccinated animals were protected from the lethal challenge with H1N1, H3N2, and H9N2 influenza viruses. Moreover, the sera from immunized mice were able to neutralize 13 subtypes of influenza viruses. Mice vaccinated with HAd5 vector expressing HA of A/HK/156/97 were effectively protected from the homologous challenge with A/HK/156/97, as well as from the challenge with an antigenically distinct A/VN/1203/04 virus even in the absence of effective neutralizing antibodies [70]. The inclusion of HA from two different HA subtypes in Ad vector-based vaccine formulation broadens the protective efficacy against influenza viruses [115]. The i.m. immunization of mice with HAd5 vector expressing NP of H5N1 virus resulted in approximately 2.4, 1.9, 2.3, 2.4, or 1.4 log reductions in lung virus titers of H1, H3, H5, H7, and H9 influenza viruses, respectively [115]. These results suggest the role of NP in inducing broadly protective immunity against influenza viruses. The HAd5 vector, expressing the relatively conserved domains [M2 ectodomain (M2e), HA fusion domain, HA alpha-helix domain, and a T-cell epitope of NP] of an H5N1 influenza virus, was developed and used for i.m. immunization of mice [114]. The expected humoral and/or CMI responses against these domains were observed, and 1.5, 1.2, and 1.8 log reductions in the lung viral titers of H5N2, H7N9, and H9N2 influenza virus, respectively, were observed in immunized mice following challenge [114]. These results suggest that broadly protective immune responses against influenza viruses can be induced by Ad vectors expressing selected influenza antigens or domains.
9. Conclusions and Future Directions
The majority of currently available seasonal influenza vaccines are strain-specific. The influenza virus is prone to continuous antigen changes due to antigenic drift and occasionally antigenic shift, leading to a pandemic influenza virus. Alternatively, an avian or porcine influenza virus can undergo mutations, leading to its replication in humans and eventually efficient human-to-human transmission, thereby starting an influenza pandemic. Recently, an H1N1 swine influenza virus with pandemic potential was identified in China [130]. The yearly updated seasonal influenza vaccines produced by egg-based technology are capable of meeting the global demand under normal circumstances. In a pandemic situation, it would be almost impossible for the current influenza vaccine manufacturing technology to produce enough doses in time to meet the global demand.
The first-generation Ad vectors (with E1 and/or E3 deletions) and the second-generation Ad vectors (E1 and E3 deletions plus E2 and/or E4 deletions) are used successfully as vaccine vectors. However, the first-generation Ad vectors are more immunogenic than the second-generation Ad vectors. Ad vectors with low seroprevalence among the population are more effective and preferred as vaccine vectors. Therefore, the first-generation Ad vectors with low seroprevalence in humans (rare human Ad and non-human Ad vectors) are highly recommended for developing pre-pandemic and pandemic influenza vaccines. First-generation Ad vector-based vaccines for the Ebola virus and SARS-CoV-2 were successfully developed [131,132,133,134,135,136]. Third-generation (gutless) Ad vectors have been developed mainly for gene therapy to reduce the host immune response against the Ad vector-transduced cells. However, gutless Ad vectors have been studied as a vaccine vector for human immunodeficiency virus (HIV) [137,138].
To supplement the current influenza vaccine technology and to meet the global demand in the event of an influenza pandemic, Ad vector-based vaccine platforms have the potential to play a vital role. A large number of Ad vector-based vaccine doses at a low cost can be produced within a short timeframe. Inclusion of an immuno-stimulatory molecule with an Ad vector-based influenza vaccine can induce protective immunity one week post immunization [139] and enhance the protective efficacy for the elderly [140]. The characteristics of the next pandemic influenza virus cannot be predicted; thus, it is imperative to develop pre-pandemic vaccines and stockpile them for pandemic preparedness. The Ad vector platform has advantages over the current influenza vaccine technology to serve as a system for developing pre-pandemic vaccines, which will be critical in inhibiting the virus transmission and infection-associated morbidity and mortality during the early phase of a pandemic and before a strain-matched vaccine is available.
The availability of several human and nonhuman Ad vector systems provides versatility in Ad vector-based influenza vaccine design for developing both seasonal and pandemic vaccines. In addition to the humoral immune response, the importance of the CMI response in generating broader protection has been realized. The role of NP-specific immune responses in protection has been demonstrated. Along with NP, the relatively conserved protective epitopes have been identified in HA2 and M2e domains; therefore, the probability of designing a universal influenza vaccine seems very high. Additional work is needed to explore whether Ad vector-based influenza vaccines could be used in all segments of the population. The use of the prime-boost approach with two different Ad vectors [54,141], Ad and another viral vector [142,143,144,145,146,147], or Ad vector and DNA immunization [148,149,150,151] will confer a considerably higher level of immune responses, especially in the elderly.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This work was supported by Public Health Service grant AI059374 from the National Institute of Allergy and Infectious Diseases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Human Adenovirus Working Group. Available online: http://hadvwg.gmu.edu/ (accessed on 25 August 2020).
- Harrach, B.; Benkö, M.; Both, G.W.; Brown, M.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kajon, A.; Lehmkuhl, H.D.; et al. Adenoviridae—dsDNA Viruses. In Virus Taxonomy; Ninth Report of the International Committee on Taxonomy of Viruses; International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- MacLachlan, N.J.; Dubovi, E.J. Adenoviridae. In Fenner’s Veterinary Virology, 5th ed.; MacLachlan, N.J., Dubovi, E.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 217–227. [Google Scholar]
- Liu, L. Fields Virology, 6th Edition. Clin. Infect. Dis. 2014, 59, 613. [Google Scholar] [CrossRef]
- Vectors Used in Gene Therapy Clinical Trials. Available online: http://www.abedia.com/wiley/vectors.php (accessed on 20 July 2020).
- Appledorn, D.M.; Patial, S.; McBride, A.; Godbehere, S.; Van Rooijen, N.; Parameswaran, N.; Amalfitano, A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008, 181, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Fejer, G.; Freudenberg, M.; Greber, U.F.; Gyory, I. Adenovirus-triggered innate signalling pathways. Eur. J. Microbiol. Immunol. 2011, 1, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Yang, Y. Innate Immune Response to Adenoviral Vectors is Mediated by both Toll-Like Receptor-Dependent and -Independent Pathways. J. Virol. 2007, 81, 3170–3180. [Google Scholar] [CrossRef]
- Kovesdi, I.; Hedley, S.J. Adenoviral Producer Cells. Viruses 2010, 2, 1681–1703. [Google Scholar] [CrossRef]
- Payne, S. Family Orthomyxoviridae. In Viruses; Chapter 23; Payne, S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 197–208. [Google Scholar]
- International Committee on Taxonomy of Viruses (ICTV). Orthomyxoviridae; Negative Sense RNA Viruses; Negative Sense RNA Viruses. 2011. Available online: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/negative-sense-rna-viruses-2011/w/negrna_viruses/209/orthomyxoviridae (accessed on 20 July 2020).
- Global Influenza Strategy 2019–2030. Available online: https://www.who.int/influenza/Global_Influenza_Strategy_2019_2030_Summary_English.pdf? (accessed on 20 July 2020).
- CDC. Frequently Asked Influenza (Flu) Questions: 2020–2021 Season. Available online: https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm (accessed on 20 July 2020).
- CDC. People 65 Years and Older & Influenza. Available online: https://www.cdc.gov/flu/highrisk/65over.htm (accessed on 20 July 2020).
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.F.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef]
- Belongia, E.A.; Kieke, B.A.; Donahue, J.G.; Greenlee, R.T.; Balish, A.; Foust, A.; Lindstrom, S.; Shay, D.K.; Marshfield Influenza Study Group. Effectiveness of Inactivated Influenza Vaccines Varied Substantially with Antigenic Match from the 2004–2005 Season to the 2006–2007 Season. J. Infect. Dis. 2009, 199, 159–167. [Google Scholar] [CrossRef]
- Zost, S.; Parkhouse, K.; Gumina, M.; Kim, K.; Diaz, P.S.; Wilson, P.; Treanor, J.; Sant, A.; Cobey, S.; Hensley, S. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef]
- Vesikari, T.; Pépin, S.; Kusters, I.; Hoffenbach, A.; Denis, M. Assessment of squalene adjuvanted and non-adjuvanted vaccines against pandemic H1N1 influenza in children 6 months to 17 years of age. Hum. Vaccines Immunother. 2012, 8, 1283–1292. [Google Scholar] [CrossRef]
- Petrovsky, N. Vaxine. Hum. Vaccines Immunother. 2016, 12, 2726–2728. [Google Scholar] [CrossRef][Green Version]
- Vemula, S.V.; Mittal, S.K. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin. Biol. Ther. 2010, 10, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Ahi, Y.S.; Bangari, D.S.; Mittal, S.K. Adenoviral vector immunity: Its implications and circumvention strategies. Curr. Gene Ther. 2011, 11, 307–320. [Google Scholar] [CrossRef]
- Sharma, A.; Tandon, M.; Ahi, Y.S.; Bangari, D.S.; Vemulapalli, R.; Mittal, S.K. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010, 17, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Muruve, D.A. The Innate Immune Response to Adenovirus Vectors. Hum. Gene Ther. 2004, 15, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Larregina, A.T.; Ganster, R.W.; Zahorchak, A.F.; Plowey, J.M.; Takayama, T.; Logar, A.J.; Robbins, P.D.; Falo, L.D.; Thomson, A.W. Recombinant Adenovirus Induces Maturation of Dendritic Cells via an NF-κB-Dependent Pathway. J. Virol. 2000, 74, 9617–9628. [Google Scholar] [CrossRef]
- Muruve, D.A.; Barnes, M.J.; Stillman, I.E.; Libermann, T.A. Adenoviral Gene Therapy Leads to Rapid Induction of Multiple Chemokines and Acute Neutrophil-Dependent Hepatic Injury in Vivo. Hum. Gene Ther. 1999, 10, 965–976. [Google Scholar] [CrossRef]
- Gregory, S.M.; Nazir, S.A.; Metcalf, J.P. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol. 2011, 6, 357–374. [Google Scholar] [CrossRef]
- Atasheva, S.; Shayakhmetov, D.M. Adenovirus sensing by the immune system. Curr. Opin. Virol. 2016, 21, 109–113. [Google Scholar] [CrossRef]
- Sharma, A.; Bangari, D.S.; Tandon, M.; HogenEsch, H.; Mittal, S.K. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res. 2010, 153, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nociari, M.; Ocheretina, O.; Schoggins, J.W.; Falck-Pedersen, E. Sensing Infection by Adenovirus: Toll-Like Receptor-Independent Viral DNA Recognition Signals Activation of the Interferon Regulatory Factor 3 Master Regulator. J. Virol. 2007, 81, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Muruve, D.A. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003, 10, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nunes, F.; Berencsi, K.; Furth, E.; Gönczöl, E.; Wilson, J. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 1994, 91, 4407–4411. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ertl, H.C.; Wilson, J.M. MHC class I-cestricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1994, 1, 433–442. [Google Scholar] [CrossRef]
- Yang, Y.; Jooss, K.U.; Su, Q.; Ertl, H.C.; Wilson, J.M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996, 3, 137–144. [Google Scholar]
- Yang, Y.; Wilson, J.M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J. Immunol. 1995, 155, 2564–2570. [Google Scholar]
- Yang, Y.; Li, Q.; Ertl, H.C.; Wilson, J.M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995, 69, 2004–2015. [Google Scholar] [CrossRef]
- Merkley, S.D.; Chock, C.J.; Yang, X.O.; Harris, J.; Castillo, E.F. Modulating T Cell Responses via Autophagy: The Intrinsic Influence Controlling the Function of Both Antigen-Presenting Cells and T Cells. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef]
- Saini, N.K.; Baena, A.; Ng, T.W.; Venkataswamy, M.M.; Kennedy, S.C.; Kunnath-Velayudhan, S.; Carreño, L.J.; Xu, J.; Chan, J.; Larsen, M.H.; et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat. Microbiol. 2016, 1, 16133. [Google Scholar] [CrossRef]
- Nwanegbo, E.; Vardas, E.; Gao, W.; Whittle, H.; Sun, H.; Rowe, D.; Robbins, P.D.; Gambotto, A. Prevalence of Neutralizing Antibodies to Adenoviral Serotypes 5 and 35 in the Adult Populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004, 11, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, Y.; Wu, H.; Wang, Z.; Zhan, Y.; Feng, X.; Geng, R.; Wu, Y.; Kong, W.; Yu, X. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J. Med Virol. 2012, 84, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Thorner, A.R.; Vogels, R.; Kaspers, J.; Weverling, G.J.; Holterman, L.; Lemckert, A.A.C.; Dilraj, A.; McNally, L.M.; Jeena, P.M.; Jepsen, S.; et al. Age Dependence of Adenovirus-Specific Neutralizing Antibody Titers in Individuals from Sub-Saharan Africa. J. Clin. Microbiol. 2006, 44, 3781–3783. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Kik, S.V.; Weverling, G.J.; Dilan, R.; King, S.L.; Maxfield, L.F.; Clark, S.; Ng’Ang’A, D.; Brandariz, K.L.; Abbink, P.; et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011, 29, 5203–5209. [Google Scholar] [CrossRef] [PubMed]
- Mast, T.C.; Kierstead, L.; Gupta, S.B.; Nikas, A.; Kallas, E.G.; Novitsky, V.; Mbewe, B.; Pitisuttithum, P.; Schechter, M.; Vardas, E.; et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010, 28, 950–957. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, N.; Vemula, S.V.; Couëtil, L.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Impact of Preexisting Adenovirus Vector Immunity on Immunogenicity and Protection Conferred with an Adenovirus-Based H5N1 Influenza Vaccine. PLoS ONE 2012, 7, e33428. [Google Scholar] [CrossRef]
- Bangari, D.S.; Mittal, S.K. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004, 105, 127–136. [Google Scholar] [CrossRef]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.C.; Vogels, R.; Custers, J.H.H.V.; Addo, M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; et al. Neutralizing Antibodies to Adenovirus Serotype 5 Vaccine Vectors are Directed Primarily against the Adenovirus Hexon Protein. J. Immunol. 2005, 174, 7179–7185. [Google Scholar] [CrossRef]
- Aldhamen, Y.A.; Seregin, S.S.; Amalfitano, A. Immune Recognition of Gene Transfer Vectors: Focus on Adenovirus as a Paradigm. Front. Immunol. 2011, 2, 40. [Google Scholar] [CrossRef]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell. Biochem. 2009, 108, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.E.; Timares, L.; Matthews, Q.L. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev. Vaccines 2009, 8, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Sayedahmed, E.E.; Kumari, R.; Shukla, S.; Hassan, A.O.; Mohammed, S.I.; York, I.A.; Gangappa, S.; Sambhara, S.; Mittal, S.K. Longevity of adenovirus vector immunity in mice and its implications for vaccine efficacy. Vaccine 2018, 36, 6744–6751. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kobinger, G.P.; Lin, J.; Figueredo, J.; Calcedo, R.; Kobasa, D.; Wilson, J.M. Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein. Vaccine 2007, 25, 6845–6851. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, A.; Jayashankar, L.; Mittal, S.K. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol. Ther. 2008, 16, 965–971. [Google Scholar] [CrossRef]
- Vogels, R.; Zuijdgeest, D.; van Rijnsoever, R.; Hartkoorn, E.; Damen, I.; de Béthune, M.P.; Kostense, S.; Penders, G.; Helmus, N.; Koudstaal, W.; et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: Efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003, 77, 8263–8271. [Google Scholar] [CrossRef]
- Holterman, L.; Vogels, R.; van der Vlugt, R.; Sieuwerts, M.; Grimbergen, J.; Kaspers, J.; Geelen, E.; van der Helm, E.; Lemckert, A.; Gillissen, G.; et al. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: Low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 2004, 78, 13207–13215. [Google Scholar] [CrossRef]
- Abbink, P.; Lemckert, A.A.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef]
- Kahl, C.A.; Bonnell, J.; Hiriyanna, S.; Fultz, M.; Nyberg-Hoffman, C.; Chen, P.; King, C.R.; Gall, J.G. Potent immune responses and in vitro pro-inflammatory cytokine suppression by a novel adenovirus vaccine vector based on rare human serotype 28. Vaccine 2010, 28, 5691–5702. [Google Scholar] [CrossRef]
- Lapuente, D.; Ruzsics, Z.; Thirion, C.; Tenbusch, M. Evaluation of adenovirus 19a as a novel vector for mucosal vaccination against influenza A viruses. Vaccine 2018, 36, 2712–2720. [Google Scholar] [CrossRef]
- Crosby, C.M.; Matchett, W.E.; Anguiano-Zarate, S.S.; Parks, C.A.; Weaver, E.A.; Pease, L.R.; Webby, R.J.; Barry, M.A. Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Barry, M.A. Low seroprevalent species D adenovirus vectors as influenza vaccines. PLoS ONE 2013, 8, e73313. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Prevec, L.; Graham, F.L.; Babiuk, L.A. Development of a bovine adenovirus type 3-based expression vector. J. Gen. Virol. 1995, 76, 93–102. [Google Scholar] [CrossRef]
- Kremer, E.J.; Boutin, S.; Chillon, M.; Danos, O. Canine adenovirus vectors: An alternative for adenovirus-mediated gene transfer. J. Virol. 2000, 74, 505–512. [Google Scholar] [CrossRef]
- Mittal, S.K.; Ahi, Y.S.; Vemula, S.V. Xenogenic Adenoviral Vectors. In Adenoviral Vectors for Gene Therapy, 2nd ed.; Chapter 19; Curiel, D.T., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 495–528. [Google Scholar]
- Sailaja, G.; HogenEsch, H.; North, A.; Hays, J.; Mittal, S.K. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002, 9, 1722–1729. [Google Scholar] [CrossRef]
- Mittal, S.K.; Aggarwal, N.; Sailaja, G.; van Olphen, A.; HogenEsch, H.; North, A.; Hays, J.; Moffatt, S. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: Effect of route of inoculation on immune response. Vaccine 2000, 19, 253–263. [Google Scholar] [CrossRef]
- Gall, J.G.; Crystal, R.G.; Falck-Pedersen, E. Construction and characterization of hexon-chimeric adenoviruses: Specification of adenovirus serotype. J. Virol. 1998, 72, 10260–10264. [Google Scholar] [CrossRef]
- Tang, M.; Harp, J.A.; Wesley, R.D. Recombinant adenovirus encoding the HA gene from swine H3N2 influenza virus partially protects mice from challenge with heterologous virus: A/HK/1/68 (H3N2). Arch. Virol. 2002, 147, 2125–2141. [Google Scholar] [CrossRef]
- Gao, W.; Soloff, A.C.; Lu, X.; Montecalvo, A.; Nguyen, D.C.; Matsuoka, Y.; Robbins, P.D.; Swayne, D.E.; Donis, R.O.; Katz, J.M.; et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 2006, 80, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, M.A.; Garg, S.; Bangari, D.S.; Belser, J.A.; Lu, X.; Stephenson, I.; Bright, R.A.; Katz, J.M.; Mittal, S.K.; Sambhara, S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 2006, 367, 475–481. [Google Scholar] [CrossRef]
- Steitz, J.; Barlow, P.; Hossain, J.; Kim, E.; Okada, K.; Kenniston, T.; Rea, S.; Donis, R.; Gambotto, A. A candidate H1N1 pandemic influenza vaccine elicits protective immunity in mice. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, M.; Jayashankar, L.; Garg, S.; Veguilla, V.; lu, X.; Singh, N.; Katz, J.; Mittal, S.; Sambhara, S. New Pre-pandemic Influenza Vaccines: An Egg-and Adjuvant-independent Human Adenoviral Vector Strategy Induces Long-lasting Protective Immune Responses in Mice. Clin. Pharmacol. Ther. 2007, 82, 665–671. [Google Scholar] [CrossRef]
- Hoelscher, M.A.; Singh, N.; Garg, S.; Jayashankar, L.; Veguilla, V.; Pandey, A.; Matsuoka, Y.; Katz, J.M.; Donis, R.; Mittal, S.K.; et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J. Infect. Dis. 2008, 197, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.H.; Wang, D.; Raja, N.U.; Luo, M.; Moore, K.M.; Woraratanadharm, J.; Mytle, N.; Dong, J.Y. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine 2008, 26, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.L.; Kong, W.P.; Misplon, J.A.; Lo, C.Y.; Tumpey, T.M.; Xu, L.; Nabel, G.J. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 2005, 23, 5404–5410. [Google Scholar] [CrossRef]
- Tompkins, S.M.; Zhao, Z.S.; Lo, C.Y.; Misplon, J.A.; Liu, T.; Ye, Z.; Hogan, R.J.; Wu, Z.; Benton, K.A.; Tumpey, T.M.; et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg. Infect. Dis. 2007, 13, 426–435. [Google Scholar] [CrossRef]
- Leung, H.C.; Chan, C.C.; Poon, V.K.; Zhao, H.J.; Cheung, C.Y.; Ng, F.; Huang, J.D.; Zheng, B.J. An H5N1-based matrix protein 2 ectodomain tetrameric peptide vaccine provides cross-protection against lethal infection with H7N9 influenza virus. Emerg. Microbes Infect. 2015, 4, e22. [Google Scholar] [CrossRef]
- Scallan, C.D.; Lindbloom, J.D.; Tucker, S.N. Oral Modeling of an Adenovirus-Based Quadrivalent Influenza Vaccine in Ferrets and Mice. Infect. Dis. Ther. 2016, 5, 165–183. [Google Scholar] [CrossRef]
- Cao, W.; Liepkalns, J.S.; Hassan, A.O.; Kamal, R.P.; Hofstetter, A.R.; Amoah, S.; Kim, J.H.; Reber, A.J.; Stevens, J.; Katz, J.M.; et al. A highly immunogenic vaccine against A/H7N9 influenza virus. Vaccine 2016, 34, 744–749. [Google Scholar] [CrossRef][Green Version]
- Sayedahmed, E.E.; Hassan, A.O.; Kumari, R.; Cao, W.; Gangappa, S.; York, I.; Sambhara, S.; Mittal, S.K. A bovine adenoviral vector-based H5N1 influenza -vaccine provides enhanced immunogenicity and protection at a significantly low dose. Mol. Ther. Methods Clin. Dev. 2018, 10, 210–222. [Google Scholar] [CrossRef]
- Rao, S.; Kong, W.; Wei, C.; Van Hoeven, N.; Gorres, J.; Nason, M.; Andersen, H.; Tumpey, T.; Nabel, G. Comparative efficacy of hemagglutinin, nucleoprotein, and matrix 2 protein gene-based vaccination against H5N1 influenza in mouse and ferret. PLoS ONE 2010, 5, e9812. [Google Scholar] [CrossRef] [PubMed]
- Price, G.E.; Soboleski, M.R.; Lo, C.Y.; Misplon, J.A.; Pappas, C.; Houser, K.V.; Tumpey, T.M.; Epstein, S.L. Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses. Vaccine 2009, 27, 6512–6521. [Google Scholar] [CrossRef] [PubMed]
- Ciaran, D.S.; Debora, W.T.; Jonathan, D.L.; James, S.T.; Sean, N.T. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin Vaccine Immunol 2013, 20, 85–94. [Google Scholar] [CrossRef]
- Wesley, R.D.; Tang, M.; Lager, K.M. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine 2004, 22, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Wesley, R.; Lager, K. Evaluation of a recombinant human adenovirus-5 vaccine administered via needle-free device and intramuscular injection for vaccination of pigs against swine influenza virus. Am. J. Vet. Res. 2005, 66, 1943–1947. [Google Scholar] [CrossRef]
- Braucher, D.; Henningson, J.; Loving, C.; Vincent, A.; Kim, E.; Steitz, J.; Gambotto, A.; Kehrli, M. Intranasal vaccination with replication-defective adenovirus type 5 encoding influenza virus hemagglutinin elicits protective immunity to homologous challenge and partial protection to heterologous challenge in pigs. Clin. Vaccine Immunol. 2012, 19, 1722–1729. [Google Scholar] [CrossRef]
- Alexander, J.; Ward, S.; Mendy, J.; Manayani, D.; Farness, P.; Avanzini, J.; Guenther, B.; Garduno, F.; Jow, L.; Snarsky, V.; et al. Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin. PLoS ONE 2012, 7, e31177. [Google Scholar] [CrossRef]
- Vitelli, A.; Quirion, M.R.; Lo, C.Y.; Misplon, J.A.; Grabowska, A.K.; Pierantoni, A.; Ammendola, V.; Price, G.E.; Soboleski, M.R.; Cortese, R.; et al. Vaccination to conserved influenza antigens in mice using a novel simian adenovirus vector, PanAd3, derived from the bonobo Pan paniscus. PLoS ONE 2013, 8, e55435. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, X.; Song, Y.; Tang, X.; Zhang, C.; Zhang, H.; Jin, X.; Zhou, D. Chimpanzee adenovirus vector-based avian influenza vaccine completely protects mice against lethal challenge of H5N1. Vaccine 2016, 34, 4875–4883. [Google Scholar] [CrossRef]
- Wang, X.; Fu, W.; Yuan, S.; Yang, X.; Song, Y.; Liu, L.; Chi, Y.; Cheng, T.; Xing, M.; Zhang, Y.; et al. Both haemagglutinin-specific antibody and T cell responses induced by a chimpanzee adenoviral vaccine confer protection against influenza H7N9 viral challenge. Sci. Rep. 2017, 7, 1854. [Google Scholar] [CrossRef]
- Li, X.; Bangari, D.S.; Sharma, A.; Mittal, S.K. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology 2009, 392, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Roelvink, P.; Lizonova, A.; Lee, J.; Li, Y.; Bergelson, J.; Finberg, R.; Brough, D.; Kovesdi, I.; Wickham, T. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [CrossRef]
- Nestić, D.; Uil, T.; Ma, J.; Roy, S.; Vellinga, J.; Baker, A.; Custers, J.; Majhen, D. αvβ3 integrin is required for efficient infection of epithelial cells with human adenovirus type 26. J. Virol. 2018, 93, e01474-18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Xiang, Z.; Gao, G.; Ertl, H.; Wilson, J.; Bergelson, J. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 2002, 83, 151–155. [Google Scholar] [CrossRef]
- Patel, A.; Tikoo, S.; Kobinger, G. A porcine adenovirus with low human seroprevalence is a promising alternative vaccine vector to human adenovirus 5 in an H5N1 virus disease model. PLoS ONE 2010, 5, e15301. [Google Scholar] [CrossRef]
- Peters, W.; Brandl, J.; Lindbloom, J.; Martinez, C.; Scallan, C.; Trager, G.; Tingley, D.; Kabongo, M.; Tucker, S. Oral administration of an adenovirus vector encoding both an avian influenza A hemagglutinin and a TLR3 ligand induces antigen specific granzyme B and IFN-γ T cell responses in humans. Vaccine 2013, 31, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Liebowitz, D.; Lindbloom, J.D.; Brandl, J.R.; Garg, S.J.; Tucker, S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: A phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1041–1048. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Safety and Immunogenicity Study of Adenovirus-Vectored, Intranasal Pandemic Influenza Vaccine; Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT00755703 (accessed on 20 July 2020).
- Liebowitz, D.; Gottlieb, K.; Kolhatkar, N.S.; Garg, S.J.; Asher, J.M.; Nazareno, J.; Kim, K.; McIlwain, D.R.; Tucker, S.N. Efficacy, immunogenicity, and safety of an oral influenza vaccine: A placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 2020, 20, 435–444. [Google Scholar] [CrossRef]
- Kim, L.; Martinez, C.J.; Hodgson, K.A.; Trager, G.R.; Brandl, J.R.; Sandefer, E.P.; Doll, W.J.; Liebowitz, D.; Tucker, S.N. Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Kolhatkar, N.; Gottlieb, K.; Kasparek, K.; Hodgson, K.; Tucker, S.; Liebowitz, D. 1947. Influenza Vaccination via Oral Tablet is Protective and Induces a Unique Mucosal Immune Response. Open Forum Infect. Dis. 2018, 5, S561–S562. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Single-Ascending-Dose Study of the Safety and Immunogenicity of NasoVAX—Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03232567 (accessed on 20 July 2020).
- Gurwith, M.; Lock, M.; Taylor, E.M.; Ishioka, G.; Alexander, J.; Mayall, T.; Ervin, J.E.; Greenberg, R.N.; Strout, C.; Treanor, J.J.; et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: A randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2013, 13, 238–250. [Google Scholar] [CrossRef]
- Khurana, S.; Coyle, E.M.; Manischewitz, J.; King, L.R.; Ishioka, G.; Alexander, J.; Smith, J.; Gurwith, M.; Golding, H. Oral priming with replicating adenovirus serotype 4 followed by subunit H5N1 vaccine boost promotes antibody affinity maturation and expands H5N1 cross-clade neutralization. PLoS ONE 2015, 10, e0115476. [Google Scholar] [CrossRef]
- Antrobus, R.D.; Berthoud, T.K.; Mullarkey, C.E.; Hoschler, K.; Coughlan, L.; Zambon, M.; Hill, A.V.; Gilbert, S.C. Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol. Ther. 2014, 22, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.; Cottingham, M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, R.D.; Coughlan, L.; Berthoud, T.K.; Dicks, M.D.; Hill, A.V.; Lambe, T.; Gilbert, S.C. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol. Ther. 2014, 22, 668–674. [Google Scholar] [CrossRef]
- Coughlan, L.; Sridhar, S.; Payne, R.; Edmans, M.; Milicic, A.; Venkatraman, N.; Lugonja, B.; Clifton, L.; Qi, C.; Folegatti, P.M.; et al. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 2018, 29, 146–154. [Google Scholar] [CrossRef]
- Nakajima, K. The mechanism of antigenic shift and drift of human influenza virus. Nihon Rinsho Jpn. J. Clin. Med. 2003, 61, 1897–1903. [Google Scholar]
- Gregory, V.; Bennett, M.; Orkhan, M.H.; Al Hajjar, S.; Varsano, N.; Mendelson, E.; Zambon, M.; Ellis, J.; Hay, A.; Lin, Y.P. Emergence of influenza A H1N2 reassortant viruses in the human population during 2001. Virology 2002, 300, 1–7. [Google Scholar] [CrossRef]
- Al Faress, S.; Cartet, G.; Ferraris, O.; Norder, H.; Valette, M.; Lina, B. Divergent genetic evolution of hemagglutinin in influenza A H1N1 and A H1N2 subtypes isolated in the south-France since the winter of 2001–2002. J. Clin. Virol. 2005, 33, 230–236. [Google Scholar] [CrossRef]
- Lin, Y.P.; Gregory, V.; Bennett, M.; Hay, A. Recent changes among human influenza viruses. Virus Res. 2004, 103, 47–52. [Google Scholar] [CrossRef]
- Lewnard, J.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Amen, O.; Sayedahmed, E.E.; Vemula, S.V.; Amoah, S.; York, I.; Gangappa, S.; Sambhara, S.; Mittal, S.K. Adenovirus vector-based multi-epitope vaccine provides partial protection against H5, H7, and H9 avian influenza viruses. PLoS ONE 2017, 12, e0186244. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.V.; Ahi, Y.S.; Swaim, A.M.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS ONE 2013, 8, e62496. [Google Scholar] [CrossRef] [PubMed]
- Price, G.E.; Lo, C.Y.; Misplon, J.A.; Epstein, S.L. Mucosal immunization with a candidate universal influenza vaccine reduces virus transmission in a mouse model. J. Virol. 2014. [Google Scholar] [CrossRef]
- Fan, X.; Hashem, A.M.; Chen, Z.; Li, C.; Doyle, T.; Zhang, Y.; Yi, Y.; Farnsworth, A.; Xu, K.; Li, Z.; et al. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol. 2015, 8, 211–220. [Google Scholar] [CrossRef]
- Price, G.E.; Lo, C.Y.; Misplon, J.A.; Epstein, S.L. Reduction of influenza virus transmission from mice immunized against conserved viral antigens is influenced by route of immunization and choice of vaccine antigen. Vaccine 2018, 36, 4910–4918. [Google Scholar] [CrossRef]
- Lapuente, D.; Bonsmann, M.S.G.; Maaske, A.; Stab, V.; Heinecke, V.; Watzstedt, K.; Heß, R.; Westendorf, A.M.; Bayer, W.; Ehrhardt, C.; et al. IL-1β as mucosal vaccine adjuvant: The specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal Immunol. 2018, 11, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Tutykhina, I.; Esmagambetov, I.; Bagaev, A.; Pichugin, A.; Lysenko, A.; Shcherbinin, D.; Sedova, E.; Logunov, D.; Shmarov, M.; Ataullakhanov, R.; et al. Vaccination potential of B and T epitope-enriched NP and M2 against Influenza A viruses from different clades and hosts. PLoS ONE 2018, 13, e0191574. [Google Scholar] [CrossRef]
- Lingel, A.; Bullard, B.L.; Weaver, E.A. Efficacy of an adenoviral vectored multivalent centralized influenza Vaccine. Sci. Rep. 2017, 7, 14912. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Han, G.Y.; Nguyen, H. An adenovirus-vectored influenza vaccine induces durable cross-protective hemagglutinin stalk antibody responses in mice. Viruses 2017, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, Y.; Xia, X.; Zhang, C.; Yang, X.; Song, Y.; Dai, X.; Wang, M.; Zhou, D. Recombinant Adenoviruses Displaying Matrix 2 Ectodomain Epitopes on Their Fiber Proteins as Universal Influenza Vaccines. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Uddback, I.E.; Pedersen, L.M.; Pedersen, S.R.; Steffensen, M.A.; Holst, P.J.; Thomsen, A.R.; Christensen, J.P. Combined local and systemic immunization is essential for durable T-cell mediated heterosubtypic immunity against influenza A virus. Sci. Rep. 2016, 6, 20137. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, H.J.; Han, G.Y.; Song, M.K.; Pereboev, A.; Hong, J.S.; Chang, J.; Byun, Y.H.; Seong, B.L.; Nguyen, H.H. Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice. J. Virol. 2014, 88, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.Y.; Choi, Y.; Nguyen, H.H.; Song, M.K.; Chang, J. Mucosal vaccination with recombinant adenovirus encoding nucleoprotein provides potent protection against influenza virus infection. PLoS ONE 2013, 8, e75460. [Google Scholar] [CrossRef]
- Hashem, A.; Jaentschke, B.; Gravel, C.; Tocchi, M.; Doyle, T.; Rosu-Myles, M.; He, R.; Li, X. Subcutaneous immunization with recombinant adenovirus expressing influenza A nucleoprotein protects mice against lethal viral challenge. Hum. Vaccin. Immunother. 2012, 8, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wu, T.L.; Lasaro, M.O.; Latimer, B.P.; Parzych, E.M.; Bian, A.; Li, Y.; Li, H.; Erikson, J.; Xiang, Z.; et al. A universal influenza A vaccine based on adenovirus expressing matrix-2 ectodomain and nucleoprotein protects mice from lethal challenge. Mol. Ther. 2010, 18, 2182–2189. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Sun, H.; Xiao, Y.; Liu, J.; Wang, D.; Li, F.; Wang, C.; Li, C.; Zhu, J.; Song, J.; Sun, H.; et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef]
- Zhu, F.C.; Wurie, A.H.; Hou, L.H.; Liang, Q.; Li, Y.H.; Russell, J.B.; Wu, S.P.; Li, J.X.; Hu, Y.M.; Guo, Q.; et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: A single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 389, 621–628. [Google Scholar] [CrossRef]
- De Santis, O.; Audran, R.; Pothin, E.; Warpelin-Decrausaz, L.; Vallotton, L.; Wuerzner, G.; Cochet, C.; Estoppey, D.; Steiner-Monard, V.; Lonchampt, S.; et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: A randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 2016, 16, 311–320. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; DeZure, A.D.; Stanley, D.A.; Coates, E.E.; Novik, L.; Enama, M.E.; Berkowitz, N.M.; Hu, Z.; Joshi, G.; Ploquin, A.; et al. Chimpanzee Adenovirus Vector Ebola Vaccine. N. Engl. J. Med. 2017, 376, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Dolzhikova, I.V.; Tokarskaya, E.A.; Dzharullaeva, A.S.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Voronina, O.L.; Syromyatnikova, S.I.; Borisevich, S.V.; Pantyukhov, V.B.; Babira, V.F.; et al. Virus-Vectored Ebola Vaccines. Acta Naturae 2017, 9, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Senac, J.S.; Palmer, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 2009, 4, e5059. [Google Scholar] [CrossRef]
- Weaver, E.A.; Rubrum, A.M.; Webby, R.J.; Barry, M.A. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS ONE 2011, 6, e18314. [Google Scholar] [CrossRef]
- Vemula, S.V.; Amen, O.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res. 2013, 178, 398–403. [Google Scholar] [CrossRef]
- Vemula, S.V.; Pandey, A.; Singh, N.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Adenoviral vector expressing murine β-defensin 2 enhances immunogenicity of an adenoviral vector based H5N1 influenza vaccine in aged mice. Virus Res. 2013, 177, 55–61. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Wang, X.; Song, Y.; Zhou, P.; Li, D.; Zhang, C.; Jin, X.; Huang, Z.; Zhou, D. Chimpanzee adenoviral vector prime-boost regimen elicits potent immune responses against Ebola virus in mice and rhesus macaques. Emerg. Microbes Infect. 2019, 8, 1086–1097. [Google Scholar] [CrossRef]
- Reyes-Sandoval, A.; Berthoud, T.; Alder, N.; Siani, L.; Gilbert, S.C.; Nicosia, A.; Colloca, S.; Cortese, R.; Hill, A.V. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect. Immun. 2010, 78, 145–153. [Google Scholar] [CrossRef]
- Rollier, C.S.; Hill, A.V.S.; Reyes-Sandoval, A. Influence of adenovirus and MVA vaccines on the breadth and hierarchy of T cell responses. Vaccine 2016, 34, 4470–4474. [Google Scholar] [CrossRef]
- Vierboom, M.P.M.; Chenine, A.L.; Darrah, P.A.; Vervenne, R.A.W.; Boot, C.; Hofman, S.O.; Sombroek, C.C.; Dijkman, K.; Khayum, M.A.; Stammes, M.A.; et al. Evaluation of heterologous prime-boost vaccination strategies using chimpanzee adenovirus and modified vaccinia virus for TB subunit vaccination in rhesus macaques. NPJ Vaccines 2020, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Liu, W.C.; Lin, Y.F.; Huang, Y.H.; Liu, J.H.; Wu, S.C. Heterologous prime-boost immunization regimens using adenovirus vector and virus-like particles induce broadly neutralizing antibodies against H5N1 avian influenza viruses. Biotechnol. J. 2013, 8, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Lambe, T.; Carey, J.B.; Li, Y.; Spencer, A.J.; van Laarhoven, A.; Mullarkey, C.E.; Vrdoljak, A.; Moore, A.C.; Gilbert, S.C. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci. Rep. 2013, 3, 1443. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.C.; Ruiz-Hernandez, R.; Peroval, M.Y.; Carson, C.; Balkissoon, D.; Staines, K.; Turner, A.V.; Hill, A.V.; Gilbert, S.C.; Butter, C. Towards a universal vaccine for avian influenza: Protective efficacy of modified Vaccinia virus Ankara and Adenovirus vaccines expressing conserved influenza antigens in chickens challenged with low pathogenic avian influenza virus. Vaccine 2013, 31, 670–675. [Google Scholar] [CrossRef]
- Lo, C.; Wu, Z.; Misplon, J.; Price, G.; Pappas, C.; Kong, W.; Tumpey, T.; Epstein, S. Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: Cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine 2008, 26, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Sedegah, M.; Hollingdale, M.R.; Farooq, F.; Ganeshan, H.; Belmonte, M.; Kim, Y.; Peters, B.; Sette, A.; Huang, J.; McGrath, S.; et al. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes. PLoS ONE 2014, 9, e106241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, D.; Xu, B.; Liang, W.; Sui, J.; Chen, Y.; Yang, H.; Chen, H.; Wei, P.; Qiao, C. Immune efficacy of an adenoviral vector-based swine influenza vaccine against antigenically distinct H1N1 strains in mice. Antivir. Res. 2017, 147, 29–36. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Misplon, J.A.; Price, G.E.; Lo, C.Y.; Epstein, S.L. Age Dependence of Immunity Induced by a Candidate Universal Influenza Vaccine in Mice. PLoS ONE 2016, 11, e0153195. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).