Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination
Abstract
1. Introduction
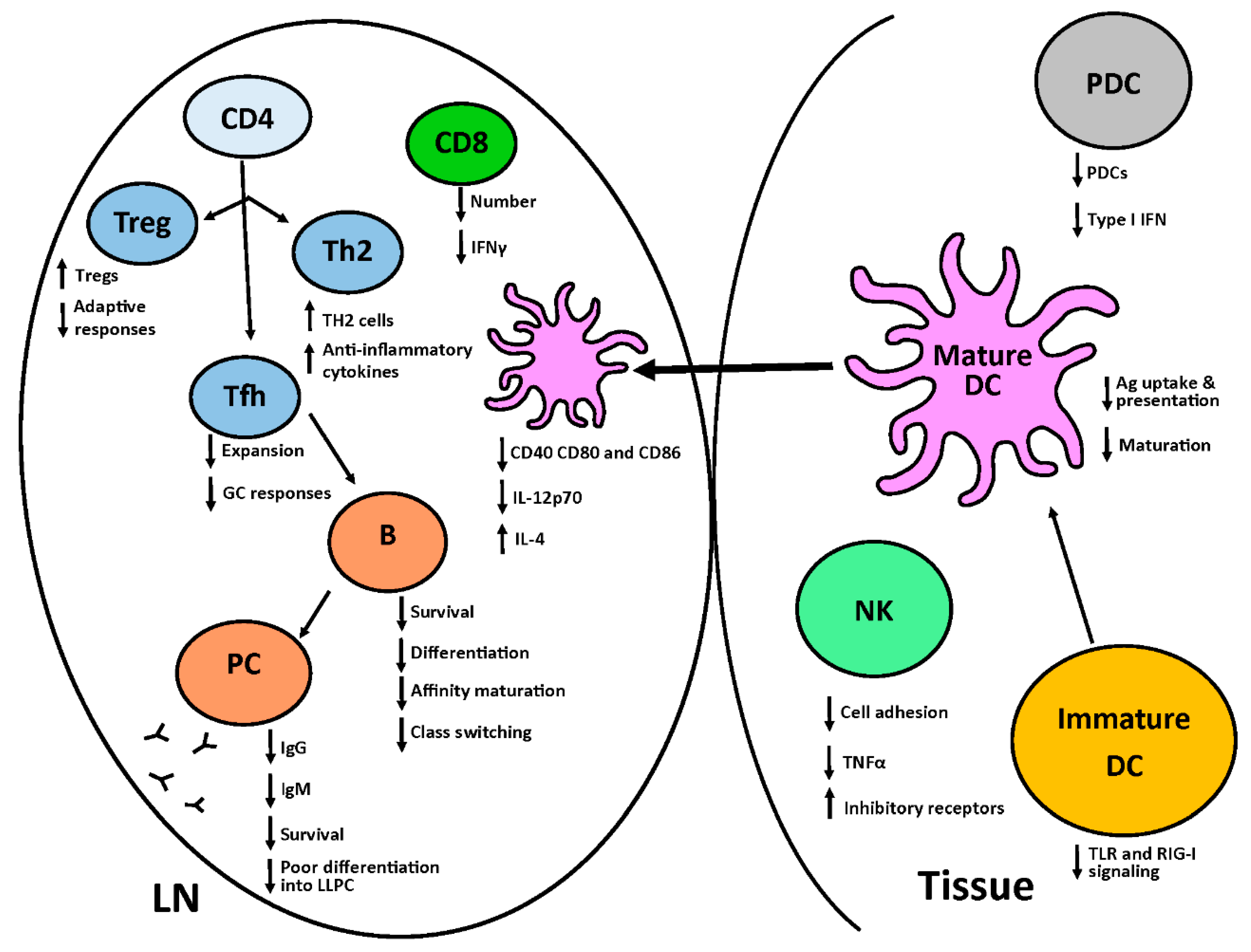
2. The Neonatal Immune System
2.1. Innate Immune System
2.2. Adaptive Immune System
3. The Newborn Response to Respiratory Virus Infection
3.1. Influenza Virus
3.2. Respiratory Syncytial Virus
3.3. Other Respiratory Viruses
4. Vaccines as an Approach to Protect Infants against Respiratory Viruses
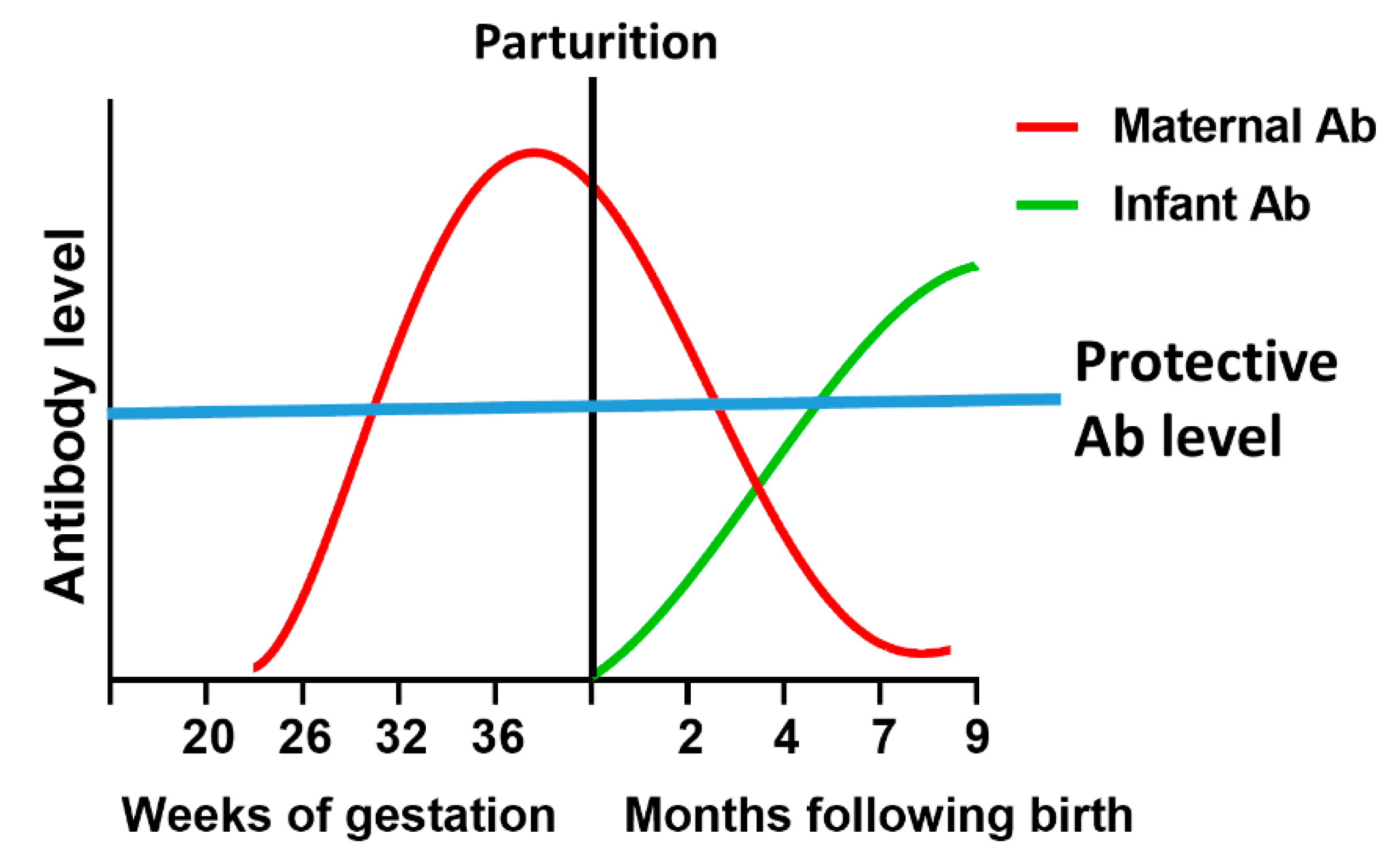
4.1. Maternal Vaccination
4.2. Newborn Vaccination
4.2.1. Adjuvants for Newborns
4.2.2. Additional Challenges Associated with Newborn Vaccination
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Nair, H.; Simoes, E.A.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang, J.S.; Feikin, D.R.; Mackenzie, G.A.; Moisi, J.C.; Roca, A.; et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 2013, 381, 1380–1390. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, A.K. Severe childhood respiratory viral infections. Adv. Pediatr. 2009, 56, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- Dowling, D.J.; Levy, O. Ontogeny of early life immunity. Trends Immunol. 2014, 35, 299–310. [Google Scholar] [CrossRef]
- Mohr, E.; Siegrist, C.A. Vaccination in early life: Standing up to the challenges. Curr. Opin. Immunol. 2016, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Marr, N.; Wang, T.I.; Kam, S.H.; Hu, Y.S.; Sharma, A.A.; Lam, A.; Markowski, J.; Solimano, A.; Lavoie, P.M.; Turvey, S.E. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J. Immunol. 2014, 192, 948–957. [Google Scholar] [CrossRef]
- Willems, F.; Vollstedt, S.; Suter, M. Phenotype and function of neonatal DC. Eur. J. Immunol. 2009, 39, 26–35. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Crabtree, J.; Rein-Weston, A.; Blimkie, D.; Thommai, F.; Wang, X.Y.; Lavoie, P.M.; Furlong, J.; Fortuno, E.S., 3rd; Hajjar, A.M.; et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009, 183, 7150–7160. [Google Scholar] [CrossRef]
- De Wit, D.; Tonon, S.; Olislagers, V.; Goriely, S.; Boutriaux, M.; Goldman, M.; Willems, F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 2003, 21, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Philbin, V.J.; Dowling, D.J.; Gallington, L.C.; Cortes, G.; Tan, Z.; Suter, E.E.; Chi, K.W.; Shuckett, A.; Stoler-Barak, L.; Tomai, M.; et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J. Allergy Clin. Immunol. 2012, 130, 195.e199–204.e199. [Google Scholar] [CrossRef] [PubMed]
- Aldrimer, M.; Ridefelt, P.; Rödöö, P.; Niklasson, F.; Gustafsson, J.; Hellberg, D. Population-based pediatric reference intervals for hematology, iron and transferrin. Scand. J. Clin. Lab. Investig. 2013, 73, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Georgountzou, A.; Papadopoulos, N.G. Postnatal innate immune development: From birth to adulthood. Front. Immunol. 2017, 8, 957. [Google Scholar] [CrossRef]
- Wilson, C.B. Immunologic basis for increased susceptibility of the neonate to infection. J. Pediatr. 1986, 108, 1–12. [Google Scholar] [CrossRef]
- Velilla, P.A.; Rugeles, M.T.; Chougnet, C.A. Defective antigen-presenting cell function in human neonates. Clin. Immunol. 2006, 121, 251–259. [Google Scholar] [CrossRef]
- Müller, K.; Zak, M.; Nielsen, S.; Pedersen, F.K.; de Nully, P.; Bendtzen, K. In vitro cytokine production and phenotype expression by blood mononuclear cellsfrom umbilical cords, children and adults. Pediatr. Allergy Immunol. 1996, 7, 117–124. [Google Scholar] [CrossRef]
- Zaghouani, H.; Hoeman, C.M.; Adkins, B. Neonatal immunity: Faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009, 30, 585–591. [Google Scholar] [CrossRef]
- Goriely, S.; Vincart, B.; Stordeur, P.; Vekemans, J.; Willems, F.; Goldman, M.; De Wit, D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 2001, 166, 2141–2146. [Google Scholar] [CrossRef]
- Langrish, C.L.; Buddle, J.C.; Thrasher, A.J.; Goldblatt, D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 2002, 128, 118–123. [Google Scholar] [CrossRef]
- Krumbiegel, D.; Zepp, F.; Meyer, C.U. Combined toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum. Immunol. 2007, 68, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Marodi, L. Innate cellular immune responses in newborns. Clin. Immunol. 2006, 118, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Encabo, A.; Solves, P.; Carbonell-Uberos, F.; Miñana, M.D. The functional immaturity of dendritic cells can be relevant to increased tolerance associated with cord blood transplantation. Transfusion 2007, 47, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.; Huppertz, H.I.; Jiang, H.J.; Petty, R.E. Studies of human cord blood dendritic cells: Evidence for functional immaturity. Blood 1994, 84, 4333–4343. [Google Scholar] [PubMed]
- De Wit, D.; Olislagers, V.; Goriely, S.; Vermeulen, F.; Wagner, H.; Goldman, M.; Willems, F. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 2004, 103, 1030–1032. [Google Scholar] [CrossRef]
- Simpson, C.C.; Woods, G.M.; Muller, H.K. Impaired CD40-signalling in Langerhans’ cells from murine neonatal draining lymph nodes: Implications for neonatally induced cutaneous tolerance. Clin. Exp. Immunol. 2003, 132, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Borràs, F.E.; Matthews, N.C.; Lowdell, M.W.; Navarrete, C.V. Identification of both myeloid CD11c+ and lymphoid CD11c- dendritic cell subsets in cord blood. Br. J. Haematol. 2001, 113, 925–931. [Google Scholar] [CrossRef]
- Dakic, A.; Shao, Q.X.; D’Amico, A.; O’Keeffe, M.; Chen, W.F.; Shortman, K.; Wu, L. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 2004, 172, 1018–1027. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Malloy, A.M.; Morabito, K.M.; Graham, B.S. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog. 2014, 10, e1003934. [Google Scholar]
- Ali, S.; Mann-Nüttel, R.; Schulze, A.; Richter, L.; Alferink, J.; Scheu, S. Sources of type I interferons in infectious immunity: Plasmacytoid dendritic cells not always in the driver’s seat. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Schüller, S.S.; Sadeghi, K.; Wisgrill, L.; Dangl, A.; Diesner, S.C.; Prusa, A.R.; Klebermasz-Schrehof, K.; Greber-Platzer, S.; Neumüller, J.; Helmer, H.; et al. Preterm neonates display altered plasmacytoid dendritic cell function and morphology. J. Leukoc. Biol. 2013, 93, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Roux, X.; Remot, A.; Petit-Camurdan, A.; Nahori, M.A.; Kiefer-Biasizzo, H.; Marchal, G.; Lagranderie, M.; Riffault, S. Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells. Eur. J. Immunol. 2011, 41, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Gazit, R.; Gruda, R.; Elboim, M.; Arnon, T.I.; Katz, G.; Achdout, H.; Hanna, J.; Qimron, U.; Landau, G.; Greenbaum, E.; et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006, 7, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, S.J. Neonatal natural killer cell function: Relevance to antiviral immune defense. Clin. Dev. Immunol. 2013, 2013, 427696. [Google Scholar] [CrossRef] [PubMed]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Guilmot, A.; Hermann, E.; Braud, V.M.; Carlier, Y.; Truyens, C. Natural killer cell responses to infections in early life. J. Innate Immun. 2011, 3, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.B.; Rathore, D.K.; Nair, D.; Chaudhary, A.; Raza, S.; Kanodia, P.; Sopory, S.; George, A.; Rath, S.; Bal, V.; et al. Comparison of human neonatal and adult blood leukocyte subset composition phenotypes. PLoS ONE 2016, 11, e0162242. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell. Mol. Immunol. 2007, 4, 377–382. [Google Scholar]
- Lin, S.J.; Yan, D.C. ICAM-1 (CD54) expression on T lymphocytes and natural killer cells from umbilical cord blood: Regulation with interleukin-12 and interleukin-15. Cytokines Cell. Mol. Ther. 2000, 6, 161–164. [Google Scholar] [CrossRef]
- Krampera, M.; Tavecchia, L.; Benedetti, F.; Nadali, G.; Pizzolo, G. Intracellular cytokine profile of cord blood T-, and NK- cells and monocytes. Haematologica 2000, 85, 675–679. [Google Scholar]
- Yabuhara, A.; Kawai, H.; Komiyama, A. Development of natural killer cytotoxicity during childhood: Marked increases in number of natural killer cells with adequate cytotoxic abilities during infancy to early childhood. Pediatr. Res. 1990, 28, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Makoni, M.; Eckert, J.; Anne Pereira, H.; Nizet, V.; Lawrence, S.M. Alterations in neonatal neutrophil function attributable to increased immature forms. Early Hum. Dev. 2016, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carr, R. Neutrophil production and function in newborn infants. Br. J. Haematol. 2000, 110, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.M.; Corriden, R.; Nizet, V. Age-appropriate functions and dysfunctions of the neonatal neutrophil. Front. Pediatr. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Junqueira, M.I.; Peçanha, L.M.F.; da Silva-Filho, V.L.; de Almeida Cardoso, M.C.; Tosta, C.E. Novel microtechnique for assessment of postnatal maturation of the phagocytic function of neutrophils and monocytes. Clin. Diagn. Lab. Immunol. 2003, 10, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Lawand, M.; Déchanet-Merville, J.; Dieu-Nosjean, M.C. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front. Immunol. 2017, 8, 761. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Haque, S.F.; Silberzahn, T.; Hamilton, K.; Langford, C.; Ellis, P.; Carr, R.; Hayday, A.C. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur. J. Immunol. 2009, 39, 1794–1806. [Google Scholar] [CrossRef]
- Chen, N.; Field, E.H. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation 1995, 59, 933–941. [Google Scholar] [CrossRef]
- Forsthuber, T.; Yip, H.C.; Lehmann, P.V. Induction of TH1 and TH2 immunity in neonatal mice. Science 1996, 271, 1728–1730. [Google Scholar] [CrossRef]
- Bendelja, K.; Gagro, A.; Bace, A.; Lokar-Kolbas, R.; Krsulovic-Hresic, V.; Drazenovic, V.; Mlinaric-Galinovic, G.; Rabatic, S. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin. Exp. Immunol. 2000, 121, 332–338. [Google Scholar] [CrossRef]
- Li, L.; Lee, H.H.; Bell, J.J.; Gregg, R.K.; Ellis, J.S.; Gessner, A.; Zaghouani, H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 2004, 20, 429–440. [Google Scholar] [CrossRef]
- McCarron, M.J.; Reen, D.J. Neonatal CD8+ T-cell differentiation is dependent on interleukin-12. Hum. Immunol. 2010, 71, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.T.; Schumacher, M.J.; Locascio, J.; Besencon, F.J.; Olson, G.B.; DeLuca, D.; Shenker, L.; Bard, J.; Boyse, E.A. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 10006–10010. [Google Scholar] [CrossRef]
- Chen, L.; Cohen, A.C.; Lewis, D.B. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol. Blood Marrow Transpl. 2006, 12, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, S.; Penix, L.A.; Edwards, C.P.; Lewis, D.B.; Ito, S.; Aruffo, A.; Wilson, C.B.; Ochs, H.D. Diminished expression of CD40 ligand by activated neonatal T cells. J. Clin. Investig. 1995, 95, 66–75. [Google Scholar] [CrossRef]
- Adkins, B.; Leclerc, C.; Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004, 4, 553–564. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, Z.P.; Lv, P.; Gao, X.M. Phenotypic and functional analysis of human T lymphocytes in early second- and third-trimester fetuses. Clin. Exp. Immunol. 2002, 129, 302–308. [Google Scholar] [CrossRef]
- Clerici, M.; DePalma, L.; Roilides, E.; Baker, R.; Shearer, G.M. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J. Clin. Investig. 1993, 91, 2829–2836. [Google Scholar] [CrossRef]
- Miscia, S.; Di Baldassarre, A.; Sabatino, G.; Bonvini, E.; Rana, R.A.; Vitale, M.; Di Valerio, V.; Manzoli, F.A. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J. Immunol. 1999, 163, 2416–2424. [Google Scholar]
- Mastelic, B.; Kamath, A.T.; Fontannaz, P.; Tougne, C.; Rochat, A.F.; Belnoue, E.; Combescure, C.; Auderset, F.; Lambert, P.H.; Tacchini-Cottier, F.; et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J. Immunol. 2012, 189, 5764–5772. [Google Scholar] [CrossRef]
- Debock, I.; Jaworski, K.; Chadlaoui, H.; Delbauve, S.; Passon, N.; Twyffels, L.; Leo, O.; Flamand, V. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J. Immunol. 2013, 191, 1231–1239. [Google Scholar] [CrossRef]
- Palin, A.C.; Ramachandran, V.; Acharya, S.; Lewis, D.B. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J. Immunol. 2013, 190, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Isakov, N.; Altman, A. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 2002, 20, 761–794. [Google Scholar] [CrossRef] [PubMed]
- Shulman, Z.; Gitlin, A.D.; Weinstein, J.S.; Lainez, B.; Esplugues, E.; Flavell, R.A.; Craft, J.E.; Nussenzweig, M.C. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 2014, 345, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, H.; Shih, C.; Wan, Z.; Ma, X.; Ma, W.; Luo, D.; Qi, H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 2015, 517, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Michaelsson, J.; Mold, J.E.; McCune, J.M.; Nixon, D.F. Regulation of T cell responses in the developing human fetus. J. Immunol. 2006, 176, 5741–5748. [Google Scholar] [CrossRef] [PubMed]
- Nettenstrom, L.; Alderson, K.; Raschke, E.E.; Evans, M.D.; Sondel, P.M.; Olek, S.; Seroogy, C.M. An optimized multi-parameter flow cytometry protocol for human T regulatory cell analysis on fresh and viably frozen cells, correlation with epigenetic analysis, and comparison of cord and adult blood. J. Immunol. Methods 2013, 387, 81–88. [Google Scholar] [CrossRef]
- Renno, C.; Nadaf, M.I.; Zago, C.A.; Carneiro-Sampaio, M.; Palmeira, P. Healthy preterm newborns show an increased frequency of CD4+ CD25high CD127low FOXP3+ regulatory T cells with a naive phenotype and high expression of gut-homing receptors. Scand. J. Immunol. 2016, 83, 445–455. [Google Scholar] [CrossRef]
- Grindebacke, H.; Stenstad, H.; Quiding-Jarbrink, M.; Waldenstrom, J.; Adlerberth, I.; Wold, A.E.; Rudin, A. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J. Immunol. 2009, 183, 4360–4370. [Google Scholar] [CrossRef]
- Van Gent, R.; van Tilburg, C.M.; Nibbelke, E.E.; Otto, S.A.; Gaiser, J.F.; Janssens-Korpela, P.L.; Sanders, E.A.; Borghans, J.A.; Wulffraat, N.M.; Bierings, M.B.; et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin. Immunol. 2009, 133, 95–107. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohno, N.; Okada, S.; Kobayashi, M. Significant augmentation of regulatory T cell numbers occurs during the early neonatal period. Clin. Exp. Immunol. 2017, 190, 268–279. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Hayward, S.L.; Blevins, L.K.; Kock, N.; Aycock, T.; Parks, G.D.; Alexander-Miller, M.A. Nonhuman primate infants have an impaired respiratory but not systemic IgG antibody response following influenza virus infection. Virology 2015, 476, 124–133. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Alexander-Miller, M.A. Higher frequency and increased expresssion of molecules associated with suppression on T regulatory cells from newborn compared with adult nonhuman primates. J. Immunol. 2020. [Google Scholar] [CrossRef]
- Mold, J.E.; Michaelsson, J.; Burt, T.D.; Muench, M.O.; Beckerman, K.P.; Busch, M.P.; Lee, T.H.; Nixon, D.F.; McCune, J.M. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008, 322, 1562–1565. [Google Scholar] [CrossRef]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaëlsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Chappert, P.; Leboeuf, M.; Rameau, P.; Lalfer, M.; Desbois, S.; Liblau, R.S.; Danos, O.; Davoust, J.M.; Gross, D.A. Antigen-specific Treg impair CD8+ T-cell priming by blocking early T-cell expansion. Eur. J. Immunol. 2010, 40, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Haeryfar, S.M.; DiPaolo, R.J.; Tscharke, D.C.; Bennink, J.R.; Yewdell, J.W. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 2005, 174, 3344–3351. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Puttur, F.K.; Wang, Y.M.; Howden, W.; Alexander, S.I.; Jones, C.A. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J. Immunol. 2008, 180, 1556–1564. [Google Scholar] [CrossRef]
- Wang, G.; Miyahara, Y.; Guo, Z.; Khattar, M.; Stepkowski, S.M.; Chen, W. “Default” generation of neonatal regulatory T cells. J. Immunol. 2010, 185, 71–78. [Google Scholar] [CrossRef]
- Ndure, J.; Flanagan, K.L. Targeting regulatory T cells to improve vaccine immunogenicity in early life. Front. Microbiol. 2014, 5, 477. [Google Scholar] [CrossRef]
- Randolph, D.A. The neonatal adaptive immune system. NeoReviews 2005, 6, e454–e462. [Google Scholar] [CrossRef]
- Gans, H.; DeHovitz, R.; Forghani, B.; Beeler, J.; Maldonado, Y.; Arvin, A.M. Measles and mumps vaccination as a model to investigate the developing immune system: Passive and active immunity during the first year of life. Vaccine 2003, 21, 3398–3405. [Google Scholar] [CrossRef]
- Brandenburg, A.H.; Groen, J.; van Steensel-Moll, H.A.; Claas, E.C.; Rothbarth, P.H.; Neijens, H.J.; Osterhaus, A.D. Respiratory syncytial virus specific serum antibodies in infants under six months of age: Limited serological response upon infection. J. Med. Virol. 1997, 52, 97–104. [Google Scholar] [CrossRef]
- Rickert, R.C.; Jellusova, J.; Miletic, A.V. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 2011, 244, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Pihlgren, M.; McGaha, T.L.; Tougne, C.; Rochat, A.F.; Bossen, C.; Schneider, P.; Huard, B.; Lambert, P.H.; Siegrist, C.A. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 2008, 111, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, L.; Cheng, Q.; Radbruch, A.; Hiepe, F. The maintenance of memory plasma cells. Front. Immunol. 2019, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Pihlgren, M.; Friedli, M.; Tougne, C.; Rochat, A.F.; Lambert, P.H.; Siegrist, C.A. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J. Immunol. 2006, 176, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Schwarze, J. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef]
- Lafond, K.E.; Nair, H.; Rasooly, M.H.; Valente, F.; Booy, R.; Rahman, M.; Kitsutani, P.; Yu, H.; Guzman, G.; Coulibaly, D.; et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: A systematic analysis. PLoS Med. 2016, 13, e1001977. [Google Scholar] [CrossRef]
- Peltola, V.; Heikkinen, T.; Ruuskanen, O. Clinical courses of croup caused by influenza and parainfluenza viruses. Pediatr. Infect. Dis. J. 2002, 21, 76–78. [Google Scholar] [CrossRef]
- Neuzil, K.M.; Mellen, B.G.; Wright, P.F.; Mitchel, E.F., Jr.; Griffin, M.R. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 2000, 342, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Zhu, Y.; Griffin, M.R.; Edwards, K.M.; Thompson, J.M.; Tollefson, S.J.; Wright, P.F. Burden of interpandemic influenza in children younger than 5 years: A 25-year prospective study. J. Infect. Dis. 2002, 185, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.R.; Napalkov, P.N.; Wegmuller, Y.; Jefferson, T.; Jick, H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Thompson, W.W.; Kramarz, P.; Shay, D.K.; Davis, R.L.; DeStefano, F.; Black, S.; Shinefield, H.; Fukuda, K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N. Engl. J. Med. 2000, 342, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M. Influenza virus infection in infancy and early childhood. Paediatr. Respir. Rev. 2003, 4, 99–104. [Google Scholar] [CrossRef]
- Khandaker, G.; Zurynski, Y.; Ridley, G.; Buttery, J.; Marshall, H.; Richmond, P.C.; Royle, J.; Gold, M.; Walls, T.; Whitehead, B.; et al. Clinical epidemiology and predictors of outcome in children hospitalised with influenza A(H1N1)pdm09 in 2009: A prospective national study. Influenza Other Respir. Viruses 2014, 8, 636–645. [Google Scholar] [CrossRef]
- Collie, M.H.; Sweet, C.; Smith, H. Infection of neonatal and adult mice with non-passaged influenza viruses. Brief report. Arch. Virol. 1980, 65, 77–81. [Google Scholar] [CrossRef]
- Lines, J.L.; Hoskins, S.; Hollifield, M.; Cauley, L.S.; Garvy, B.A. The migration of T cells in response to influenza virus is altered in neonatal mice. J. Immunol. 2010, 185, 2980–2988. [Google Scholar]
- Chan, M.C.; Cheung, C.Y.; Chui, W.H.; Tsao, S.W.; Nicholls, J.M.; Chan, Y.O.; Chan, R.W.; Long, H.T.; Poon, L.L.; Guan, Y.; et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005, 6, 135. [Google Scholar] [CrossRef]
- Chan, M.C.; Chan, R.W.; Yu, W.C.; Ho, C.C.; Chui, W.H.; Lo, C.K.; Yuen, K.M.; Guan, Y.I.; Nicholls, J.M.; Peiris, J.S. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir. Res. 2009, 10, 102. [Google Scholar] [CrossRef]
- Wang, J.; Nikrad, M.P.; Phang, T.; Gao, B.; Alford, T.; Ito, Y.; Edeen, K.; Travanty, E.A.; Kosmider, B.; Hartshorn, K.; et al. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 582–591. [Google Scholar] [CrossRef]
- Yu, W.C.; Chan, R.W.; Wang, J.; Travanty, E.A.; Nicholls, J.M.; Peiris, J.S.; Mason, R.J.; Chan, M.C. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J. Virol. 2011, 85, 6844–6855. [Google Scholar] [CrossRef]
- Pulendran, B.; Maddur, M.S. Innate immune sensing and response to influenza. Curr. Top. Microbiol. Immunol. 2015, 386, 23–71. [Google Scholar] [CrossRef]
- Clay, C.C.; Reader, J.R.; Gerriets, J.E.; Wang, T.T.; Harrod, K.S.; Miller, L.A. Enhanced viral replication and modulated innate immune responses in infant airway epithelium following H1N1 infection. J. Virol. 2014, 88, 7412–7425. [Google Scholar] [CrossRef]
- Lin, S.J.; Cheng, P.J.; Lin, T.Y.; Lee, P.T.; Hsiao, H.S.; Kuo, M.L. Effect of influenza A infection on umbilical cord blood natural killer function regulation with interleukin-15. J. Infect. Dis. 2012, 205, 745–756. [Google Scholar] [CrossRef]
- Bender, B.S.; Croghan, T.; Zhang, L.; Small, P.A., Jr. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J. Exp. Med. 1992, 175, 1143–1145. [Google Scholar] [CrossRef]
- Graham, M.B.; Braciale, T.J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 1997, 186, 2063–2068. [Google Scholar] [CrossRef]
- Welliver, T.P.; Garofalo, R.P.; Hosakote, Y.; Hintz, K.H.; Avendano, L.; Sanchez, K.; Velozo, L.; Jafri, H.; Chavez-Bueno, S.; Ogra, P.L.; et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 2007, 195, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Ripple, M.; Balakrishna, S.; Troxclair, D.; Sandquist, D.; Ding, L.; Ahlert, T.A.; Cormier, S.A. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J. Immunol. 2008, 181, 3486–3494. [Google Scholar] [CrossRef]
- Lin, S.J.; Lee, Y.C. Effect of influenza A infection on maturation and function of neonatal monocyte-derived dendritic cells. Viral Immunol. 2014, 27, 277–284. [Google Scholar] [CrossRef]
- Oliphant, S.; Lines, J.L.; Hollifield, M.L.; Garvy, B.A. Regulatory T cells are critical for clearing influenza A virus in neonatal mice. Viral Immunol. 2015, 28, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.; Fouchier, R.A.; Rimmelzwaan, G.F. Immune responses to influenza virus infection. Virus Res. 2011, 162, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Coudret, C.L.; Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 1995, 69, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.M.; Alter, G. Extra-neutralizing FcR-mediated antibody functions for a universal influenza vaccine. Front. Immunol. 2019, 10, 440. [Google Scholar] [CrossRef]
- Von Holle, T.A.; Moody, M.A. Influenza and antibody-dependent cellular cytotoxicity. Front. Immunol. 2019, 10, 1457. [Google Scholar] [CrossRef]
- Murphy, B.R.; Nelson, D.L.; Wright, P.F.; Tierney, E.L.; Phelan, M.A.; Chanock, R.M. Secretory and systemic immunological response in children infected with live attenuated influenza a virus vaccines. Infect. Immunol. 1982, 36, 1102–1108. [Google Scholar] [CrossRef]
- Irvin, C.G.; Bates, J.H. Measuring the lung function in the mouse: The challenge of size. Respir. Res. 2003, 4, 4. [Google Scholar] [CrossRef]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Kusser, K.; Hartson, L.; Sprague, F.; Goodrich, S.; Woodland, D.L.; Lund, F.E.; Randall, T.D. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004, 10, 927–934. [Google Scholar] [CrossRef]
- Altman, M.O.; Angeletti, D.; Yewdell, J.W. Antibody immunodominance: The key to understanding influenza virus antigenic drift. Viral Immunol. 2018, 31, 142–149. [Google Scholar] [CrossRef]
- Angeletti, D.; Yewdell, J.W. Understanding and manipulating viral immunity: Antibody immunodominance enters center stage. Trends Immunol. 2018, 39, 549–561. [Google Scholar] [CrossRef]
- Andrews, S.F.; Huang, Y.; Kaur, K.; Popova, L.I.; Ho, I.Y.; Pauli, N.T.; Henry Dunand, C.J.; Taylor, W.M.; Lim, S.; Huang, M.; et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015, 7, 316ra192. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tan, G.S.; Mullarkey, C.E.; Lee, A.J.; Lam, M.M.; Krammer, F.; Henry, C.; Wilson, P.C.; Ashkar, A.A.; Palese, P.; et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc. Natl. Acad. Sci. USA 2016, 113, 11931–11936. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Koutsonanos, D.; Li, G.M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, 208, 181–193. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza A virus hemagglutinin specific antibodies interfere with virion neuraminidase activity via two distinct mechanisms. Virology 2017, 500, 178–183. [Google Scholar] [CrossRef]
- Angeletti, D.; Gibbs, J.S.; Angel, M.; Kosik, I.; Hickman, H.D.; Frank, G.M.; Das, S.R.; Wheatley, A.K.; Prabhakaran, M.; Leggat, D.J.; et al. Defining B cell immunodominance to viruses. Nat. Immunol. 2017, 18, 456–463. [Google Scholar] [CrossRef]
- Clemens, E.; Angeletti, D.; Holbrook, B.C.; Kanekiyo, M.; Jorgensen, M.J.; Graham, B.S.; Yewdell, J.; Alexander-Miller, M.A. Influenza-infected newborn and adult monkeys exhibit a strong primary antibody response to hemagglutinin stem. JCI Insight 2020, 5, 135449. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Graham, B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Rostad, C.A. Respiratory syncytial virus: Spectrum of clinical manifestations and complications in children. Pediatr. Ann. 2019, 48, e349–e353. [Google Scholar] [CrossRef]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef]
- Sigurs, N.; Bjarnason, R.; Sigurbergsson, F.; Kjellman, B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000, 161, 1501–1507. [Google Scholar] [CrossRef]
- Mejias, A.; Dimo, B.; Suarez, N.M.; Garcia, C.; Suarez-Arrabal, M.C.; Jartti, T.; Blankenship, D.; Jordan-Villegas, A.; Ardura, M.I.; Xu, Z.; et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013, 10, e1001549. [Google Scholar] [CrossRef]
- Shindo, H.; Yasui, K.; Yamamoto, K.; Honma, K.; Yui, K.; Kohno, T.; Ma, Y.; Chua, K.J.; Kubo, Y.; Aihara, H.; et al. Interferon regulatory factor-4 activates IL-2 and IL-4 promoters in cooperation with c-Rel. Cytokine 2011, 56, 564–572. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A.; Iwasaki, Y.; Kubo, T.; Aoyama, K.; Mori, T.; Yamazaki, T.; Kobayashi, N.; Ishiguro, A. Neutrophil-mediated inflammation in respiratory syncytial viral bronchiolitis. Pediatr. Int. 2005, 47, 190–195. [Google Scholar] [CrossRef]
- McNamara, P.S.; Ritson, P.; Selby, A.; Hart, C.A.; Smyth, R.L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child. 2003, 88, 922–926. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Salazar-Mather, T.P.; Dalod, M.Y.; Van Deusen, J.B.; Wei, X.Q.; Liew, F.Y.; Caligiuri, M.A.; Durbin, J.E.; Biron, C.A. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002, 169, 4279–4287. [Google Scholar] [CrossRef]
- Smit, J.J.; Rudd, B.D.; Lukacs, N.W. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 2006, 203, 1153–1159. [Google Scholar] [CrossRef]
- Cormier, S.A.; Shrestha, B.; Saravia, J.; Lee, G.I.; Shen, L.; DeVincenzo, J.P.; Kim, Y.I.; You, D. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J. Virol. 2014, 88, 9350–9360. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Chiu, C. Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol. 2013, 3, 468–474. [Google Scholar] [CrossRef]
- Graham, B.S.; Bunton, L.A.; Wright, P.F.; Karzon, D.T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 1991, 88, 1026–1033. [Google Scholar] [CrossRef]
- Cannon, M.J.; Openshaw, P.J.; Askonas, B.A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1988, 168, 1163–1168. [Google Scholar] [CrossRef]
- Lambert, L.; Sagfors, A.M.; Openshaw, P.J.; Culley, F.J. Immunity to RSV in early-life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef]
- Legg, J.P.; Hussain, I.R.; Warner, J.A.; Johnston, S.L.; Warner, J.O. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2003, 168, 633–639. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Dworzak, M.N.; Mandl, C.W.; Rebhandl, W.; Vollnhofer, G.; Kundi, M.; Popow-Kraupp, T. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1263–1268. [Google Scholar] [CrossRef]
- Semple, M.G.; Dankert, H.M.; Ebrahimi, B.; Correia, J.B.; Booth, J.A.; Stewart, J.P.; Smyth, R.L.; Hart, C.A. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS ONE 2007, 2, e1038. [Google Scholar] [CrossRef]
- Vercelli, D.; Jabara, H.H.; Arai, K.; Geha, R.S. Induction of human IgE synthesis requires interleukin 4 and T/B cell interactions involving the T cell receptor/CD3 complex and MHC class II antigens. J. Exp. Med. 1989, 169, 1295–1307. [Google Scholar] [CrossRef]
- Welliver, R.C.; Wong, D.T.; Sun, M.; Middleton, E., Jr.; Vaughan, R.S.; Ogra, P.L. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N. Engl. J. Med. 1981, 305, 841–846. [Google Scholar] [CrossRef]
- Pène, J.; Rousset, F.; Brière, F.; Chrétien, I.; Bonnefoy, J.Y.; Spits, H.; Yokota, T.; Arai, N.; Arai, K.; Banchereau, J.; et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1988, 85, 6880–6884. [Google Scholar] [CrossRef]
- Stoppelenburg, A.J.; de Roock, S.; Hennus, M.P.; Bont, L.; Boes, M. Elevated Th17 response in infants undergoing respiratory viral infection. Am. J. Pathol. 2014, 184, 1274–1279. [Google Scholar] [CrossRef]
- Stoppelenburg, A.J.; Salimi, V.; Hennus, M.; Plantinga, M.; In’t Veld, H.R.; Walk, J.; Meerding, J.; Coenjaerts, F.; Bont, L.; Boes, M. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS ONE 2013, 8, e78461. [Google Scholar] [CrossRef]
- Mukherjee, S.; Lindell, D.M.; Berlin, A.A.; Morris, S.B.; Shanley, T.P.; Hershenson, M.B.; Lukacs, N.W. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am. J. Pathol. 2011, 179, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.A.; Hervé, P.L.; Dériaud, E.; et al. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 2017, 46, 301–314. [Google Scholar] [CrossRef]
- Gern, J.E.; Busse, W.W. Association of rhinovirus infections with asthma. Clin. Microbiol. Rev. 1999, 12, 9–18. [Google Scholar]
- Troy, N.M.; Bosco, A. Respiratory viral infections and host responses; insights from genomics. Respir. Res. 2016, 17, 156. [Google Scholar] [CrossRef]
- Olenec, J.P.; Kim, W.K.; Lee, W.M.; Vang, F.; Pappas, T.E.; Salazar, L.E.; Evans, M.D.; Bork, J.; Roberg, K.; Lemanske, R.F.; et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J. Allergy Clin. Immunol. 2010, 125, 1001.e1–1006.e1. [Google Scholar] [CrossRef]
- Schneider, D.; Hong, J.Y.; Popova, A.P.; Bowman, E.R.; Linn, M.J.; McLean, A.M.; Zhao, Y.; Sonstein, J.; Bentley, J.K.; Weinberg, J.B.; et al. Neonatal rhinovirus infection induces mucous metaplasia and airways hyperresponsiveness. J. Immunol. 2012, 188, 2894–2904. [Google Scholar] [CrossRef]
- Branche, A.R.; Falsey, A.R. Parainfluenza virus infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554. [Google Scholar] [CrossRef]
- Fry, A.M.; Curns, A.T.; Harbour, K.; Hutwagner, L.; Holman, R.C.; Anderson, L.J. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clin. Infect. Dis. 2006, 43, 1016–1022. [Google Scholar] [CrossRef]
- Tremonti, L.P.; Lin, J.S.; Jackson, G.G. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J. Immunol. 1968, 101, 572–577. [Google Scholar]
- Smith, C.B.; Purcell, R.H.; Bellanti, J.A.; Chanock, R.M. Protective effect of antibody to parainfluenza type 1 virus. N. Engl. J. Med. 1966, 275, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, R.; McIntosh, K. Secretory immunological response in infants and children to parainfluenza virus types 1 and 2. Infect. Immun. 1980, 30, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A. Neonatal and early life vaccinology. Vaccine 2001, 19, 3331–3346. [Google Scholar] [CrossRef]
- Winkelstein, J.A.; Marino, M.C.; Lederman, H.M.; Jones, S.M.; Sullivan, K.; Burks, A.W.; Conley, M.E.; Cunningham-Rundles, C.; Ochs, H.D. X-linked agammaglobulinemia: Report on a United States registry of 201 patients. Medicine 2006, 85, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Ward, E.S. Transcytosis and catabolism of antibody. Immunol. Res. 2002, 25, 97–113. [Google Scholar] [CrossRef]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Bjornson, G.; Jones, C.E.; Halperin, S.A.; Edwards, K.M.; et al. Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 2017, 17, e197–e208. [Google Scholar] [CrossRef]
- Hobbs, J.R.; Davis, J.A. Serum gamma-G-globulin levels and gestational age in premature babies. Lancet 1967, 1, 757–759. [Google Scholar] [CrossRef]
- Conway, S.P.; Dear, P.R.; Smith, I. Immunoglobulin profile of the preterm baby. Arch. Dis. Child. 1985, 60, 208–212. [Google Scholar] [CrossRef]
- Salimonu, L.S.; Ladipo, O.A.; Adeniran, S.O.; Osukoya, B.O. Serum immunoglobulin levels in normal, premature and postmature newborns and their mothers. Int. J. Gynaecol. Obstet. 1978, 16, 119–123. [Google Scholar] [CrossRef]
- Linder, N.; Handsher, R.; Fruman, O.; Shiff, E.; Ohel, G.; Reichman, B.; Dagan, R. Effect of maternal immunization with oral poliovirus vaccine on neonatal immunity. Pediatr. Infect. Dis. J. 1994, 13, 959–962. [Google Scholar] [CrossRef]
- Munoz, F.M. Current challenges and achievements in maternal immunization research. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Englund, J.A.; Glezen, W.P.; Turner, C.; Harvey, J.; Thompson, C.; Siber, G.R. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate Haemophilus influenzae type b vaccines. J. Infect. Dis. 1995, 171, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef]
- Poehling, K.A.; Szilagyi, P.G.; Staat, M.A.; Snively, B.M.; Payne, D.C.; Bridges, C.B.; Chu, S.Y.; Light, L.S.; Prill, M.M.; Finelli, L.; et al. Impact of maternal immunization on influenza hospitalizations in infants. Am. J. Obstet. Gynecol. 2011, 204, S141–S148. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Zhao, H.; Dabrera, G.; Andrews, N.; Thomas, S.L.; Tsang, C.; Ellis, J.; Donati, M.; Pebody, R.G. Assessment of effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants in England, 2013–2014 and 2014–2015. J. Infect. Dis. 2020, 221, 16–20. [Google Scholar] [CrossRef]
- Jackson, L.A.; Patel, S.M.; Swamy, G.K.; Frey, S.E.; Creech, C.B.; Munoz, F.M.; Artal, R.; Keitel, W.A.; Noah, D.L.; Petrie, C.R.; et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J. Infect. Dis. 2011, 204, 854–863. [Google Scholar] [CrossRef]
- Nunes, M.C.; Madhi, S.A. Prevention of influenza-related illness in young infants by maternal vaccination during pregnancy. F1000Research 2018, 7, 122. [Google Scholar] [CrossRef]
- Ochola, R.; Sande, C.; Fegan, G.; Scott, P.D.; Medley, G.F.; Cane, P.A.; Nokes, D.J. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS ONE 2009, 4, e8088. [Google Scholar] [CrossRef]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef]
- Tapia, L.I.; Shaw, C.A.; Aideyan, L.O.; Jewell, A.M.; Dawson, B.C.; Haq, T.R.; Piedra, P.A. Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLoS ONE 2014, 9, e90786. [Google Scholar] [CrossRef]
- Beeler, J.A.; van Wyke Coelingh, K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: Effect of mutation upon fusion function. J. Virol. 1989, 63, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.A.; Settembre, E.C.; Shaw, C.A.; Dey, A.K.; Rappuoli, R.; Mandl, C.W.; Dormitzer, P.R.; Carfi, A. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc. Natl. Acad. Sci. USA 2011, 108, 9619–9624. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Raghunandan, R.; Wu, Y.; Liu, Y.; Massare, M.; Nathan, M.; Zhou, B.; Lu, H.; Boddapati, S.; Li, J.; et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE 2012, 7, e50852. [Google Scholar] [CrossRef]
- August, A.; Glenn, G.M.; Kpamegan, E.; Hickman, S.P.; Jani, D.; Lu, H.; Thomas, D.N.; Wen, J.; Piedra, P.A.; Fries, L.F. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017, 35, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, R.; Lu, H.; Zhou, B.; Xabier, M.G.; Massare, M.J.; Flyer, D.C.; Fries, L.F.; Smith, G.E.; Glenn, G.M. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine 2014, 32, 6485–6492. [Google Scholar] [CrossRef] [PubMed]
- Killikelly, A.M.; Kanekiyo, M.; Graham, B.S. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci. Rep. 2016, 6, 34108. [Google Scholar] [CrossRef]
- Carbonell-Estrany, X.; Simões, E.A.; Dagan, R.; Hall, C.B.; Harris, B.; Hultquist, M.; Connor, E.M.; Losonsky, G.A. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics 2010, 125, e35–e51. [Google Scholar] [CrossRef]
- Feltes, T.F.; Sondheimer, H.M.; Tulloh, R.M.; Harris, B.S.; Jensen, K.M.; Losonsky, G.A.; Griffin, M.P. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr. Res. 2011, 70, 186–191. [Google Scholar] [CrossRef]
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simoes, E.A.F.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory Syncytial Virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Agerskov, K.; Meakins, T.; Aaby, P.; Simoes, E.A. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 2009, 123, 398–403. [Google Scholar] [CrossRef]
- Eick, A.; Karron, R.; Shaw, J.; Thumar, B.; Reid, R.; Santosham, M.; O’Brien, K.L. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 2008, 27, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.G.; Graham, B.S.; Traore, A.; Haidara, F.C.; Chen, M.; Morabito, K.; Lin, B.C.; Sow, S.O.; Levine, M.M.; Pasetti, M.F.; et al. Respiratory Syncytial Virus (RSV) neutralizing antibodies at birth predict protection from RSV illness in infants in the first 3 months of life. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, E6566–E6575. [Google Scholar] [CrossRef] [PubMed]
- Brann, E.; Edvinsson, A.; Rostedt Punga, A.; Sundstrom-Poromaa, I.; Skalkidou, A. Inflammatory and anti-inflammatory markers in plasma: From late pregnancy to early postpartum. Sci. Rep. 2019, 9, 1863. [Google Scholar] [CrossRef]
- Graham, C.; Chooniedass, R.; Stefura, W.P.; Becker, A.B.; Sears, M.R.; Turvey, S.E.; Mandhane, P.J.; Subbarao, P.; Investigators, C.S.; HayGlass, K.T. In vivo immune signatures of healthy human pregnancy: Inherently inflammatory or anti-inflammatory? PLoS ONE 2017, 12, e0177813. [Google Scholar] [CrossRef]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Feyaerts, D.; Benner, M.; van Cranenbroek, B.; van der Heijden, O.W.H.; Joosten, I.; van der Molen, R.G. Human uterine lymphocytes acquire a more experienced and tolerogenic phenotype during pregnancy. Sci. Rep. 2017, 7, 2884. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef]
- Gnjatic, S.; Sawhney, N.B.; Bhardwaj, N. Toll-like receptor agonists: Are they good adjuvants? Cancer J. 2010, 16, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [PubMed]
- Castilow, E.M.; Olson, M.R.; Varga, S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 2007, 39, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.Y.; Mitzner, W.; et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef]
- Johnson, T.R.; Rao, S.; Seder, R.A.; Chen, M.; Graham, B.S. TLR9 agonist, but not TLR7/8, functions as an adjuvant to diminish FI-RSV vaccine-enhanced disease, while either agonist used as therapy during primary RSV infection increases disease severity. Vaccine 2009, 27, 3045–3052. [Google Scholar] [CrossRef]
- Gilles, S.; Fekete, A.; Zhang, X.; Beck, I.; Blume, C.; Ring, J.; Schmidt-Weber, C.; Behrendt, H.; Schmitt-Kopplin, P.; Traidl-Hoffmann, C. Pollen metabolome analysis reveals adenosine as a major regulator of dendritic cell-primed T(H) cell responses. J. Allergy Clin. Immunol. 2011, 127, 454–461. [Google Scholar] [CrossRef]
- Desrosiers, M.D.; Cembrola, K.M.; Fakir, M.J.; Stephens, L.A.; Jama, F.M.; Shameli, A.; Mehal, W.Z.; Santamaria, P.; Shi, Y. Adenosine deamination sustains dendritic cell activation in inflammation. J. Immunol. 2007, 179, 1884–1892. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P.; Deitch, E.A.; Vizi, E.S. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol. Ther. 2007, 113, 264–275. [Google Scholar] [CrossRef]
- Holbrook, B.C.; D’Agostino, R.B., Jr.; Tyler Aycock, S.; Jorgensen, M.J.; Hadimani, M.B.; Bruce King, S.; Alexander-Miller, M.A. Adjuvanting an inactivated influenza vaccine with conjugated R848 improves the level of antibody present at 6months in a nonhuman primate neonate model. Vaccine 2017, 35, 6137–6142. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Kim, J.R.; Blevins, L.K.; Jorgensen, M.J.; Kock, N.D.; D’Agostino, R.B., Jr.; Aycock, S.T.; Hadimani, M.B.; King, S.B.; Parks, G.D.; et al. A novel R848-conjugated inactivated influenza virus vaccine is efficacious and safe in a neonate nonhuman primate model. J. Immunol. 2016, 197, 555–564. [Google Scholar] [CrossRef]
- Francica, J.R.; Lynn, G.M.; Laga, R.; Joyce, M.G.; Ruckwardt, T.J.; Morabito, K.M.; Chen, M.; Chaudhuri, R.; Zhang, B.; Sastry, M.; et al. Thermoresponsive polymer nanoparticles co-deliver RSV F trimers with a TLR-7/8 adjuvant. Bioconjug. Chem. 2016, 27, 2372–2385. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiao, Y.Y.; Yu, Y.Z.; Jiang, N.; Hua, Y.; Zhang, X.J.; Fu, Y.H.; Peng, X.L.; Zheng, Y.P.; Anderson, L.J.; et al. A built-in CpG adjuvant in RSV F protein DNA vaccine drives a Th1 polarized and enhanced protective immune response. Viruses 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Brazolot Millan, C.L.; Weeratna, R.; Krieg, A.M.; Siegrist, C.A.; Davis, H.L. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15553–15558. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Wang, C.C.; Wu, T.C.; Wu, K.G.; Lee, C.C.; Peng, H.J. Neonatal sublingual vaccination with Salmonella proteins and adjuvant cholera toxin or CpG oligodeoxynucleotides induces mucosal and systemic immunity in mice. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Warger, T.; Osterloh, P.; Rechtsteiner, G.; Fassbender, M.; Heib, V.; Schmid, B.; Schmitt, E.; Schild, H.; Radsak, M.P. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 2006, 108, 544–550. [Google Scholar] [CrossRef]
- McCarron, M.; Reen, D.J. Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J. Immunol. 2009, 182, 55–62. [Google Scholar] [CrossRef]
- Randhawa, A.K.; Hawn, T.R. Toll-like receptors: Their roles in bacterial recognition and respiratory infections. Expert Rev. Anti Infect. Ther. 2008, 6, 479–495. [Google Scholar] [CrossRef]
- Willis, E.; Pardi, N.; Parkhouse, K.; Mui, B.L.; Tam, Y.K.; Weissman, D.; Hensley, S.E. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Aaby, P.; Martins, C.L.; Garly, M.L.; Andersen, A.; Fisker, A.B.; Claesson, M.H.; Ravn, H.; Rodrigues, A.; Whittle, H.C.; Benn, C.S. Measles vaccination in the presence or absence of maternal measles antibody: Impact on child survival. Clin. Infect. Dis. 2014, 59, 484–492. [Google Scholar] [CrossRef]
- Samb, B.; Aaby, P.; Whittle, H.C.; Seck, A.M.; Rahman, S.; Bennett, J.; Markowitz, L.; Simondon, F. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr. Infect. Dis. J. 1995, 14, 203–209. [Google Scholar] [CrossRef][Green Version]
- Mooi, F.R.; de Greeff, S.C. The case for maternal vaccination against pertussis. Lancet Infect. Dis. 2007, 7, 614–624. [Google Scholar] [CrossRef]
- Halasa, N.B.; Gerber, M.A.; Chen, Q.; Wright, P.F.; Edwards, K.M. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J. Infect. Dis. 2008, 197, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015, 33, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- Vono, M.; Eberhardt, C.S.; Auderset, F.; Mastelic-Gavillet, B.; Lemeille, S.; Christensen, D.; Andersen, P.; Lambert, P.H.; Siegrist, C.A. Maternal antibodies inhibit neonatal and infant responses to vaccination by shaping the early-life B cell repertoire within germinal centers. Cell. Rep. 2019, 28, 1773.e5–1784.e5. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B. Feedback regulation by IgG antibodies. Immunol. Lett. 2003, 88, 157–161. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van Snick, J. Enhancement of IgG anti-carrier responses by IgG2 anti-hapten antibodies in mice. Eur. J. Immunol. 1985, 15, 793–798. [Google Scholar] [CrossRef]
- Wernersson, S.; Karlsson, M.C.; Dahlstrom, J.; Mattsson, R.; Verbeek, J.S.; Heyman, B. IgG-mediated enhancement of antibody responses is low in Fc receptor gamma chain-deficient mice and increased in Fc gamma RII-deficient mice. J. Immunol. 1999, 163, 618–622. [Google Scholar]
- Iwasaki, A.; Yang, Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 339–341. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 1977, 265, 739–741. [Google Scholar] [CrossRef]
- Ochiai, H.; Kurokawa, M.; Hayashi, K.; Niwayama, S. Antibody-mediated growth of influenza a NWS virus in macrophagelike cell line P388D1. J. Virol. 1988, 62, 20–26. [Google Scholar] [CrossRef]
- Polack, F.P.; Teng, M.N.; Collins, P.L.; Prince, G.A.; Exner, M.; Regele, H.; Lirman, D.D.; Rabold, R.; Hoffman, S.J.; Karp, C.L.; et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 2002, 196, 859–865. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Zhang, A.J.; Hung, I.F.; Xu, T.; Ip, W.C.; Wong, R.T.; Ng, J.C.; Chan, J.F.; Chan, K.H.; Yuen, K.Y. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin. Vaccine. Immunol. 2012, 19, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Winarski, K.L.; Tang, J.; Klenow, L.; Lee, J.; Coyle, E.M.; Manischewitz, J.; Turner, H.L.; Takeda, K.; Ward, A.B.; Golding, H.; et al. Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc. Natl. Acad. Sci. USA 2019, 116, 15194–15199. [Google Scholar] [CrossRef] [PubMed]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.E., Jr.; Kawamura, T.; Gorny, M.K.; Lake, D.; Xu, J.Y.; Matsumoto, Y.; Sugano, T.; Masuho, Y.; Mitchell, W.M.; Hersh, E.; et al. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 3185–3189. [Google Scholar] [CrossRef]
- Takada, A.; Watanabe, S.; Okazaki, K.; Kida, H.; Kawaoka, Y. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J. Virol. 2001, 75, 2324–2330. [Google Scholar] [CrossRef]
- Huisman, W.; Martina, B.E.; Rimmelzwaan, G.F.; Gruters, R.A.; Osterhaus, A.D. Vaccine-induced enhancement of viral infections. Vaccine 2009, 27, 505–512. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al Thani, A.A.; Yassine, H.M. Viral-induced enhanced disease illness. Front. Microbiol. 2018, 9, 2991. [Google Scholar] [CrossRef]
- Vincent, A.L.; Lager, K.M.; Janke, B.H.; Gramer, M.R.; Richt, J.A. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet. Microbiol. 2008, 126, 310–323. [Google Scholar] [CrossRef]
- Gauger, P.C.; Vincent, A.L.; Loving, C.L.; Lager, K.M.; Janke, B.H.; Kehrli, M.E., Jr.; Roth, J.A. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (δ-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 2011, 29, 2712–2719. [Google Scholar] [CrossRef]
- Rajao, D.S.; Sandbulte, M.R.; Gauger, P.C.; Kitikoon, P.; Platt, R.; Roth, J.A.; Perez, D.R.; Loving, C.L.; Vincent, A.L. Heterologous challenge in the presence of maternally-derived antibodies results in vaccine-associated enhanced respiratory disease in weaned piglets. Virology 2016, 491, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.Z.; Skowronski, D.M.; Hottes, T.S.; Osei, W.; Adams, E.; Petric, M.; Sabaiduc, S.; Chan, T.; Mak, A.; Lem, M.; et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: First detection of the association in British Columbia, Canada. Clin. Infect. Dis. 2010, 51, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G.; Crowcroft, N.S.; Janjua, N.Z.; Boulianne, N.; Hottes, T.S.; Rosella, L.C.; Dickinson, J.A.; Gilca, R.; Sethi, P.; et al. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: Four observational studies from Canada. PLoS Med. 2010, 7, e1000258. [Google Scholar] [CrossRef]
- Hur, Y.G.; Gorak-Stolinska, P.; Lalor, M.K.; Mvula, H.; Floyd, S.; Raynes, J.; Ben-Smith, A.; Fitchett, J.R.; Flanagan, K.L.; Burl, S.; et al. Factors affecting immunogenicity of BCG in infants, a study in Malawi, The Gambia and the UK. BMC Infect. Dis. 2014, 14, 184. [Google Scholar] [CrossRef]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the microbiota in the modulation of vaccine immune responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef]
- Jamieson, A.M. Influence of the microbiome on response to vaccination. Hum. Vaccin. Immunother. 2015, 11, 2329–2331. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef]
- Lamousé-Smith, E.S.; Tzeng, A.; Starnbach, M.N. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS ONE 2011, 6, e27662. [Google Scholar] [CrossRef]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crofts, K.F.; Alexander-Miller, M.A. Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination. Vaccines 2020, 8, 558. https://doi.org/10.3390/vaccines8040558
Crofts KF, Alexander-Miller MA. Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination. Vaccines. 2020; 8(4):558. https://doi.org/10.3390/vaccines8040558
Chicago/Turabian StyleCrofts, Kali F., and Martha A. Alexander-Miller. 2020. "Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination" Vaccines 8, no. 4: 558. https://doi.org/10.3390/vaccines8040558
APA StyleCrofts, K. F., & Alexander-Miller, M. A. (2020). Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination. Vaccines, 8(4), 558. https://doi.org/10.3390/vaccines8040558




