The Role of the Tumor Microenvironment in Developing Successful Therapeutic and Secondary Prophylactic Breast Cancer Vaccines
Abstract
1. Introduction
2. Breast Cancer Vaccines and the Microenvironment Hurdle
3. T-Cells
3.1. Checkpoint Blockades as Adjuvants to BC Vaccines
3.2. Tregs
4. Myeloid Cells
4.1. DC Vaccines
4.2. DC-Targeting In Situ Vaccination
4.3. MDSCs
5. Oncolytic Viruses
5.1. In Situ TME Changes with Oncolytic Viruses
5.2. Oncolytic Viruses Combined with Peptide Vaccines
6. Microenvironment Antigen Vaccines
6.1. Tumor Endothelia
6.2. CAFs
7. Summary and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Garcia-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822. [Google Scholar] [CrossRef]
- Schlom, J.; Hodge, J.W.; Palena, C.; Tsang, K.Y.; Jochems, C.; Greiner, J.W.; Farsaci, B.; Madan, R.A.; Heery, C.R.; Gulley, J.L. Therapeutic cancer vaccines. Adv. Cancer Res. 2014, 121, 67–124. [Google Scholar] [CrossRef]
- Tower, H.; Ruppert, M.; Britt, K. The Immune Microenvironment of Breast Cancer Progression. Cancers 2019, 11, 1375. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Allan, A.L.; Raouf, A. Role of the Microenvironment in Regulating Normal and Cancer Stem Cell Activity: Implications for Breast Cancer Progression and Therapy Response. Cancers 2019, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Locy, H.; de Mey, S.; de Mey, W.; De Ridder, M.; Thielemans, K.; Maenhout, S.K. Immunomodulation of the Tumor Microenvironment: Turn Foe Into Friend. Front. Immunol. 2018, 9, 2909. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.; Eruslanov, E.; Judy, B.; Kapoor, V.; Cheng, G.; Wang, L.C.; Sun, J.; Moon, E.K.; Fridlender, Z.G.; Albelda, S.; et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc. Natl. Acad. Sci. USA 2013, 110, E415–E424. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.; Tari, G.; Bagirova, M.; Abamor, E.S. Current Approaches in Development of Immunotherapeutic Vaccines for Breast Cancer. J. Breast Cancer 2018, 21, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bu, X. Progress in Vaccine Therapies for Breast Cancer. Adv. Exp. Med. Biol. 2017, 1026, 315–330. [Google Scholar] [CrossRef]
- Ren, Y.; Cherukuri, Y.; Wickland, D.P.; Sarangi, V.; Tian, S.; Carter, J.M.; Mansfield, A.S.; Block, M.S.; Sherman, M.E.; Knutson, K.L.; et al. HLA class-I and class-II restricted neoantigen loads predict overall survival in breast cancer. Oncoimmunology 2020, 9. [Google Scholar] [CrossRef]
- Costa, R.; Zaman, S.; Sharpe, S.; Helenowski, I.; Shaw, C.; Han, H.; Soliman, H.; Czerniecki, B. A brief report of toxicity end points of HER2 vaccines for the treatment of patients with HER2(+) breast cancer. Drug Des. Devel. Ther. 2019, 13, 309–316. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Amendola, P.G.; Reuten, R.; Erler, J.T. Interplay Between LOX Enzymes and Integrins in the Tumor Microenvironment. Cancers 2019, 11, 729. [Google Scholar] [CrossRef]
- Muller, W.A. Localized signals that regulate transendothelial migration. Curr. Opin. Immunol. 2016, 38, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Muller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Canale, F.P.; Ramello, M.C.; Nunez, N.; Araujo Furlan, C.L.; Bossio, S.N.; Gorosito Serran, M.; Tosello Boari, J.; Del Castillo, A.; Ledesma, M.; Sedlik, C.; et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells. Cancer Res. 2018, 78, 115–128. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef]
- Chung, W.; Eum, H.H.; Lee, H.O.; Lee, K.M.; Lee, H.B.; Kim, K.T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Ren, X.; Wu, H.; Lu, J.; Zhang, Y.; Luo, Y.; Xu, Q.; Shen, S.; Liang, Z. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol. Ther. 2018, 19, 373–380. [Google Scholar] [CrossRef]
- Huang, W.; Ran, R.; Shao, B.; Li, H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019, 178, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Anbazhagan, R.; Fujii, H.; Gabrielson, E. Microsatellite instability is uncommon in breast cancer. Clin. Cancer Res. 1999, 5, 839–844. [Google Scholar]
- Ravaioli, S.; Limarzi, F.; Tumedei, M.M.; Palleschi, M.; Maltoni, R.; Bravaccini, S. Are we ready to use TMB in breast cancer clinical practice? Cancer Immunol. Immunother. 2020. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Keenan, T.E.; Pernas, S.; Exman, P.; Jain, E.; Garrido-Castro, A.C.; Hughes, M.; Bychkovsky, B.; Umeton, R.; Files, J.L.; et al. Tumor Mutational Burden and PTEN Alterations as Molecular Correlates of Response to PD-1/L1 Blockade in Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Mei, P.; Freitag, C.E.; Wei, L.; Zhang, Y.; Parwani, A.V.; Li, Z. High tumor mutation burden is associated with DNA damage repair gene mutation in breast carcinomas. Diagn. Pathol. 2020, 15, 50. [Google Scholar] [CrossRef]
- Hollern, D.P.; Xu, N.; Thennavan, A.; Glodowski, C.; Garcia-Recio, S.; Mott, K.R.; He, X.; Garay, J.P.; Carey-Ewend, K.; Marron, D.; et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 2019, 179, 1191–1206.e1121. [Google Scholar] [CrossRef]
- Chung, V.; Kos, F.J.; Hardwick, N.; Yuan, Y.; Chao, J.; Li, D.; Waisman, J.; Li, M.; Zurcher, K.; Frankel, P.; et al. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers. Clin. Transl. Oncol. 2019, 21, 363–372. [Google Scholar] [CrossRef]
- Knudson, K.M.; Hicks, K.C.; Luo, X.; Chen, J.Q.; Schlom, J.; Gameiro, S.R. M7824, a novel bifunctional anti-PD-L1/TGFbeta Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology 2018, 7, e1426519. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer. Mol. Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Goldoy-Carderon, M.; Gonzalez-Marcano, E.; Carballo, J.; Convit, A.F. Evaluation of a ConvitVax/anti-PD-1 combined immunotherapy for breast cancer treatment. Oncotarget 2019, 10, 6546–6560. [Google Scholar]
- Tan, Z.; Chiu, M.S.; Yan, C.W.; Wong, Y.C.; Huang, H.; Man, K.; Chen, Z. Antimesothelioma Immunotherapy by CTLA-4 Blockade Depends on Active PD1-Based TWIST1 Vaccination. Mol. Ther. Oncolytics 2020, 16, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Karyampudi, L.; Lamichhane, P.; Scheid, A.D.; Kalli, K.R.; Shreeder, B.; Krempski, J.W.; Behrens, M.D.; Knutson, K.L. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014, 74, 2974–2985. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, K.; Feng, L.; Su, R.; Lai, N.; Yang, Z.; Kang, S. Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer. Cancer Sci. 2018, 109, 1753–1763. [Google Scholar] [CrossRef]
- Hassannia, H.; Ghasemi Chaleshtari, M.; Atyabi, F.; Nosouhian, M.; Masjedi, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Mohammadi, H.; Ghalamfarsa, G.; et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology 2020, 159, 75–87. [Google Scholar] [CrossRef]
- Kodumudi, K.N.; Ramamoorthi, G.; Snyder, C.; Basu, A.; Jia, Y.; Awshah, S.; Beyer, A.P.; Wiener, D.; Lam, L.; Zhang, H.; et al. Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response. Front. Immunol. 2019, 10, 1939. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4 + and CD8 + T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef]
- Foote, J.B.; Kok, M.; Leatherman, J.M.; Armstrong, T.D.; Marcinkowski, B.C.; Ojalvo, L.S.; Kanne, D.B.; Jaffee, E.M.; Dubensky, T.W., Jr.; Emens, L.A. A STING Agonist Given with OX40 Receptor and PD-L1 Modulators Primes Immunity and Reduces Tumor Growth in Tolerized Mice. Cancer Immunol. Res. 2017, 5, 468–479. [Google Scholar] [CrossRef]
- Kim, J.M.; Rasmussen, J.P.; Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007, 8, 191–197. [Google Scholar] [CrossRef]
- Arab, A.; Nicastro, J.; Slavcev, R.; Razazan, A.; Barati, N.; Nikpoor, A.R.; Brojeni, A.A.M.; Mosaffa, F.; Badiee, A.; Jaafari, M.R.; et al. Lambda phage nanoparticles displaying HER2-derived E75 peptide induce effective E75-CD8(+) T response. Immunol. Res. 2018, 66, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Hueman, M.T.; Stojadinovic, A.; Storrer, C.E.; Foley, R.J.; Gurney, J.M.; Shriver, C.D.; Ponniah, S.; Peoples, G.E. Levels of circulating regulatory CD4+CD25+ T cells are decreased in breast cancer patients after vaccination with a HER2/neu peptide (E75) and GM-CSF vaccine. Breast Cancer Res. Treat. 2006, 98, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Madondo, M.T.; Quinn, M.; Plebanski, M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat. Rev. 2016, 42, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 957. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Domschke, C.; Stoiber, N.; Schott, S.; Heil, J.; Rom, J.; Blumenstein, M.; Thum, J.; Sohn, C.; Schneeweiss, A.; et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: Immunological effects and clinical outcome. Cancer Immunol. Immunother. 2012, 61, 353–362. [Google Scholar] [CrossRef]
- Chen, G.; Gupta, R.; Petrik, S.; Laiko, M.; Leatherman, J.M.; Asquith, J.M.; Daphtary, M.M.; Garrett-Mayer, E.; Davidson, N.E.; Hirt, K.; et al. A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol. Res. 2014, 2, 949–961. [Google Scholar] [CrossRef]
- Le, D.T.; Jaffee, E.M. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: A current perspective. Cancer Res. 2012, 72, 3439–3444. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, W.; Yang, Z.G.; Zhao, G.F. DC targeting DNA vaccines induce protective and therapeutic antitumor immunity in mice. Int. J. Clin. Exp. Med. 2015, 8, 17565–17577. [Google Scholar]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef]
- Cai, H.; Wang, C.; Shukla, S.; Steinmetz, N.F. Cowpea Mosaic Virus Immunotherapy Combined with Cyclophosphamide Reduces Breast Cancer Tumor Burden and Inhibits Lung Metastasis. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tuve, S.; Persson, J.; Beyer, I.; Yumul, R.; Li, Z.Y.; Tragoolpua, K.; Hellström, K.E.; Roffler, S.; Lieber, A. Adenovirus-mediated intratumoral expression of immunostimulatory proteins in combination with systemic Treg inactivation induces tumor-destructive immune responses in mouse models. Cancer Gene Ther. 2011, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Moreno Ayala, M.A.; Gottardo, M.F.; Imsen, M.; Asad, A.S.; Bal de Kier Joffé, E.; Casares, N.; Lasarte, J.J.; Seilicovich, A.; Candolfi, M. Therapeutic blockade of Foxp3 in experimental breast cancer models. Breast Cancer Res. Treat. 2017, 166, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Rech, A.J.; Mick, R.; Martin, S.; Recio, A.; Aqui, N.A.; Powell, D.J., Jr.; Colligon, T.A.; Trosko, J.A.; Leinbach, L.I.; Pletcher, C.H.; et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med. 2012, 4, 134ra162. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Sprague, L.; McGinty, J.; Pate, M.; Muccioli, M. Dendritic cells the tumor microenvironment and the challenges for an effective antitumor vaccination. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Fainaru, O.; Almog, N.; Yung, C.W.; Nakai, K.; Montoya-Zavala, M.; Abdollahi, A.; D’Amato, R.; Ingber, D.E. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J. 2010, 24, 1411–1418. [Google Scholar] [CrossRef]
- Gervais, A.; Levêque, J.; Bouet-Toussaint, F.; Burtin, F.; Lesimple, T.; Sulpice, L.; Patard, J.J.; Genetet, N.; Catros-Quemener, V. Dendritic cells are defective in breast cancer patients: A potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005, 7, R326–R335. [Google Scholar] [CrossRef]
- Aspord, C.; Pedroza-Gonzalez, A.; Gallegos, M.; Tindle, S.; Burton, E.C.; Su, D.; Marches, F.; Banchereau, J.; Palucka, A.K. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 2007, 204, 1037–1047. [Google Scholar] [CrossRef]
- Chen, X.; Shao, Q.; Hao, S.; Zhao, Z.; Wang, Y.; Guo, X.; He, Y.; Gao, W.; Mao, H. CTLA-4 positive breast cancer cells suppress dendritic cells maturation and function. Oncotarget 2017, 8, 13703–13715. [Google Scholar] [CrossRef]
- Treilleux, I.; Blay, J.Y.; Bendriss-Vermare, N.; Ray-Coquard, I.; Bachelot, T.; Guastalla, J.P.; Bremond, A.; Goddard, S.; Pin, J.J.; Barthelemy-Dubois, C.; et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 2004, 10, 7466–7474. [Google Scholar] [CrossRef]
- da Cunha, A.; Michelin, M.A.; Murta, E.F. Pattern response of dendritic cells in the tumor microenvironment and breast cancer. World J. Clin. Oncol. 2014, 5, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bryson, P.D.; Han, X.; Truong, N.; Wang, P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine 2017, 35, 5842–5849. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Koldovsky, U.; Xu, S.; Mick, R.; Roses, R.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 2012, 118, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, Y.; Zhang, Q.C.; Zhang, P.; Wu, J.K.; Wang, J.N.; Ruan, Y.; Huang, Y. Antitumor efficacy of the Runx2-dendritic cell vaccine in triple-negative breast cancer in vitro. Oncol. Lett. 2018, 16, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.C.; DeInnocentes, P.; Church Bird, A.E.; Lutful Kabir, F.M.; Martinez-Romero, E.G.; Smith, A.N.; Smith, B.F. Autologous hybrid cell fusion vaccine in a spontaneous intermediate model of breast carcinoma. J. Vet. Sci. 2019, 20, e48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, W.; Wang, Y.; Chen, J.; Liu, Y.; Zhang, Y. Enhanced antitumor immunity of nanoliposome-encapsulated heat shock protein 70 peptide complex derived from dendritic tumor fusion cells. Oncol. Rep. 2015, 33, 2695–2702. [Google Scholar] [CrossRef]
- Avigan, D. Fusions of breast cancer and dendritic cells as a novel cancer vaccine. Clin Breast Cancer 2003, 3 (Suppl. 4), S158–S163. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, A.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Lowenfeld, L.; Mick, R.; Datta, J.; Xu, S.; Fitzpatrick, E.; Fisher, C.S.; Fox, K.R.; DeMichele, A.; Zhang, P.J.; Weinstein, S.P.; et al. Dendritic Cell Vaccination Enhances Immune Responses and Induces Regression of HER2(pos) DCIS Independent of Route: Results of Randomized Selection Design Trial. Clin. Cancer Res. 2017, 23, 2961–2971. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Cui, P.; Piao, C.; Xiao, M.; Liu, X.; Wang, Y.; Wu, X.; Liu, J.; Yang, L. Phase I/II clinical trial of a Wilms’ tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol. Immunother. 2019, 68, 121–130. [Google Scholar] [CrossRef]
- Soliman, H.; Khambati, F.; Han, H.S.; Ismail-Khan, R.; Bui, M.M.; Sullivan, D.M.; Antonia, S. A phase-1/2 study of adenovirus-p53 transduced dendritic cell vaccine in combination with indoximod in metastatic solid tumors and invasive breast cancer. Oncotarget 2018, 9, 10110–10117. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Kim, C.S.; Kim, S.B.; Kim, Y.M.; Kwon, S.W.; Kim, Y.; Kim, H.; Lee, H. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: Results from a phase I/II trial. J. Transl. Med. 2011, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.; Wang, F.; Wang, J.; Liu, Q.; Saaoud, F.; Wang, Y.; Singh, U.P.; Chen, H.; Luo, M.; Ai, W.; et al. Overexpression of microRNA-155 enhances the efficacy of dendritic cell vaccine against breast cancer. Oncoimmunology 2020, 9. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Y.; Zhang, Y.; Wang, Y.; Alotaibi, F.; Qiu, L.; Wang, H.; Peng, S.; Liu, Y.; Li, Q.; et al. miRNA-5119 regulates immune checkpoints in dendritic cells to enhance breast cancer immunotherapy. Cancer Immunol. Immunother. 2020, 69, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Taghikhani, A.; Hassan, Z.M.; Ebrahimi, M.; Moazzeni, S.M. microRNA modified tumor-derived exosomes as novel tools for maturation of dendritic cells. J. Cell. Physiol. 2019, 234, 9417–9427. [Google Scholar] [CrossRef] [PubMed]
- Oechsle, C.M.; Showalter, L.E.; Novak, C.M.; Czerniecki, B.J.; Koski, G.K. Statin Drugs Plus Th1 Cytokines Potentiate Apoptosis and Ras Delocalization in Human Breast Cancer Lines and Combine with Dendritic Cell-Based Immunotherapy to Suppress Tumor Growth in a Mouse Model of HER-2(pos) Disease. Vaccines 2020, 8, 72. [Google Scholar] [CrossRef]
- Showalter, L.E.; Oechsle, C.; Ghimirey, N.; Steele, C.; Czerniecki, B.J.; Koski, G.K. Th1 cytokines sensitize HER-expressing breast cancer cells to lapatinib. PLoS ONE 2019, 14, e0210209. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.; Xu, A.; Ahmeqd, S.; Sami, A.; Chibbar, R.; Freywald, A.; Zheng, C.; Xiang, J. Heterologous human/rat HER2-specific exosome-targeted T cell vaccine stimulates potent humoral and CTL responses leading to enhanced circumvention of HER2 tolerance in double transgenic HLA-A2/HER2 mice. Vaccine 2018, 36, 1414–1422. [Google Scholar] [CrossRef]
- Abdellateif, M.S.; Shaarawy, S.M.; Kandeel, E.Z.; El-Habashy, A.H.; Salem, M.L.; El-Houseini, M.E. A novel potential effective strategy for enhancing the antitumor immune response in breast cancer patients using a viable cancer cell-dendritic cell-based vaccine. Oncol. Lett. 2018, 16, 529–535. [Google Scholar] [CrossRef]
- Qi, C.J.; Ning, Y.L.; Han, Y.S.; Min, H.Y.; Ye, H.; Zhu, Y.L.; Qian, K.Q. Autologous dendritic cell vaccine for estrogen receptor (ER)/progestin receptor (PR) double-negative breast cancer. Cancer Immunol. Immunother. 2012, 61, 1415–1424. [Google Scholar] [CrossRef]
- Mastelic-Gavillet, B.; Balint, K.; Boudousquie, C.; Gannon, P.O.; Kandalaft, L.E. Personalized Dendritic Cell Vaccines-Recent Breakthroughs and Encouraging Clinical Results. Front. Immunol. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Peethambaram, P.P.; Melisko, M.E.; Rinn, K.J.; Alberts, S.R.; Provost, N.M.; Jones, L.A.; Sims, R.B.; Lin, L.R.; Frohlich, M.W.; Park, J.W. A phase I trial of immunotherapy with lapuleucel-T (APC8024) in patients with refractory metastatic tumors that express HER-2/neu. Clin. Cancer Res. 2009, 15, 5937–5944. [Google Scholar] [CrossRef] [PubMed]
- Solans, B.P.; Lopez-Diaz de Cerio, A.; Elizalde, A.; Pina, L.J.; Inoges, S.; Espinos, J.; Salgado, E.; Mejias, L.D.; Troconiz, I.F.; Santisteban, M. Assessing the impact of the addition of dendritic cell vaccination to neoadjuvant chemotherapy in breast cancer patients: A model-based characterization approach. Br. J. Clin. Pharmacol. 2019, 85, 1670–1683. [Google Scholar] [CrossRef]
- Vasir, B.; Wu, Z.; Crawford, K.; Rosenblatt, J.; Zarwan, C.; Bissonnette, A.; Kufe, D.; Avigan, D. Fusions of dendritic cells with breast carcinoma stimulate the expansion of regulatory T cells while concomitant exposure to IL-12, CpG oligodeoxynucleotides, and anti-CD3/CD28 promotes the expansion of activated tumor reactive cells. J. Immunol. 2008, 181, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Qi, H.; Lu, Z.; Chen, L.; Sun, B.; Yang, R.; Zhang, Y.; Liu, Z.; Gao, X.; You, A.; et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat. Commun. 2020, 11, 1790. [Google Scholar] [CrossRef]
- Lu, H.; Dietsch, G.N.; Matthews, M.A.; Yang, Y.; Ghanekar, S.; Inokuma, M.; Suni, M.; Maino, V.C.; Henderson, K.E.; Howbert, J.J.; et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin. Cancer Res. 2012, 18, 499–509. [Google Scholar] [CrossRef]
- Wick, D.A.; Martin, S.D.; Nelson, B.H.; Webb, J.R. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C). Vaccine 2011, 29, 984–993. [Google Scholar] [CrossRef]
- Di, S.; Zhou, M.; Pan, Z.; Sun, R.; Chen, M.; Jiang, H.; Shi, B.; Luo, H.; Li, Z. Combined Adjuvant of Poly I:C Improves Antitumor Effects of CAR-T Cells. Front. Oncol. 2019, 9, 241. [Google Scholar] [CrossRef]
- Glaffig, M.; Stergiou, N.; Schmitt, E.; Kunz, H. Immunogenicity of a Fully Synthetic MUC1 Glycopeptide Antitumor Vaccine Enhanced by Poly(I:C) as a TLR3-Activating Adjuvant. ChemMedChem 2017, 12, 722–727. [Google Scholar] [CrossRef]
- Inao, T.; Harashima, N.; Monma, H.; Okano, S.; Itakura, M.; Tanaka, T.; Tajima, Y.; Harada, M. Antitumor effects of cytoplasmic delivery of an innate adjuvant receptor ligand, poly(I:C), on human breast cancer. Breast Cancer Res. Treat. 2012, 134, 89–100. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, P.; Hong, Q.; Chen, A.X.; Liu, G.Y.; Yu, K.D.; Shao, Z.M. Toll-like receptor 3 acts as a suppressor gene in breast cancer initiation and progression: A two-stage association study and functional investigation. Oncoimmunology 2019, 8, e1593801. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Dickerson, E.; Dagia, N.; Malgor, R.; McCall, K.D. TLR signaling inhibitor, phenylmethimazole, in combination with tamoxifen inhibits human breast cancer cell viability and migration. Oncotarget 2017, 8, 113295–113302. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Marron, T.U.; Upadhyay, R.; Svensson-Arvelund, J.; Dhainaut, M.; Hussein, S.; Zhan, Y.; Ostrowski, D.; Yellin, M.; Marsh, H.; et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 2019, 25, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Mardiana, S.; House, I.G.; Sek, K.; Henderson, M.A.; Giuffrida, L.; Chen, A.X.Y.; Todd, K.L.; Petley, E.V.; Chan, J.D.; et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Clifton, G.T.; Hale, D.; Vreeland, T.J.; Hickerson, A.T.; Litton, J.K.; Alatrash, G.; Murthy, R.K.; Qiao, N.; Philips, A.V.; Lukas, J.J.; et al. Results of a Randomized Phase IIb Trial of Nelipepimut-S + Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast Cancer. Clin. Cancer Res. 2020, 26, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Clifton, G.T.; Litton, J.K.; Arrington, K.; Ponniah, S.; Ibrahim, N.K.; Gall, V.; Alatrash, G.; Peoples, G.E.; Mittendorf, E.A. Results of a Phase Ib Trial of Combination Immunotherapy with a CD8+ T Cell Eliciting Vaccine and Trastuzumab in Breast Cancer Patients. Ann. Surg Oncol. 2017, 24, 2161–2167. [Google Scholar] [CrossRef]
- Clifton, G.T.; Peace, K.M.; Holmes, J.P.; Vreeland, T.J.; Hale, D.F.; Herbert, G.S.; Litton, J.K.; Murthy, R.K.; Lukas, J.; Peoples, G.E.; et al. Initial safety analysis of a randomized phase II trial of nelipepimut-S+ GM-CSF and trastuzumab compared to trastuzumab alone to prevent recurrence in breast cancer patients with HER2 low-expressing tumors. Clin. Immunol. 2019, 201, 48–54. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Pinedo, H.M.; Buter, J.; Luykx-de Bakker, S.A.; Pohlmann, P.R.; van Hensbergen, Y.; Heideman, D.A.; van Diest, P.J.; de Gruijl, T.D.; van der Wall, E. Extended neoadjuvant chemotherapy in locally advanced breast cancer combined with GM-CSF: Effect on tumour-draining lymph node dendritic cells. Eur. J. Cancer 2003, 39, 1061–1067. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Ardavanis, A.; Symanowski, J.; Murray, J.L.; Shumway, N.M.; Litton, J.K.; Hale, D.F.; Perez, S.A.; Anastasopoulou, E.A.; Pistamaltzian, N.F.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 2016, 27, 1241–1248. [Google Scholar] [CrossRef]
- Norell, H.; Poschke, I.; Charo, J.; Wei, W.Z.; Erskine, C.; Piechocki, M.P.; Knutson, K.L.; Bergh, J.; Lidbrink, E.; Kiessling, R. Vaccination with a plasmid DNA encoding HER-2/neu together with low doses of GM-CSF and IL-2 in patients with metastatic breast carcinoma: A pilot clinical trial. J. Transl. Med. 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, C.; Gasparetto, M.; Morse, M.; Rooney, B.; Vredenburgh, J.J.; Long, G.D.; Rizzieri, D.A.; Loftis, J.; Chao, N.J.; Smith, C. Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-Ligand and G-CSF or GM-CSF. Cytokine 2002, 18, 8–19. [Google Scholar] [CrossRef]
- Kim, P.S.; Armstrong, T.D.; Song, H.; Wolpoe, M.E.; Weiss, V.; Manning, E.A.; Huang, L.Q.; Murata, S.; Sgouros, G.; Emens, L.A.; et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J. Clin. Investig. 2008, 118, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Lacher, M.D.; Bauer, G.; Fury, B.; Graeve, S.; Fledderman, E.L.; Petrie, T.D.; Coleal-Bergum, D.P.; Hackett, T.; Perotti, N.H.; Kong, Y.Y.; et al. SV-BR-1-GM, a Clinically Effective GM-CSF-Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4(+) T Lymphocytes. Front. Immunol. 2018, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Mediavilla-Varela, M.; Antonia, S.J. A GM-CSF and CD40L bystander vaccine is effective in a murine breast cancer model. Breast Cancer 2015, 7, 389–397. [Google Scholar] [CrossRef][Green Version]
- Özverel, C.S.; Uyanikgil, Y.; Karaboz, İ.; Nalbantsoy, A. Investigation of the combination of anti-PD-L1 mAb with HER2/neu-loaded dendritic cells and QS-21 saponin adjuvant: Effect against HER2 positive breast cancer in mice. Immunopharmacol. Immunotoxicol. 2020, 1–12. [Google Scholar] [CrossRef]
- De Mingo Pulido, Á.; Gardner, A.; Hiebler, S.; Soliman, H.; Rugo, H.S.; Krummel, M.F.; Coussens, L.M.; Ruffell, B. TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 2018, 33, 60–74.e66. [Google Scholar] [CrossRef]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef]
- Krombach, J.; Hennel, R.; Brix, N.; Orth, M.; Schoetz, U.; Ernst, A.; Schuster, J.; Zuchtriegel, G.; Reichel, C.A.; Bierschenk, S.; et al. Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology 2019, 8, e1523097. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.E.; Perez-Gracia, J.L.; Rodríguez, I.; Alfaro, C.; Oñate, C.; Pérez, G.; Gil-Bazo, I.; Benito, A.; Inogés, S.; López-Diaz de Cerio, A.; et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 2018, 29, 1312–1319. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kusmartsev, S.; Cheng, F.; Paolini, M.; Nefedova, Y.; Sotomayor, E.; Gabrilovich, D. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin. Cancer Res. 2003, 9, 285–294. [Google Scholar]
- Shou, D.; Wen, L.; Song, Z.; Yin, J.; Sun, Q.; Gong, W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget 2016, 7, 64505–64511. [Google Scholar] [CrossRef]
- Vetsika, E.K.; Koukos, A.; Kotsakis, A. Myeloid-Derived Suppressor Cells: Major Figures that Shape the Immunosuppressive and Angiogenic Network in Cancer. Cells 2019, 8, 1647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wilkes, D.W.; Samuel, N.; Blanco, M.A.; Nayak, A.; Alicea-Torres, K.; Gluck, C.; Sinha, S.; Gabrilovich, D.; Chakrabarti, R. DeltaNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J. Clin. Investig. 2018, 128, 5095–5109. [Google Scholar] [CrossRef]
- Forghani, P.; Waller, E.K. Poly (I: C) modulates the immunosuppressive activity of myeloid-derived suppressor cells in a murine model of breast cancer. Breast Cancer Res. Treat. 2015, 153, 21–30. [Google Scholar] [CrossRef]
- Christmas, B.J.; Rafie, C.I.; Hopkins, A.C.; Scott, B.A.; Ma, H.S.; Cruz, K.A.; Woolman, S.; Armstrong, T.D.; Connolly, R.M.; Azad, N.A.; et al. Entinostat Converts Immune-Resistant Breast and Pancreatic Cancers into Checkpoint-Responsive Tumors by Reprogramming Tumor-Infiltrating MDSCs. Cancer Immunol. Res. 2018, 6, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Bao, X.; Dong, L.; Guo, Q.Q.; Guo, J.; Xie, Y.; Zhou, Y.; Yu, B.; Wu, H.; Wu, J.X.; et al. Doxorubicin pretreatment enhances FAPα/survivin co-targeting DNA vaccine anti-tumor activity primarily through decreasing peripheral MDSCs in the 4T1 murine breast cancer model. Oncoimmunology 2020, 9. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, P.; Li, D.; Qin, M.; Nie, L.; Wang, X.; Ai, L.; Feng, Z.; Odhiambo, W.O.; Ma, Y.; et al. CD19-targeting fusion protein combined with PD1 antibody enhances anti-tumor immunity in mouse models. Oncoimmunology 2020, 9. [Google Scholar] [CrossRef]
- Ma, H.S.; Poudel, B.; Torres, E.R.; Sidhom, J.W.; Robinson, T.M.; Christmas, B.; Scott, B.; Cruz, K.; Woolman, S.; Wall, V.Z.; et al. A CD40 Agonist and PD-1 Antagonist Antibody Reprogram the Microenvironment of Nonimmunogenic Tumors to Allow T-cell-Mediated Anticancer Activity. Cancer Immunol. Res. 2019, 7, 428–442. [Google Scholar] [CrossRef]
- Farsaci, B.; Donahue, R.N.; Coplin, M.A.; Grenga, I.; Lepone, L.M.; Molinolo, A.A.; Hodge, J.W. Immune consequences of decreasing tumor vasculature with antiangiogenic tyrosine kinase inhibitors in combination with therapeutic vaccines. Cancer Immunol. Res. 2014, 2, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Song, X.; Wang, Y.; Liu, F.; Wei, J. Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment. Front. Immunol. 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Eissa, I.R.; Bustos-Villalobos, I.; Ichinose, T.; Matsumura, S.; Naoe, Y.; Miyajima, N.; Morimoto, D.; Mukoyama, N.; Zhiwen, W.; Tanaka, M.; et al. The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers. Cancers 2018, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, S.; Yang, A.; Kang, S.; Lu, J.; Kim, S.I.; Park, A.K.; Sivanandam, V.; Zhang, Z.; Woo, Y.; Warner, S.G.; et al. Oncolytic poxvirus CF33-hNIS-DeltaF14.5 favorably modulates tumor immune microenvironment and works synergistically with anti-PD-L1 antibody in a triple-negative breast cancer model. Oncoimmunology 2020, 9. [Google Scholar] [CrossRef]
- Choi, A.H.; O’Leary, M.P.; Lu, J.; Kim, S.I.; Fong, Y.; Chen, N.G. Endogenous Akt Activity Promotes Virus Entry and Predicts Efficacy of Novel Chimeric Orthopoxvirus in Triple-Negative Breast Cancer. Mol. Ther. Oncolytics 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Rodriguez Stewart, R.M.; Berry, J.T.L.; Berger, A.K.; Yoon, S.B.; Hirsch, A.L.; Guberman, J.A.; Patel, N.B.; Tharp, G.K.; Bosinger, S.E.; Mainou, B.A. Enhanced Killing of Triple-Negative Breast Cancer Cells by Reassortant Reovirus and Topoisomerase Inhibitors. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Nguyen, H.M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef]
- Lee, T.J.; Nair, M.; Banasavadi-Siddegowda, Y.; Liu, J.; Nallanagulagari, T.; Jaime-Ramirez, A.C.; Guo, J.Y.; Quadri, H.; Zhang, J.; Bockhorst, K.H.; et al. Enhancing Therapeutic Efficacy of Oncolytic Herpes Simplex Virus-1 with Integrin β1 Blocking Antibody OS2966. Mol. Cancer Ther. 2019, 18, 1127–1136. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Zou, C.L.; Chen, H.B.; Lv, Q.Y.; Wu, R.Q.; Gu, D.N. Folate-conjugated herpes simplex virus for retargeting to tumor cells. J. Gene Med. 2020, e3177. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y.; Hu, Z.; Head, M.; Mangold, K.A.; Sullivan, M.; Wang, E.; Saha, P.; Gulukota, K.; Helseth, D.L., Jr.; et al. LyP-1-Modified Oncolytic Adenoviruses Targeting Transforming Growth Factor β Inhibit Tumor Growth and Metastases and Augment Immune Checkpoint Inhibitor Therapy in Breast Cancer Mouse Models. Hum. Gene Ther. 2020. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, H.; Wang, J.; Wu, X.; Wen, W.; Liang, Y.; Wang, L.; Liu, F.; Du, X. Inhibition of breast cancer cells by targeting E2F-1 gene and expressing IL15 oncolytic adenovirus. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, H.; Kong, F.; Xu, W.; Wang, T.; Xiao, F.; Wang, L.; Huang, D.; Seth, P.; Yang, Y.; et al. Oncolytic Adenovirus rAd.DCN Inhibits Breast Tumor Growth and Lung Metastasis in an Immune-Competent Orthotopic Xenograft Model. Hum. Gene Ther. 2019, 30, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Bramante, S.; Koski, A.; Liikanen, I.; Vassilev, L.; Oksanen, M.; Siurala, M.; Heiskanen, R.; Hakonen, T.; Joensuu, T.; Kanerva, A.; et al. Oncolytic virotherapy for treatment of breast cancer, including triple-negative breast cancer. Oncoimmunology 2016, 5, e1078057. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Amin, Z.; Che Ani, M.A.; Tan, S.W.; Yeap, S.K.; Alitheen, N.B.; Syed Najmuddin, S.U.F.; Kalyanasundram, J.; Chan, S.C.; Veerakumarasivam, A.; Chia, S.L.; et al. Evaluation of a Recombinant Newcastle Disease Virus Expressing Human IL12 against Human Breast Cancer. Sci. Rep. 2019, 9, 13999. [Google Scholar] [CrossRef] [PubMed]
- Raihan, J.; Ahmad, U.; Yong, Y.K.; Eshak, Z.; Othman, F.; Ideris, A. Regression of solid breast tumours in mice by Newcastle disease virus is associated with production of apoptosis related-cytokines. BMC Cancer 2019, 19, 315. [Google Scholar] [CrossRef]
- Niavarani, S.R.; Lawson, C.; Boudaud, M.; Simard, C.; Tai, L.H. Oncolytic vesicular stomatitis virus-based cellular vaccine improves triple-negative breast cancer outcome by enhancing natural killer and CD8(+) T-cell functionality. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Lal, G.; Rajala, M.S. Combination of Oncolytic Measles Virus Armed With BNiP3, a Pro-apoptotic Gene and Paclitaxel Induces Breast Cancer Cell Death. Front. Oncol. 2018, 8, 676. [Google Scholar] [CrossRef]
- McDonald, C.J.; Erlichman, C.; Ingle, J.N.; Rosales, G.A.; Allen, C.; Greiner, S.M.; Harvey, M.E.; Zollman, P.J.; Russell, S.J.; Galanis, E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006, 99, 177–184. [Google Scholar] [CrossRef]
- Tai, C.J.; Liu, C.H.; Pan, Y.C.; Wong, S.H.; Tai, C.J.; Richardson, C.D.; Lin, L.T. Chemovirotherapeutic Treatment Using Camptothecin Enhances Oncolytic Measles Virus-Mediated Killing of Breast Cancer Cells. Sci. Rep. 2019, 9, 6767. [Google Scholar] [CrossRef]
- Mullins-Dansereau, V.; Petrazzo, G.; Geoffroy, K.; Béland, D.; Bourgeois-Daigneault, M.C. Pre-surgical oncolytic virotherapy improves breast cancer outcomes. Oncoimmunology 2019, 8, e1655363. [Google Scholar] [CrossRef]
- Koske, I.; Rossler, A.; Pipperger, L.; Petersson, M.; Barnstorf, I.; Kimpel, J.; Tripp, C.H.; Stoitzner, P.; Banki, Z.; von Laer, D. Oncolytic virotherapy enhances the efficacy of a cancer vaccine by modulating the tumor microenvironment. Int. J. Cancer 2019, 145, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dai, E.; Liu, Z.; Ma, C.; Guo, Z.S.; Bartlett, D.L. In Situ Therapeutic Cancer Vaccination with an Oncolytic Virus Expressing Membrane-Tethered IL-2. Mol. Ther. Oncolytics 2020, 17, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade. Clin. Cancer Res. 2019, 25, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Feola, S.; Capasso, C.; Fusciello, M.; Martins, B.; Tahtinen, S.; Medeot, M.; Carpi, S.; Frascaro, F.; Ylosmaki, E.; Peltonen, K.; et al. Oncolytic vaccines increase the response to PD-L1 blockade in immunogenic and poorly immunogenic tumors. Oncoimmunology 2018, 7, e1457596. [Google Scholar] [CrossRef]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef]
- Bourgeois-Daigneault, M.C.; Roy, D.G.; Aitken, A.S.; El Sayes, N.; Martin, N.T.; Varette, O.; Falls, T.; St-Germain, L.E.; Pelin, A.; Lichty, B.D.; et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; Chu, X.; Sun, P.; Huang, W.; Liu, C.; Yang, X.; Sun, W.; Bai, H.; Ma, Y. Recombinant virus-like particles presenting IL-33 successfully modify the tumor microenvironment and facilitate antitumor immunity in a model of breast cancer. Acta Biomater. 2019, 100, 316–325. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, M.; Zhang, Y.; Zhong, L.; Zheng, X. Novel Combination Oncolytic Adenoviral Gene Therapy Armed with Dm-dNK and CD40L for Breast Cancer. Curr. Gene Ther. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Schenkel, A.R.; Chew, T.W.; Muller, W.A. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J. Immunol. 2004, 173, 6403–6408. [Google Scholar] [CrossRef]
- Sokeland, G.; Schumacher, U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol. Cancer 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Kato, H.; Zardouzian, E.; Kelber, J.; Yang, J.; Shattil, S.; Klemke, R. Visualizing extravasation dynamics of metastatic tumor cells. J. Cell Sci. 2010, 123, 2332–2341. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86. [Google Scholar] [CrossRef]
- Loges, S.; Schmidt, T.; Carmeliet, P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer 2010, 1, 12–25. [Google Scholar] [CrossRef]
- Kangsamaksin, T.; Murtomaki, A.; Kofler, N.M.; Cuervo, H.; Chaudhri, R.A.; Tattersall, I.W.; Rosenstiel, P.E.; Shawber, C.J.; Kitajewski, J. NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov. 2015, 5, 182–197. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wang, Q.R.; Zhao, X.; Yang, L.; Tian, L.; Lu, Y.; Kang, B.; Lu, C.J.; Huang, M.J.; Lou, Y.Y.; et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat. Med. 2000, 6, 1160–1166. [Google Scholar] [CrossRef]
- Wagner, S.C.; Ichim, T.E.; Ma, H.; Szymanski, J.; Perez, J.A.; Lopez, J.; Bogin, V.; Patel, A.N.; Marincola, F.M.; Kesari, S. Cancer anti-angiogenesis vaccines: Is the tumor vasculature antigenically unique? J. Transl. Med. 2015, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.X.; Cheng, P.; Wei, H.Y.; Shen, G.B.; Fu, L.X.; Ni, J.; Wu, Y.; Wei, Y.Q. Active immunotherapy for mouse breast cancer with irradiated whole-cell vaccine expressing VEGFR2. Oncol. Rep. 2013, 29, 1510–1516. [Google Scholar] [CrossRef]
- Lu, M.; Yao, Q.; Liu, H.; Zhong, W.; Gao, J.; Si, C.; Zhou, L.; Zhang, S.; Xu, M. Combination of Human Umbilical Vein Endothelial Cell Vaccine and Docetaxel Generates Synergistic Anti-Breast Cancer Effects. Cancer Biother. Radiopharm. 2019, 34, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bose, A.; Komita, H.; Taylor, J.L.; Chi, N.; Lowe, D.B.; Okada, H.; Cao, Y.; Mukhopadhyay, D.; Cohen, P.A.; et al. Vaccines targeting tumor blood vessel antigens promote CD8(+) T cell-dependent tumor eradication or dormancy in HLA-A2 transgenic mice. J. Immunol. 2012, 188, 1782–1788. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, Y.Q.; Tian, L.; Zhao, X.; Yang, L.; Hu, B.; Kan, B.; Wen, Y.J.; Liu, F.; Deng, H.X.; et al. Immunogene therapy of tumors with vaccine based on xenogeneic epidermal growth factor receptor. J. Immunol. 2003, 170, 3162–3170. [Google Scholar] [CrossRef]
- Jin, D.; Yu, X.; Chen, B.; Li, Z.; Ding, J.; Zhao, X.; Qi, G. Combined immunotherapy of breast cancer with EGF and VEGF vaccines from DNA shuffling in a mouse model. Immunotherapy 2017, 9, 537–553. [Google Scholar] [CrossRef]
- Wood, L.M.; Pan, Z.K.; Guirnalda, P.; Tsai, P.; Seavey, M.; Paterson, Y. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol. Immunother. 2011, 60, 931–942. [Google Scholar] [CrossRef]
- Lee, S.H.; Mizutani, N.; Mizutani, M.; Luo, Y.; Zhou, H.; Kaplan, C.; Kim, S.W.; Xiang, R.; Reisfeld, R.A. Endoglin (CD105) is a target for an oral DNA vaccine against breast cancer. Cancer Immunol. Immunother. 2006, 55, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.D.; Kruger, J.A.; Zhou, H.; Luo, Y.; Xiang, R.; Reisfeld, R.A. A novel DNA vaccine encoding PDGFRbeta suppresses growth and dissemination of murine colon, lung and breast carcinoma. Vaccine 2006, 24, 6994–7002. [Google Scholar] [CrossRef]
- Seavey, M.M.; Maciag, P.C.; Al-Rawi, N.; Sewell, D.; Paterson, Y. An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J. Immunol. 2009, 182, 5537–5546. [Google Scholar] [CrossRef]
- Xie, K.; Bai, R.Z.; Wu, Y.; Liu, Q.; Liu, K.; Wei, Y.Q. Anti-tumor effects of a human VEGFR-2-based DNA vaccine in mouse models. Genet Vaccines Ther. 2009, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Denies, S.; Leyman, B.; Huysmans, H.; Combes, F.; Mc Cafferty, S.; Cicchelero, L.; Steppe, M.; De Temmerman, J.; Sanders, N.N. Evaluation of a xenogeneic vascular endothelial growth factor-2 vaccine in two preclinical metastatic tumor models in mice. Cancer Immunol. Immunother. 2017, 66, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, E.; Xia, W.; Lv, T.; Lu, C.; Xu, Z.; Zhu, W. Utilizing VEGF165b mutant as an effective immunization adjunct to augment antitumor immune response. Vaccine 2019, 37, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Cleuren, A.C.A.; van der Ent, M.A.; Jiang, H.; Hunker, K.L.; Yee, A.; Siemieniak, D.R.; Molema, G.; Aird, W.C.; Ganesh, S.K.; Ginsburg, D. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc. Natl. Acad. Sci. USA 2019, 116, 23618–23624. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, P.R.; Milani, C.; Brentani, M.M.; Katayama, M.L.; de Lyra, E.C.; Carraro, D.M.; Brentani, H.; Puga, R.; Lima, L.A.; Rozenchan, P.B.; et al. Transcriptional profile of fibroblasts obtained from the primary site, lymph node and bone marrow of breast cancer patients. Genet Mol. Biol. 2014, 37, 480–489. [Google Scholar] [CrossRef]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Hu, M.; Yao, J.; Cai, L.; Bachman, K.E.; van den Brûle, F.; Velculescu, V.; Polyak, K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat. Genet. 2005, 37, 899–905. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, F.F.; Geng, F.; Liu, C.L.; Xu, P.; Lu, Z.Z.; Yu, B.; Wu, H.; Wu, J.X.; Zhang, H.H.; et al. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein alpha by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model. Cancer Immunol. Immunother. 2016, 65, 613–624. [Google Scholar] [CrossRef]
- Xia, Q.; Geng, F.; Zhang, F.F.; Liu, C.L.; Xu, P.; Lu, Z.Z.; Xie, Y.; Sun, B.; Wu, H.; Yu, B.; et al. Cyclophosphamide enhances anti-tumor effects of a fibroblast activation protein α-based DNA vaccine in tumor-bearing mice with murine breast carcinoma. Immunopharmacol. Immunotoxicol. 2017, 39, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Guo, J.; Guo, Q.Q.; Xie, Y.; Dong, L.; Zhou, Y.; Liu, C.L.; Yu, B.; Wu, H.; Wu, J.X.; et al. A DNA vaccine expressing an optimized secreted FAPalpha induces enhanced anti-tumor activity by altering the tumor microenvironment in a murine model of breast cancer. Vaccine 2019, 37, 4382–4391. [Google Scholar] [CrossRef] [PubMed]
- Achyut, B.R.; Zhang, H.; Angara, K.; Mivechi, N.F.; Arbab, A.S.; Ko, L. Oncoprotein GT198 vaccination delays tumor growth in MMTV-PyMT mice. Cancer Lett. 2020, 476, 57–66. [Google Scholar] [PubMed]
- Sung Kim, T.; Cohen, E.P. Immunity to breast cancer in mice immunized with fibroblasts transfected with a cDNA expression library derived from small numbers of breast cancer cells. Cancer Gene Ther. 2005, 12, 890–899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, T.S.; Jung, M.Y.; Cho, D.; Cohen, E.P. Prolongation of the survival of breast cancer-bearing mice immunized with GM-CSF-secreting syngeneic/allogeneic fibroblasts transfected with a cDNA expression library from breast cancer cells. Vaccine 2006, 24, 6564–6573. [Google Scholar] [CrossRef]
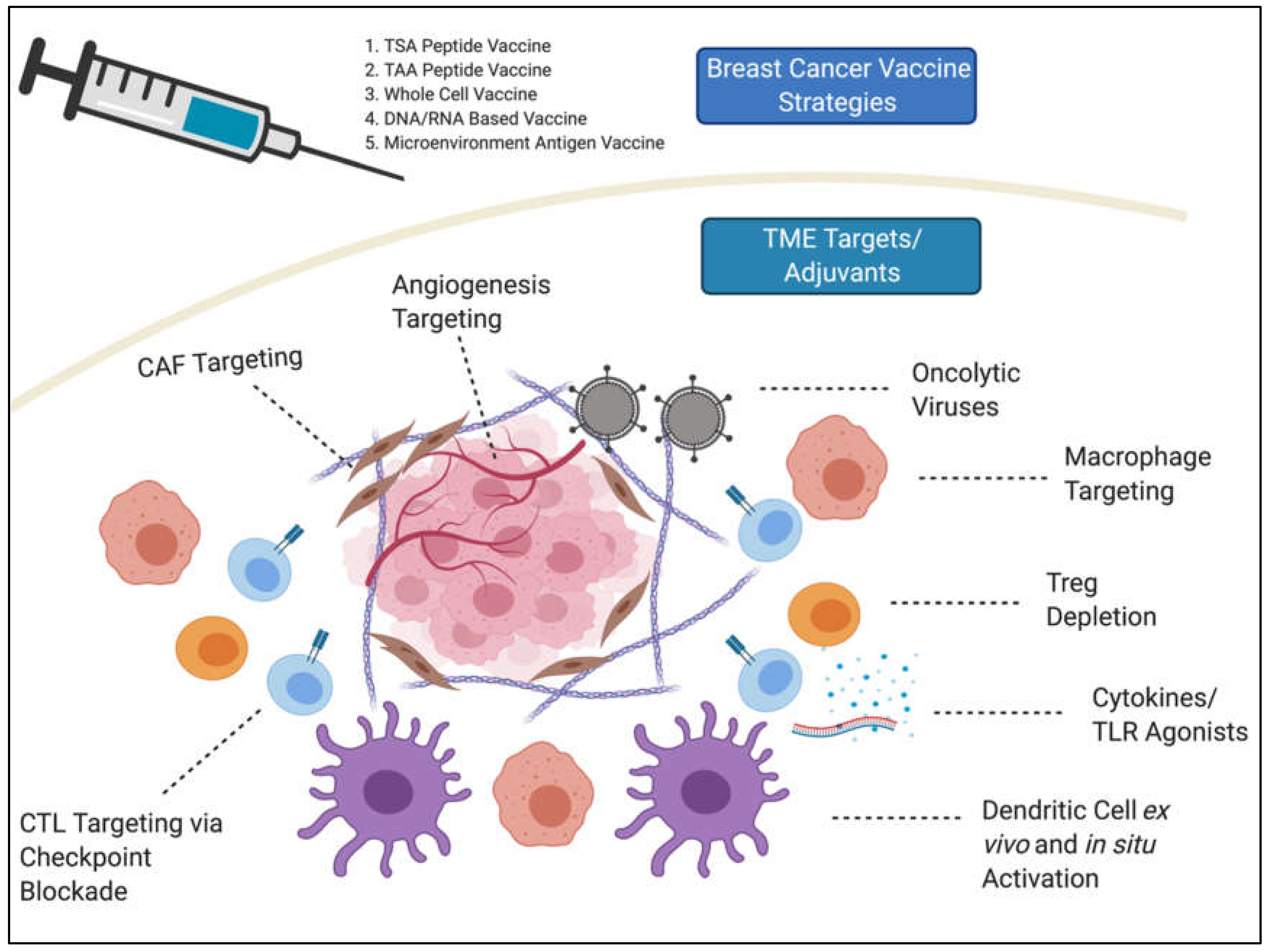
| Class | Examples |
|---|---|
| TSA Peptide Vaccines | Cancer-testis antigens, Neoantigens, Tumor virus antigens |
| TAA Peptide Vaccines | HER2/neu (eg E75, NeuVax), MUC1, p53, CEA, hTERT, Folate Binding Proteins (E39/J65), sialyl Lewisª |
| DNA Vaccines | Rat HER2/neu, Human mammaglobin-A (SCGB2A2), CD105/Yb-1/SOX2/CDH3/MDM2-polyepitpope plasmid, neoantigen DNA, pUMVC3-IGFBP2-HER2-IGF1R plasmid, pNGVL3-hICD plasmid |
| RNA Vaccines | IVAC_W_bre1_uID and IVAC_M_uID (IVAC MUTANOME), Alphavirus-like replicon particles with HER2 RNA |
| Whole Cell Vaccines | Dendritic Cell (DC) vaccines, Autologous or allogenic tumor vaccines |
| Microenvironment Targeting Vaccines | Angiogenesis-targeting vaccines, Fibroblast-targeting vaccines |
| Clinical Trial ID | BC Type | Vaccine Description | Microenvironment Target | Phase |
|---|---|---|---|---|
| NCT03362060 | mTNBC (HLA-A2+) | PVX-410 (Multi-peptide Vaccine) + Pembrolizumab (Anti-PD-1) | Checkpoint Molecules | I (recruiting) |
| NCT03066947 | Local or mBC | SV-BR-1-GM (GM-CSF Secreting BC Cell Line) + Cyclophosphamide | Dendritic Cells and Other Cells (GM-CSF); Tregs | I/II (completed) |
| NCT02479230 | BC | Type I Polarized Autologous DC Vaccine with Tumor Blood Vessel Antigen-Derived Peptides | Dendritic Cells; Angiogenesis | I (completed) |
| NCT01730118 | HER2+ BC | HER2-pulsed DC Vaccination | Dendritic Cells | I (completed) |
| NCT02018458 | TNBC | Cyclin B1/WT-1/CEF-loaded DC Vaccine + Chemotherapy (Varied) | Dendritic Cells | III (completed) |
| NCT02063724 | HER2+ BC | HER2-pulsed DC Vaccine | Dendritic Cells | I (active, not recruiting) |
| NCT02061423 | HER2+ BC | HER2-pulsed DC Vaccine + Trastuzumab | Dendritic Cells | I (active, not recruiting) |
| NCT02061332 | DCIS | HER2-pulsed DC Vaccine | Dendritic Cells | I/II (completed) |
| NCT02643303 | BC | Poly-ICLC in situ Vaccine + Durvalumab (Anti-PD-1) + Tremelimumab (Anti-CTLA-4) | TLR; Checkpoint Molecules | I/II (recruiting) |
| NCT00791037 | HER2+ BC | HER2 Peptide Vaccine + Aargramostim + Cyclophosphamide + Adoptive HER2 Specific T-cells | Dendritic Cells and Other Cells (GM-CSF); T-cells; Tregs | I/II (completed) |
| NCT02140996 | BC | Ad-sig-hMUC1/ecdCD40L Vector Vaccine | APCs (CD40L) | I (recruiting) |
| NCT02593227 | BC | Folate Receptor Alpha Peptide Vaccine + GM-CSF + Cyclophosphamide | Dendritic Cells and Other Cells (GM-CSF); Tregs | II (active, not recruiting) |
| NCT02636582 | DCIS | NeuVax (Nelipepimut-S Peptide Vaccine with Sargramostim Adjuvant) | Dendritic Cells and Other Cells (GM-CSF) | II (active, not recruiting) |
| NCT01660529 | mBC | hTERT/survivin/CMT Multipeptide Vaccine + Basiliximab (anti-CD25) | Tregs | I (completed) |
| NCT01570036 | HER2 low BC | NeuVax (Nelipepimut-S Peptide Vaccine with Sargramostim Adjuvant) + Trastuzumab | Dendritic Cells and Other Cells (GM-CSF) | II (completed) |
| NCT04215146 | ER+ HER2- mBC | Pelareorep (Reovirus-based Therapy) + Nab-paclitaxel + Avelumab (Anti-PD-L1) | Multi-target (Oncolytic Virus); Checkpoint Molecules | II (recruiting) |
| NCT02779855 | TNBC | Talimogene Laherparepvec (Herpes Virus-based Therapy) + Nab-paclitaxel | Multi-target (Oncolytic Virus) | I/II (active, not recruiting) |
| NCT04301011 | TNBC | TBio-6517 (Vaccinia Virus-based Therapy) + Pembrolizumab (Anti-PD-1) | Multi-target (Oncolytic Virus); Checkpoint Molecules | I/II (recruiting) |
| NCT03740256 | HER2+ BC | CAdVEC (Adenovirus-based Therapy) + Autologous CAR Viral Specific T-cells | Multi-target (Oncolytic Virus); T-cells | I (not yet recruiting) |
| NCT02826434 | TNBC | PVX-410 (Multi-peptide Vaccine) + Durvalumab (Anti-PD-L1) | Checkpoint Molecules | I (active, not recruiting) |
| NCT02276300 | HER2+ BC | HER2 Peptide Vaccine + Sargramostim + Cyclophosphamide + Imiquimod | Dendritic Cells and Other Cells (GM-CSF); Tregs, TLR | I (completed) |
| NCT02297698 | HER2+ BC | NeuVax (Nelipepimut-S Peptide Vaccine with Sargramostim Adjuvant) + Trastuzumab | Dendritic Cells and Other Cells (GM-CSF) | II (active, not recruiting) |
| NCT00986609 | TNBC | MUC-1 Peptide Vaccine + Poly-ICLC | TLR | I (completed) |
| NCT02019524 | BC | Folate Binding (E39 and J65) Peptide Vaccine + Sargramostim | Dendritic Cells and Other Cells (GM-CSF) | I/II (completed) |
| NCT00524277 | HER2+ BC | HER2 (GP2) Peptide Vaccine + Modified HER2 (AE37) Peptide Vaccine + Sargramostim | Dendritic Cells and Other Cells (GM-CSF) | II (completed) |
| NCT00971737 | BC | Allogenic GM-CSF-Secreting Whole Tumor Cell Vaccine + Cyclophosphamide | Dendritic Cells and Other Cells (GM-CSF); Tregs | II (completed) |
| NCT03066947 | BC | SV-BR-1-GM (GM-CSF Secreting BC Cell Line) + Cyclophosphamide + IFN-α-2b | Multiple Targets (Interferon); Tregs | I/II (completed) |
| NCT00622401 | BC | DC-Tumor Fusion Vaccine + IL-12 | Dendritic Cells; Other (IL-12) | I/II (terminated) |
| NCT00317603 | mBC | Autologous GM-CSF-Secreting BC Cell Vaccine | Dendritic Cells and Other Cells (GM-CSF) | I (active, not recruiting) |
| NCT02427581 | TNBC | Personalized Synthetic Long Peptide Breast Cancer + Poly-ICLC | TLR | I (recruiting) |
| NCT04024800 | TNBC | Modified HER2 (AE37) Peptide Vaccine + Pembrolizumab (Anti-PD-1) | Checkpoint Molecules | II (recruiting) |
| NCT00880464 | BC | Autologous GM-CSF-Secreting BC Cell Vaccine | Dendritic Cells and Other Cells (GM-CSF) | I (active, not recruiting) |
| NCT03199040 | TNBC | Neo-Antigen DNA vaccine + Durvalumab (Anti-PD-1) | Checkpoint Molecules | I (recruiting) |
| NCT03632941 | HER2+ BC | Neutralized Viral Vector Vaccine (VRP-HER2) + Pembrolizumab (Anti-PD-1) | Checkpoint Molecules | II (recruiting) |
| NCT03384914 | HER2+ BC | DC1 Vaccine + pUMVC3-IGFBP2-HER2-IGF1R (WOKVAC) DNA Vaccine | Dendritic Cells | II (recruiting) |
| NCT04270149 | ER+ BC | ESR1 Peptide Vaccine + Montanide + GM-CSF | Dendritic Cells and Other Cells (GM-CSF) | I (not yet recruiting) |
| NCT03387553 | HER2+ | HER2-pulsed DC Vaccine | Dendritic Cells | I (recruiting) |
| NCT04348747 | mTNBC | Anti-HER2/HER3 DC Vaccine + Celecoxib + Pembrolizumab (Anti-PD-1) + IFN-α-2b | Dendritic Cells; Multiple Targets (Interferon); TLR; Checkpoint Molecules | II (not yet recruiting) |
| NCT02780401 | HER2- BC | pUMVC3-IGFBP2-HER2-IGF1R (WOKVAC) DNA Vaccine + Sargramostim | Dendritic Cells and Other Cells (GM-CSF) | I (active, not recruiting) |
| NCT04105582 | TNBC | Neo-Antigen Pulsed DC Vaccine | Dendritic Cells | I (recruiting) |
| NCT03387085 | TNBC | Adenoviral and Yeast-based Vaccines (CEA, Brachyury, MUC1, Mutant RAS) + Bevacizumab (Anti-VEGF) + Avelumab (Anti-PD-L1) + N-803 (IL-15 Agonist) + NK-92 (hNK Cells) + Chemotherapy (Varied) + Radiation | Multi-target (IL-15 agonist); Angiogenesis; Checkpoint Molecules; NK Cells | I/II (active, not recruiting) |
| NCT03606967 | TNBC | Personalized Synthetic Long Peptide Vaccine + Nab-paclitaxel + Durvalumab (Anti-PD-1) + Poly-ICLC | Checkpoint Molecules; TLR | II (not yet recruiting) |
| NCT03012100 | TNBC | Folate Receptor Alpha Peptide Vaccine. + Sargramostim + Cyclophosphamide | Dendritic Cells and Other Cells (GM-CSF); Tregs | II (recruiting) |
| NCT03804944 | ER+ BC | Flt3L + Radiation Therapy + Pembrolizumab (Anti-PD-1) | Multi-target (radiation); Checkpoint Molecule; Dendritic Cells | II (active, not recruiting) |
| NCT04197687 | HER2+ BC | HER2 Peptide Vaccine (TPIV100) + Sargramostim + Pertuzumab + Trastuzumab | Dendritic Cells and other cells (GM-CSF) | II (recruiting) |
| NCT04418219 | BC | SV-BR-1-GM (GM-CSF Secreting BC Cell Line) + Cyclophosphamide + IFN-α-2b + Pembrolizumab (Anti-PD-1) | Dendritic Cells and Other Cells (GM-CSF); Multi-target (Interferon); Checkpoint Molecules | I/II (not yet recruiting) |
| NCT04296942 | mBC | Brachyury-TRICOM (Vaccinia Viral Vector Based Brachyury Vaccine) + Entinostat + Adotrastuzumab Emtansine + M7824 (PD-L1/TGF-β Fusion Protein) | Checkpoint Molecules; Myeloid Cells | I (recruiting) |
| NCT01782274 | mBC | Proteomic Approach with Allogeneic Haploidentical Hematopoietic Stem Cells (HSCs) + CTLs + DC Vaccine | Multi-target, including Dendritic Cells | II/III (enrolling by invite) |
| NCT03789097 | mBC | Flt3L + Radiation Therapy + Pembrolizumab (Anti-PD-1) + Poly-ICLC | Multi-target (radiation); Checkpoint Molecules;, Dendritic Cells; TLR | I/II (recruiting) |
| NCT00436254 | HER2+ BC | HER2 DNA Vaccine + Sargramostim | Dendritic Cells and Other Cells (GM-CSF) | I (active, not recruiting) |
| NCT04144023 | DCIS | Multi-epitope HER2 Peptide Vaccine + GM-CSF | Dendritic Cells and Other Cells (GM-CSF) | I (recruiting) |
| NCT03564782 | mBC | PVSRIPO (Oncolytic Poliovirus) | Multi-target (Oncolytic Virus) | I (recruiting) |
| NCT03328026 | mBC | SV-BR-1-GM (GM-CSF Secreting BC Cell Line) + IFN-α-2b + INCMGA00012 (PD-1 Inhibitor) + Cyclophosphamide Interferon Inoculation + Epacadostat (Indoleamine-pyrrole 2,3-dioxygenase-1 Inhibitor) | Dendritic Cells and Other Cells (GM-CSF); Checkpoint Molecules; Multi-target (Interferon); Tregs | I/II (recruiting) |
| NCT04246671 | HER2+ BC | TAEK-VAC-HerBy Vaccine + PD-1/PD-L1 Inhibition | Checkpoint Molecules | I/II (not yet recruiting) |
| NCT01376505 | HER2+ BC | HER2 Peptide Vaccine + nor-MDP (Muramyldipeptide Derivative—a Bacterial Cell wall Peptidoglycan) | Multi-target (NOD2 agonist) | I (recruiting) |
| NCT02491697 | mBC | DC-CIK Vaccine (DC Cells Co-cultured with Cytokine-Induced Killer Cells) + Capecitabine | Dendritic Cells | II (active, not recruiting) |
| NCT02432963 | mBC | Modified Vaccinia Virus Ankara Vaccine Expressing p53 + Pembrolizumab (Anti-PD-1) | Checkpoint Molecules | I (active not recruiting) |
| NCT03761914 | TNBC | Galinpepimut-S. (Wilms Tumor-1-Targeting Multivalent Heteroclitic Peptide Vaccine) + Pembrolizumab (Anti-PD-1) | Checkpoint Molecules | I/II (recruiting) |
| NCT01997190 | mBC | AdV-tk (Adenovirus-mediated Herpes Simplex Virus Thymidine Kinase Gene Therapy) + Valacyclovir | Multi-target (Oncolytic Virus) | I (active not recruiting) |
| NCT03289962 | TNBC | RO7198457 (Individualized mRNA Vaccine) + Atezolizumab (Anti-PD-L1) | Checkpoint Molecules | I (recruiting) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, B.; Gadi, V.K. The Role of the Tumor Microenvironment in Developing Successful Therapeutic and Secondary Prophylactic Breast Cancer Vaccines. Vaccines 2020, 8, 529. https://doi.org/10.3390/vaccines8030529
Gordon B, Gadi VK. The Role of the Tumor Microenvironment in Developing Successful Therapeutic and Secondary Prophylactic Breast Cancer Vaccines. Vaccines. 2020; 8(3):529. https://doi.org/10.3390/vaccines8030529
Chicago/Turabian StyleGordon, Benjamin, and Vijayakrishna K. Gadi. 2020. "The Role of the Tumor Microenvironment in Developing Successful Therapeutic and Secondary Prophylactic Breast Cancer Vaccines" Vaccines 8, no. 3: 529. https://doi.org/10.3390/vaccines8030529
APA StyleGordon, B., & Gadi, V. K. (2020). The Role of the Tumor Microenvironment in Developing Successful Therapeutic and Secondary Prophylactic Breast Cancer Vaccines. Vaccines, 8(3), 529. https://doi.org/10.3390/vaccines8030529






