Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Peptides and Recombinant Proteins Used for Immunoassays
2.3. Transient Expression of TERT in Mammalian Cells
2.4. Generation of 4T1luc2 Derivatives Expressing rtTERT by Lentiviral Transduction
2.5. Extraction of Nucleic Acids and Analysis of Genomic rtTERT Inserts in 4T1luc2 Daughter Clones
2.6. Reverse Transcription and Analysis of rtTERT mRNA Expression by Semiquantitative PCR
2.7. Analysis of Expression of Endogenous TERT in 4T1luc2 Clones by Immunofluorescent Microscopy
2.8. Assessment of Genetic Stability of 4T1luc2 Clones
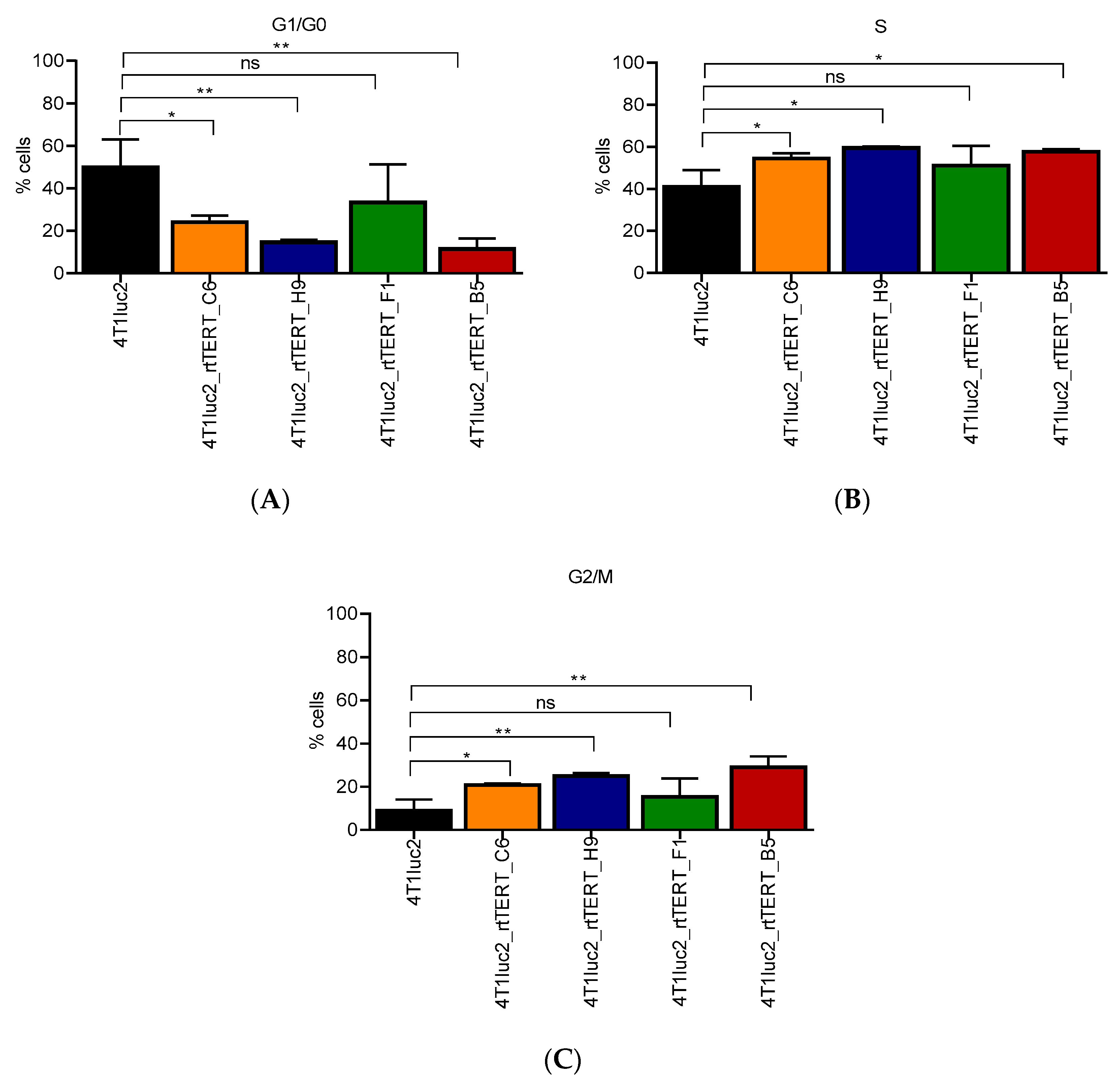
2.9. Cell Cycle Analysis of 4T1luc2 Clones
2.10. Animals and Animal Experiments
2.11. DNA Immunization of Mice
2.12. In Vivo Bioluminescent Imaging
2.13. End Point of DNA Immunization Experiment
2.14. Assessment of Cellular Immune Response
2.15. Assessment of Anti-TERT Antibody Response
2.16. Assessment of In Vivo Tumorigenicity of 4T1luc2 Clones Expressing rtTERT
2.17. End Point of Tumor Challenge Experiment, Collection of Mouse Organs, and Rapid Ex Vivo Assessment of Metastases
2.18. Evaluation of Cellular Response against TERT in Mice Implanted with 4T1luc2 Clones
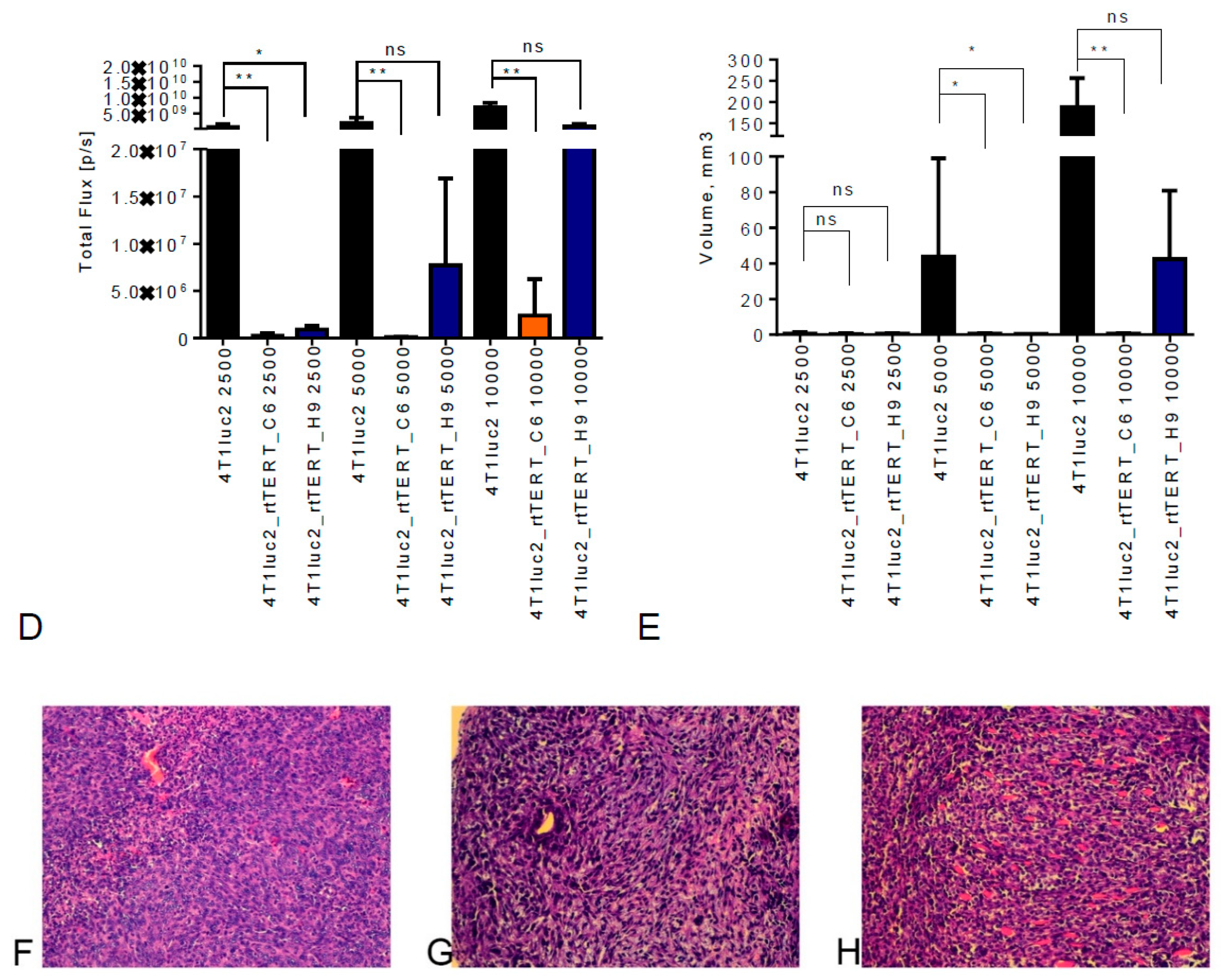
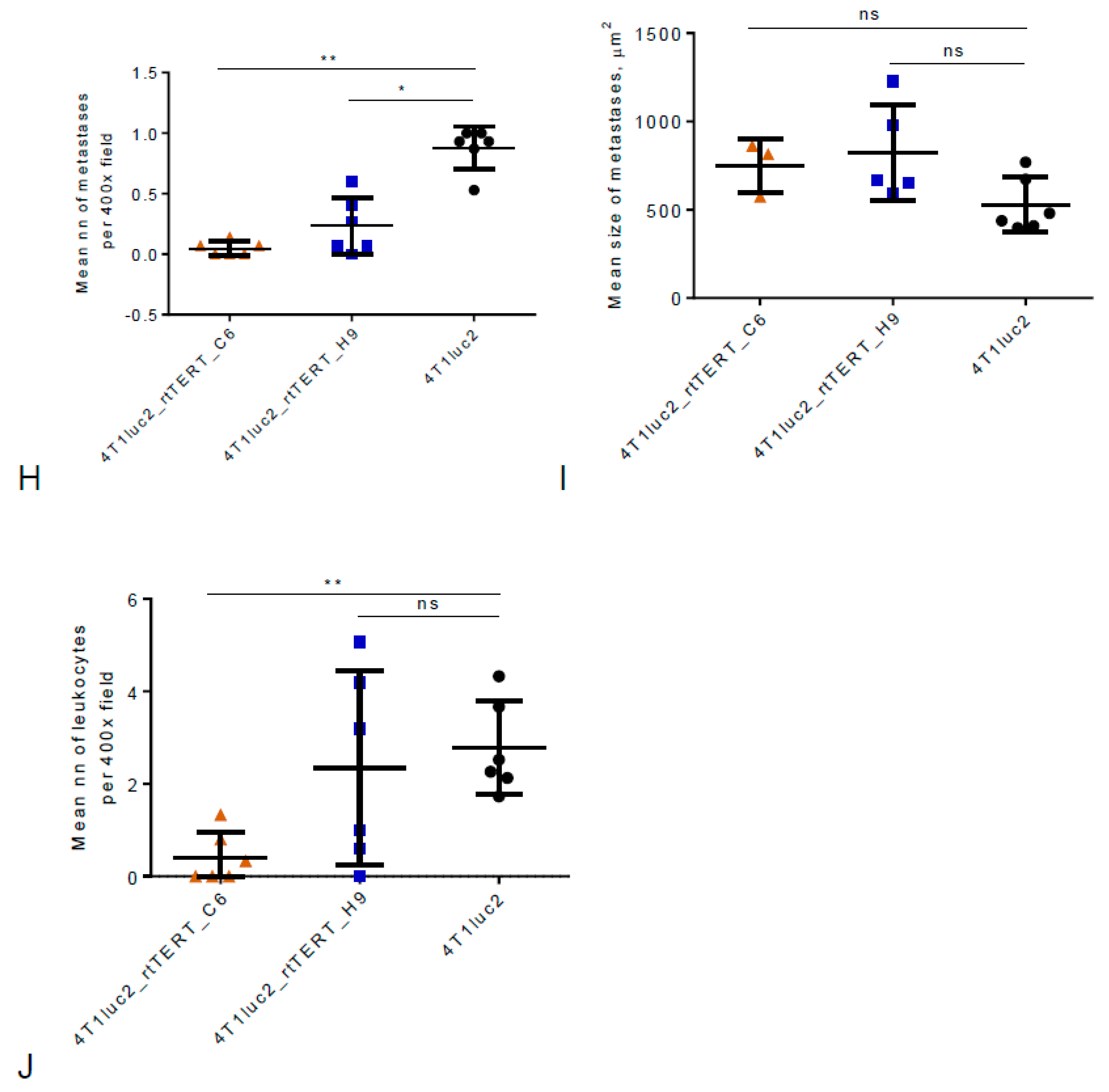
2.19. Tumor Histology and Ex Vivo Assessment of the Metastases
2.20. Statistical Analysis
3. Results
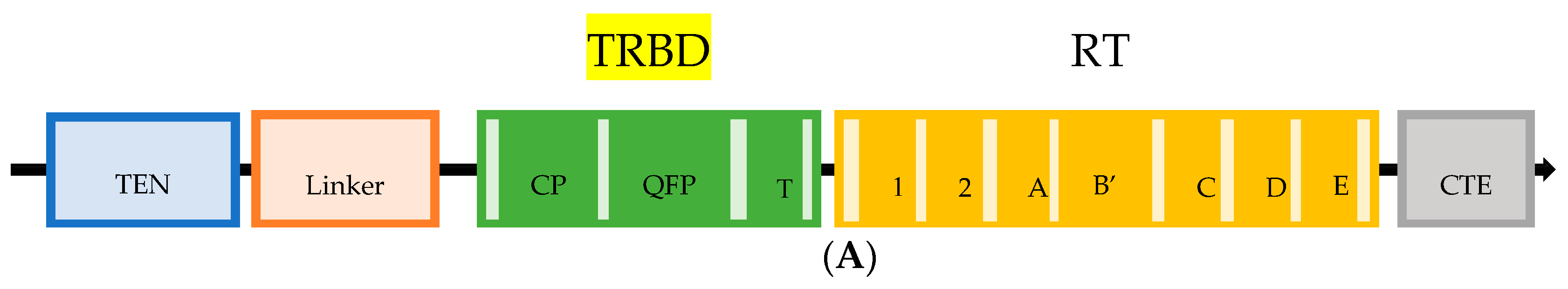
3.1. Design and Expression of Synthetic Rat TERT Gene
3.2. TERT Epitope Map and Selection of TERT-Derived Peptides for the Analysis of Anti-TERT Immune Response
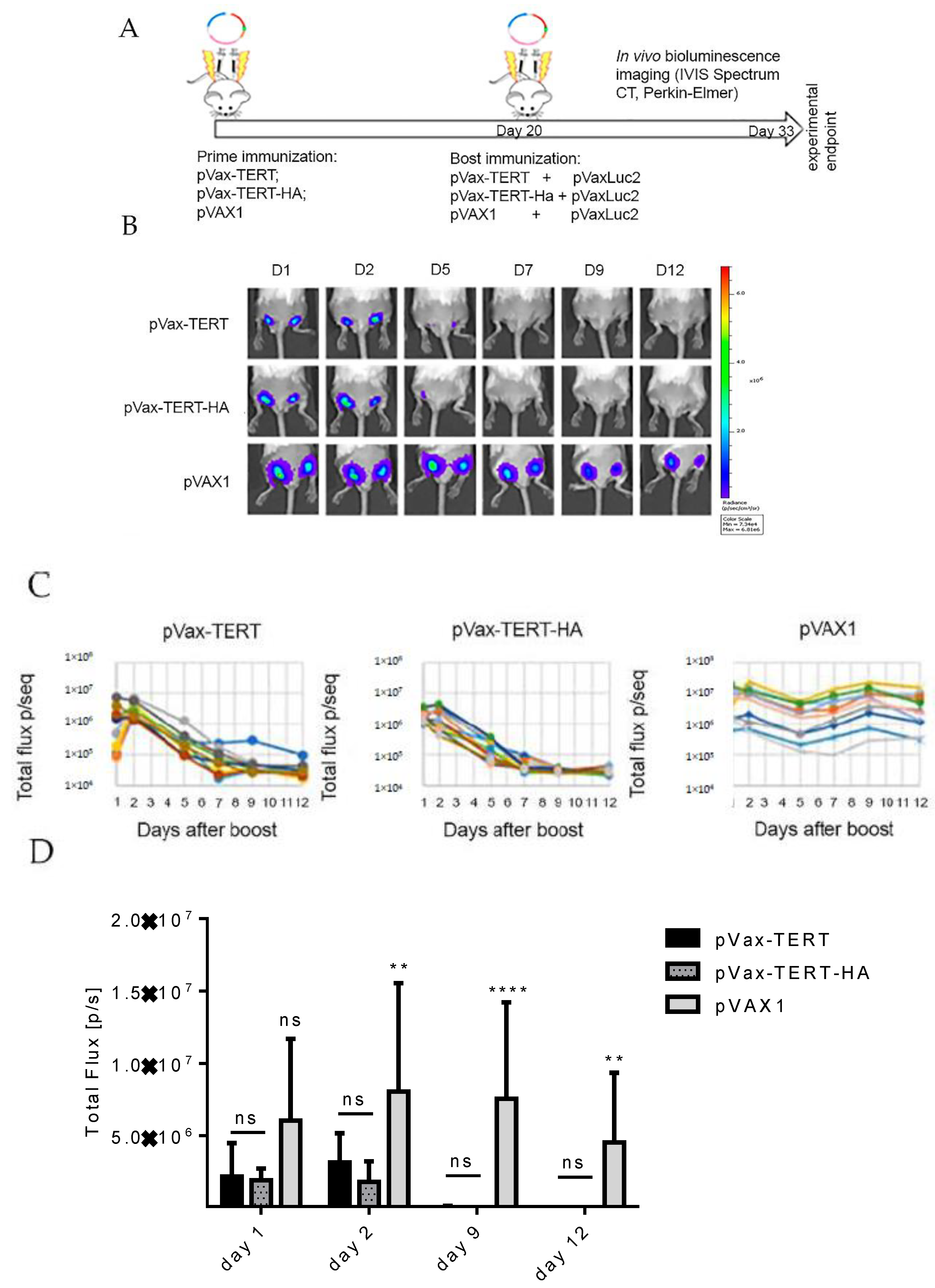
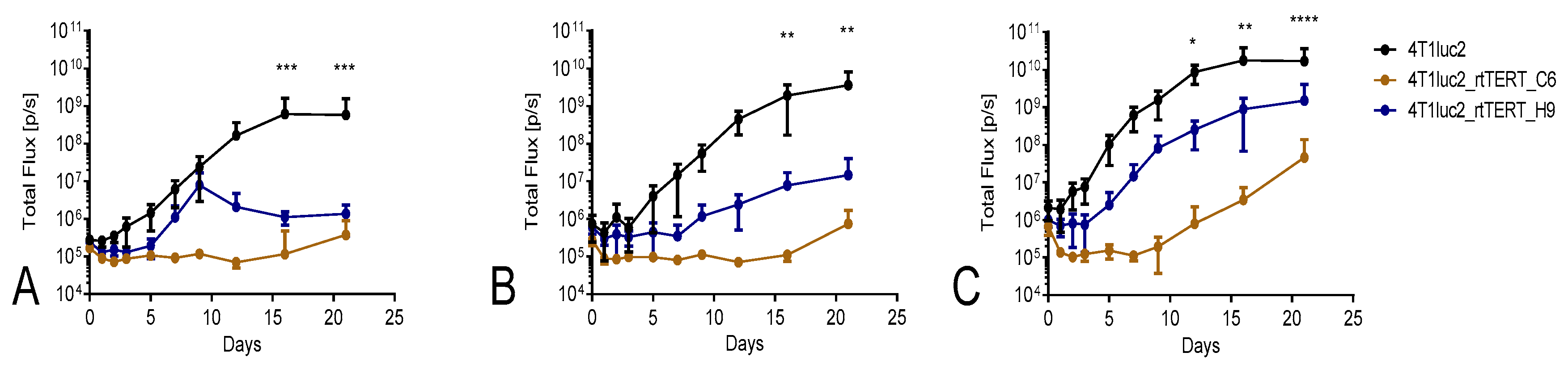
3.3. Immunization and In Vivo Assessment of the Development of Immune Response by Bioluminescent Imaging
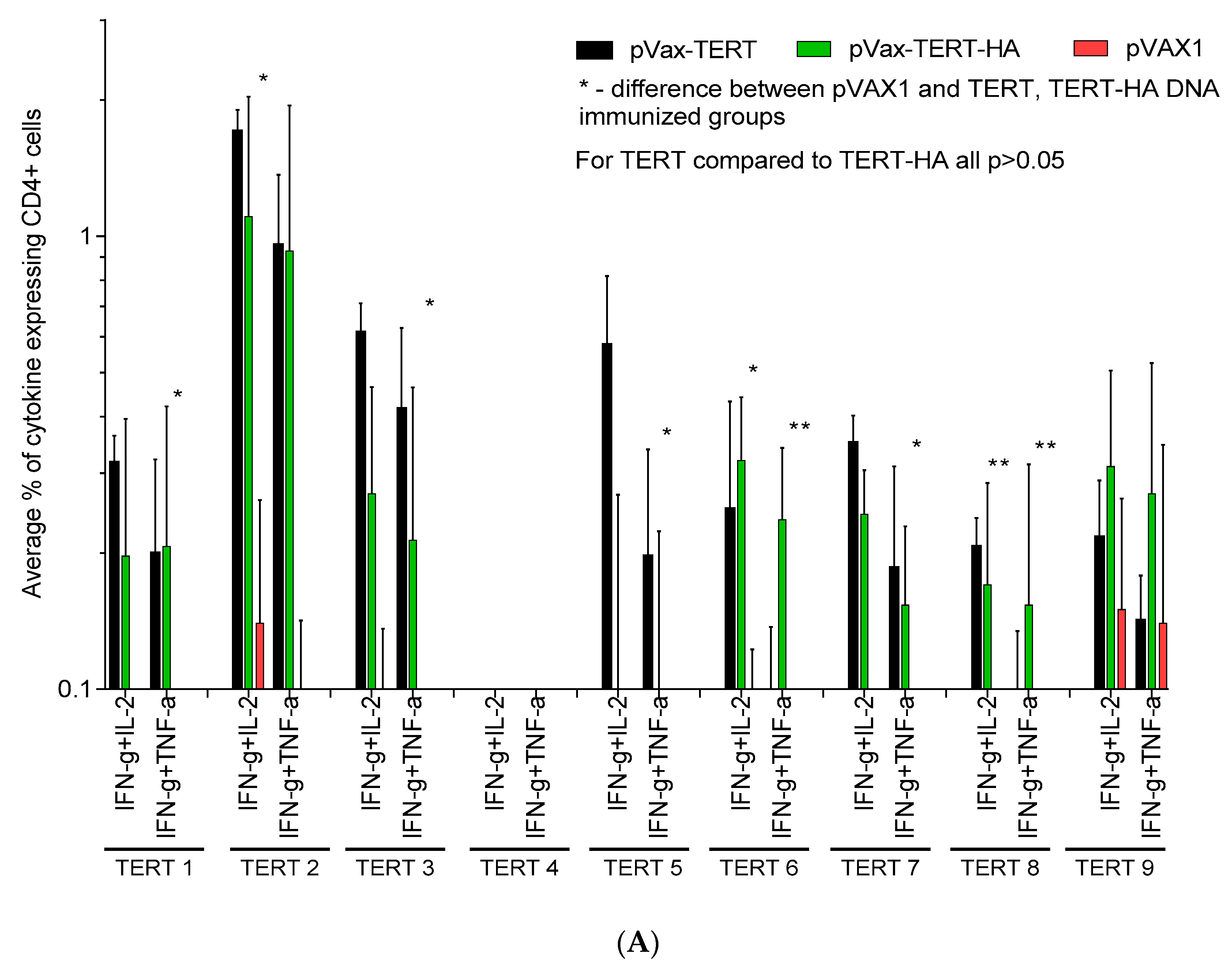
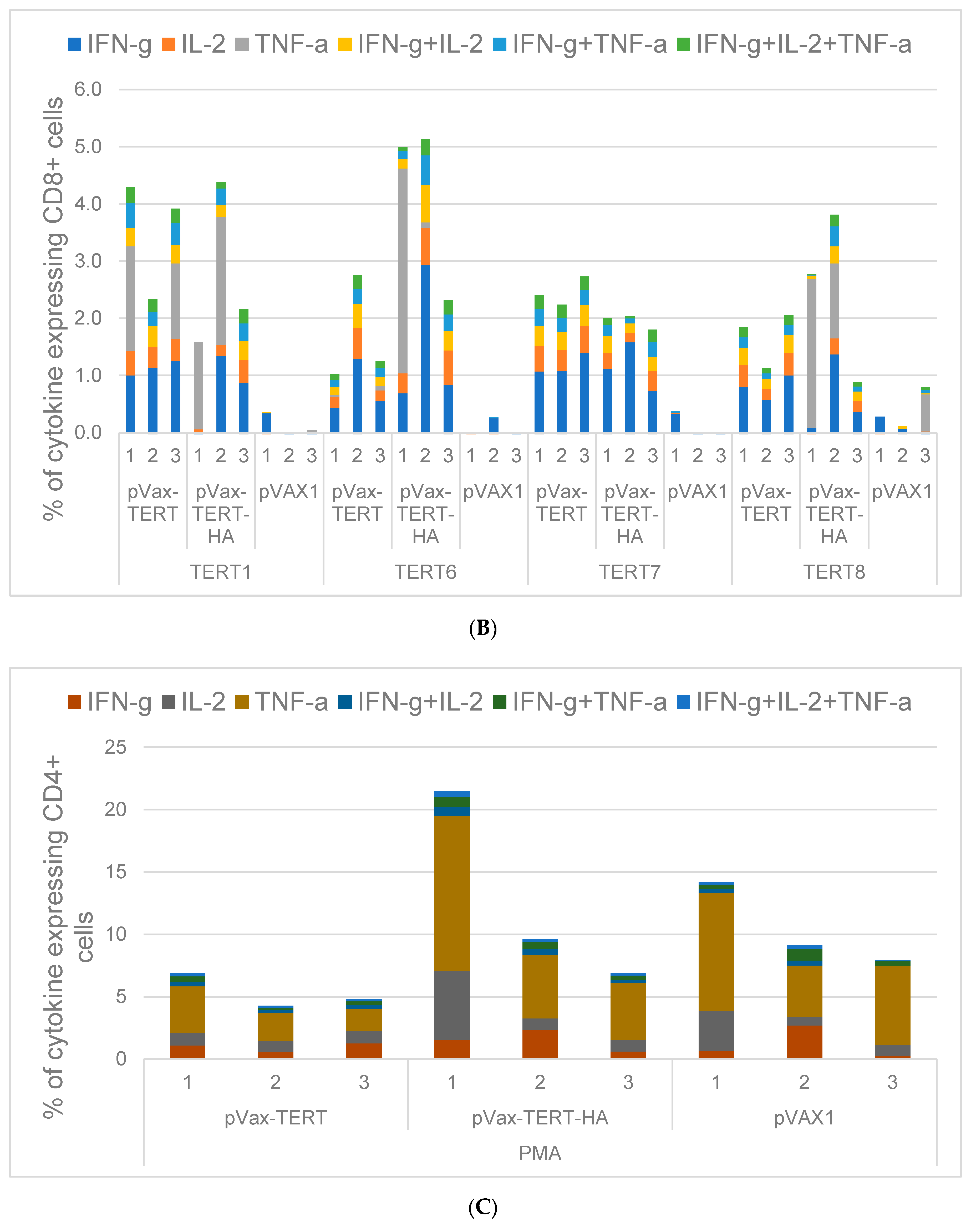
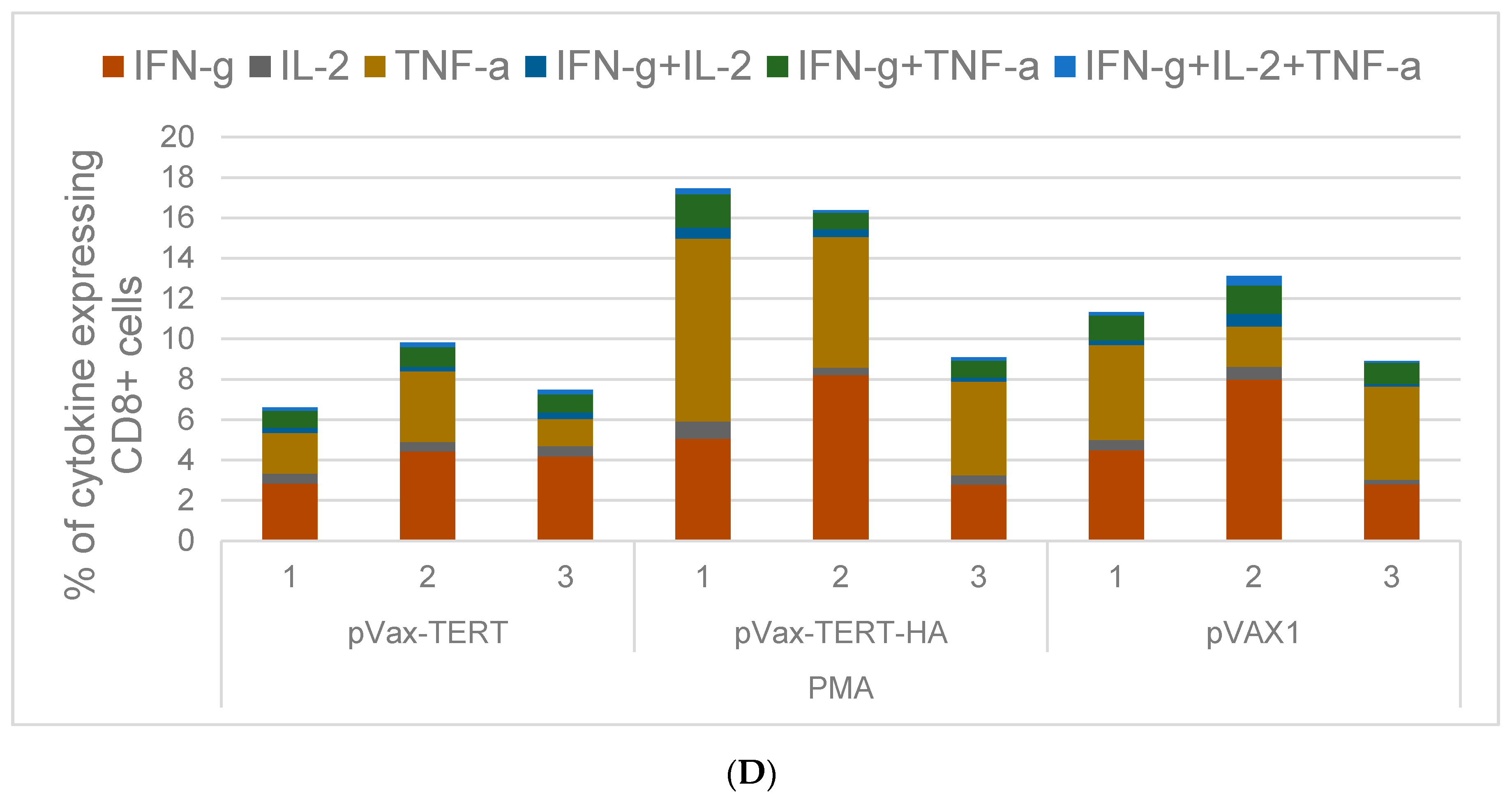
3.4. Assessment of Cellular Immune Response
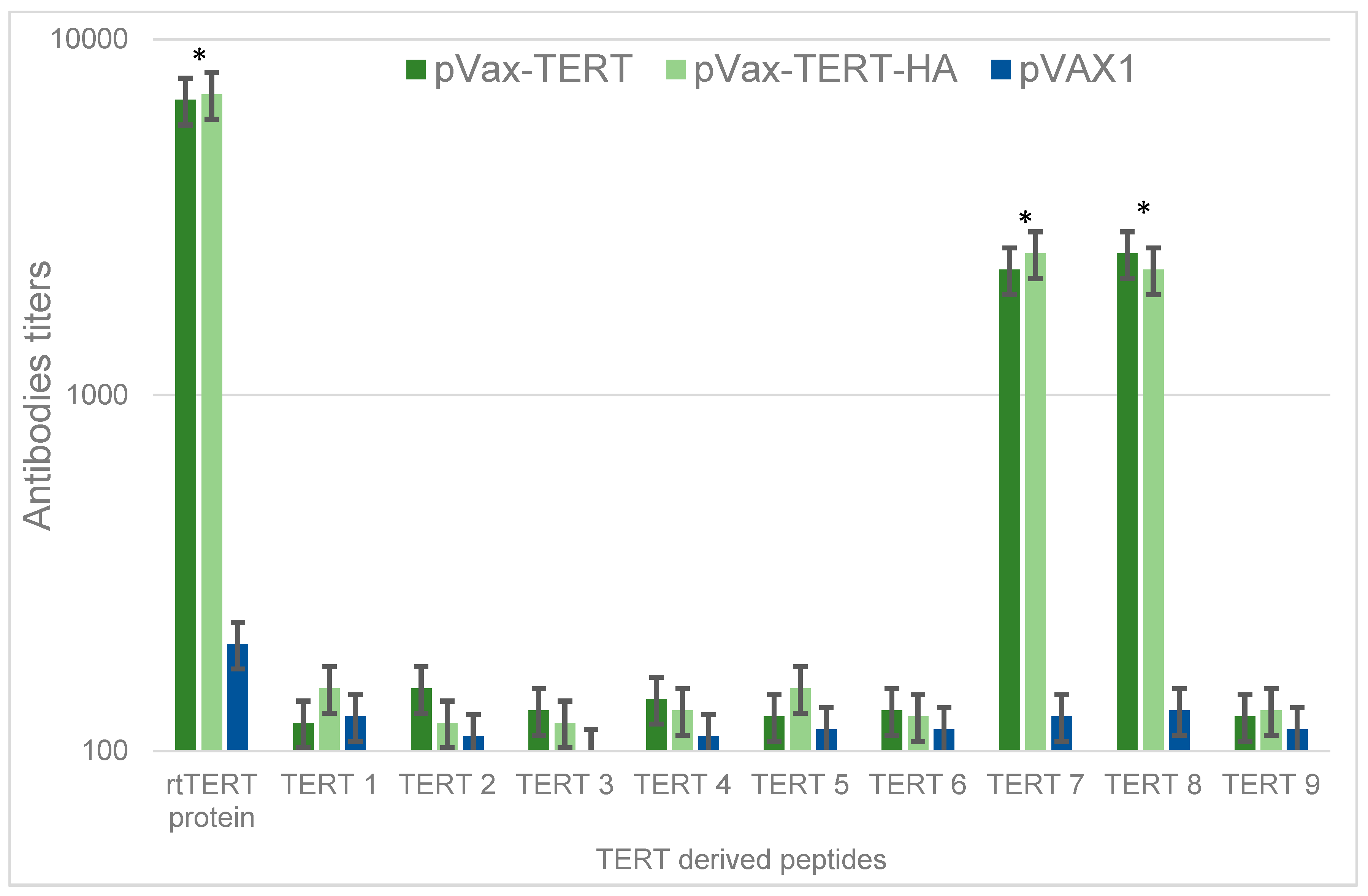
3.5. Antibody Response
3.6. Generation of 4T1luc2 Clones Expressing RT Domain of Rat TERT
3.7. Tumorigenic Potential of 4T1luc2 Clones Expressing RT-TERT
3.8. Metastatic Potential of 4T1luc2 Clones Expressing rtTERT
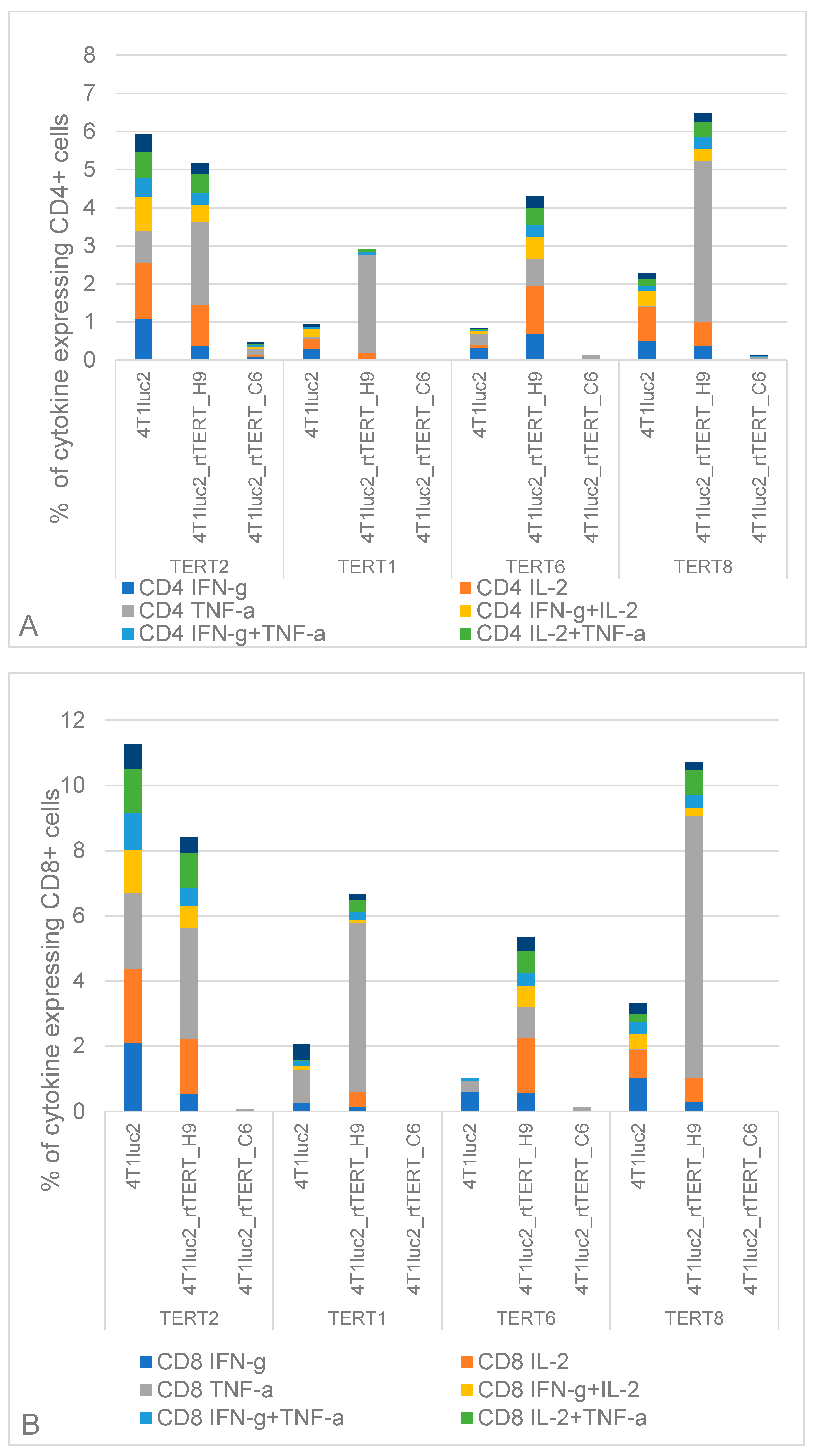
3.9. Cellular Immune Response against Epitopes of TERT in Mice Implanted with rtTERT-Expressing 4T1luc2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Liu, D.; Staveley-O’Carroll, K.F.; Li, G. Immune-based therapy clinical trials in hepatocellular carcinoma. J. Clin. Cell. Immunol. 2015, 6, 376. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; El Dika, I.; Abou-Alfa, G.K. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer 2016, 122, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Gnoni, A.; Gardini, A.C.; Pisconti, S.; Licchetta, A.; Scartozzi, M.; Memeo, R.; Palmieri, V.O.; Aprile, G.; Santini, D. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget 2017, 8, 33897. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; June, C.H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013, 39, 49–60. [Google Scholar] [CrossRef]
- Xie, Y.; Xiang, Y.; Sheng, J.; Zhang, D.; Yao, X.; Yang, Y.; Zhang, X. Immunotherapy for Hepatocellular Carcinoma: Current Advances and Future Expectations. J. Immunol. Res. 2018, 2018, 8740976. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Forner, A.; Korangy, F.; N‘Kontchou, G.; Barget, N.; Ayuso, C.; Ormandy, L.A.; Manns, M.P.; Beaugrand, M.; Bruix, J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 2010, 10, 209. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Duperret, E.K.; Wise, M.C.; Trautz, A.; Villarreal, D.O.; Ferraro, B.; Walters, J.; Yan, J.; Khan, A.; Masteller, E.; Humeau, L.; et al. Synergy of Immune Checkpoint Blockade with a Novel Synthetic Consensus DNA Vaccine Targeting TERT. Mol. Ther. 2018, 26, 435–445. [Google Scholar] [CrossRef]
- Impellizeri, J.A.; Ciliberto, G.; Aurisicchio, L. Electro-gene-transfer as a new tool for cancer immunotherapy in animals. Vet. Comp. Oncol. 2014, 12, 310–318. [Google Scholar] [CrossRef]
- Gabai, V.; Venanzi, F.M.; Bagashova, E.; Rud, O.; Mariotti, F.; Vullo, C.; Catone, G.; Sherman, M.Y.; Concetti, A.; Chursov, A. Pilot study of p62 DNA vaccine in dogs with mammary tumors. Oncotarget 2014, 5, 12803. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Aurisicchio, L.; Impellizeri, J.A.; Cavallo, F. The importance of comparative oncology in translational medicine. Cancer Immunol. Immunother. 2015, 64, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical applications of DNA vaccines: Current progress. Clin. Infect. Dis. 2011, 53, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Flingai, S.; Czerwonko, M.; Goodman, J.; Kudchodkar, S.B.; Muthumani, K.; Weiner, D.B. Synthetic DNA vaccines: Improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front. Immunol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Alvarez, R.D.; Huh, W.K.; Bae, S.; Lamb, L.S., Jr.; Conner, M.G.; Boyer, J.; Wang, C.; Hung, C.F.; Sauter, E.; Paradis, M.; et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol. Oncol. 2016, 140, 245–252. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Preat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Kaneko, S. Telomerase-Targeted Cancer Immunotherapy. Int. J. Mol. Sci. 2019, 20, 1823. [Google Scholar] [CrossRef]
- Collins, K.; Mitchell, J.R. Telomerase in the human organism. Oncogene 2002, 21, 564–579. [Google Scholar] [CrossRef]
- Cong, Y.-S.; Wright, W.E.; Shay, J.W. Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Shay, J.; Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 1997, 33, 787–791. [Google Scholar] [CrossRef]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Grandori, C.; Amacker, M.; Simon-Vermot, N.; Polack, A.; Lingner, J.; Dalla-Favera, R. Direct activation of TERT transcription by c-MYC. Nat. Genet. 1999, 21, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Populo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef]
- Kailashiya, C.; Sharma, H.B.; Kailashiya, J. Telomerase based anticancer immunotherapy and vaccines approaches. Vaccine 2017, 35, 5768–5775. [Google Scholar] [CrossRef]
- Sohn, H.J.; Lee, J.Y.; Lee, H.J.; Sohn, D.H.; Cho, H.I.; Kim, H.J.; Kim, T.G. Simultaneous in vitro generation of CD8 and CD4 T cells specific to three universal tumor associated antigens of WT1, survivin and TERT and adoptive T cell transfer for the treatment of acute myeloid leukemia. Oncotarget 2017, 8, 44059–44072. [Google Scholar] [CrossRef]
- Teixeira, L.; Medioni, J.; Garibal, J.; Adotevi, O.; Doucet, L.; Durey, M.D.; Ghrieb, Z.; Kiladjian, J.J.; Brizard, M.; Laheurte, C.; et al. A First-in-Human Phase I Study of INVAC-1, an Optimized Human Telomerase DNA Vaccine in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 588–597. [Google Scholar] [CrossRef]
- Aurisicchio, L.; Fridman, A.; Mauro, D.; Sheloditna, R.; Chiappori, A.; Bagchi, A.; Ciliberto, G. Safety, tolerability and immunogenicity of V934/V935 hTERT vaccination in cancer patients with selected solid tumors: A phase I study. J. Transl. Med. 2020, 18, 39. [Google Scholar] [CrossRef]
- Thalmensi, J.; Pliquet, E.; Liard, C.; Escande, M.; Bestetti, T.; Julithe, M.; Kostrzak, A.; Pailhes-Jimenez, A.S.; Bourges, E.; Loustau, M.; et al. Anticancer DNA vaccine based on human telomerase reverse transcriptase generates a strong and specific T cell immune response. Oncoimmunology 2016, 5, e1083670. [Google Scholar] [CrossRef]
- Yan, J.; Pankhong, P.; Shin, T.H.; Obeng-Adjei, N.; Morrow, M.P.; Walters, J.N.; Khan, A.S.; Sardesai, N.Y.; Weiner, D.B. Highly optimized DNA vaccine targeting human telomerase reverse transcriptase stimulates potent antitumor immunity. Cancer Immunol. Res. 2013, 1, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, D.; Parodi, A.; Lavieri, R.; Kalli, F.; Ferrera, F.; Tagliamacco, A.; Guastalla, A.; Lamperti, M.G.; Giacomini, M.; Filaci, G. Immunogenicity of GX301 cancer vaccine: Four (telomerase peptides) are better than one. Hum. Vaccin. Immunother. 2015, 11, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Fridman, A.; Finnefrock, A.C.; Peruzzi, D.; Pak, I.; La Monica, N.; Bagchi, A.; Casimiro, D.R.; Ciliberto, G.; Aurisicchio, L. An efficient T-cell epitope discovery strategy using in silico prediction and the iTopia assay platform. Oncoimmunology 2012, 1, 1258–1270. [Google Scholar] [CrossRef][Green Version]
- Adotevi, O.; Mollier, K.; Neuveut, C.; Dosset, M.; Ravel, P.; Fridman, W.H.; Tartour, E.; Charneau, P.; Wain-Hobson, S.; Langlade-Demoyen, P. Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8 T-cell immunity in vivo. Blood 2010, 115, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Vonderheide, R.H. Telomerase as a tumor-associated antigen for cancer immunotherapy. Cytotechnology 2004, 45, 91–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gross, D.A.; Graff-Dubois, S.; Opolon, P.; Cornet, S.; Alves, P.; Bennaceur-Griscelli, A.; Faure, O.; Guillaume, P.; Firat, H.; Chouaib, S.; et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J. Clin. Investig. 2004, 113, 425–433. [Google Scholar] [CrossRef]
- Dosset, M.; Godet, Y.; Vauchy, C.; Beziaud, L.; Lone, Y.C.; Sedlik, C.; Liard, C.; Levionnois, E.; Clerc, B.; Sandoval, F.; et al. Universal cancer peptide-based therapeutic vaccine breaks tolerance against telomerase and eradicates established tumor. Clin. Cancer Res. 2012, 18, 6284–6295. [Google Scholar] [CrossRef]
- Hernandez, J.; Garcia-Pons, F.; Lone, Y.C.; Firat, H.; Schmidt, J.D.; Langlade-Demoyen, P.; Zanetti, M. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc. Natl. Acad. Sci. USA 2002, 99, 12275–12280. [Google Scholar] [CrossRef]
- Brunsvig, P.F.; Aamdal, S.; Gjertsen, M.K.; Kvalheim, G.; Markowski-Grimsrud, C.J.; Sve, I.; Dyrhaug, M.; Trachsel, S.; Moller, M.; Eriksen, J.A.; et al. Telomerase peptide vaccination: A phase I/II study in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2006, 55, 1553–1564. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Korovina, A.N.; Tunitskaya, V.L.; Kostyuk, D.A.; Rechinsky, V.O.; Kukhanova, M.K.; Kochetkov, S.N. Development of the system ensuring a high-level expression of hepatitis C virus nonstructural NS5B and NS5A proteins. Protein Expr. Purif. 2006, 48, 14–23. [Google Scholar] [CrossRef]
- Latanova, A.; Petkov, S.; Kuzmenko, Y.; Kilpelainen, A.; Ivanov, A.; Smirnova, O.; Krotova, O.; Korolev, S.; Hinkula, J.; Karpov, V.; et al. Fusion to Flaviviral Leader Peptide Targets HIV-1 Reverse Transcriptase for Secretion and Reduces Its Enzymatic Activity and Ability to Induce Oxidative Stress but Has No Major Effects on Its Immunogenic Performance in DNA-Immunized Mice. J. Immunol. Res. 2017, 2017, 7407136. [Google Scholar]
- Bayurova, E.; Jansons, J.; Skrastina, D.; Smirnova, O.; Mezale, D.; Kostyusheva, A.; Kostyushev, D.; Petkov, S.; Podschwadt, P.; Valuev-Elliston, V. HIV-1 Reverse Transcriptase Promotes Tumor Growth and Metastasis Formation via ROS-Dependent Upregulation of Twist. Oxidative Med. Cell. Longev. 2019, 2019, 1–28. [Google Scholar] [CrossRef]
- Giry-Laterriere, M.; Verhoeyen, E.; Salmon, P. Lentiviral vectors. Methods Mol. Biol. 2011, 737, 183–209. [Google Scholar]
- Zhang, J.D.; Ruschhaupt, M.; Biczok, R. ddCt Method for qRT–PCR Data Analysis. Available online: https://www.bioconductor.org/packages/devel/bioc/vignettes/ddCt/inst/doc/rtPCR.pdf. (accessed on 27 April 2020).
- Brezgin, S.; Kostyusheva, A.; Bayurova, E.; Gordeychuk, I.; Isaguliants, M.; Goptar, I.; Nikiforova, A.; Smirnov, V.; Volchkova, E.; Glebe, D. Replenishment of Hepatitis B Virus cccDNA Pool Is Restricted by Baseline Expression of Host Restriction Factors In Vitro. Microorganisms 2019, 7, 533. [Google Scholar] [CrossRef]
- Watson, J.V.; Chambers, S.H.; Smith, P.J. A pragmatic approach to the analysis of DNA histograms with a definable G1 peak. Cytom. J. Int. Soc. Anal. Cytol. 1987, 8, 1–8. [Google Scholar] [CrossRef]
- Latanova, A.; Petkov, S.; Kilpelainen, A.; Jansons, J.; Latyshev, O.; Kuzmenko, Y.; Hinkula, J.; Abakumov, M.; Valuev-Elliston, V.; Gomelsky, M. Codon optimization and improved delivery/immunization regimen enhance the immune response against wild-type and drug-resistant hiv-1 reverse transcriptase, preserving its th2-polarity. Sci. Rep. 2018, 8, 8078. [Google Scholar] [CrossRef]
- Petkov, S.; Starodubova, E.; Latanova, A.; Kilpelainen, A.; Latyshev, O.; Svirskis, S.; Wahren, B.; Chiodi, F.; Gordeychuk, I.; Isaguliants, M. DNA immunization site determines the level of gene expression and the magnitude, but not the type of the induced immune response. PLoS ONE 2018, 13, e0197902. [Google Scholar] [CrossRef]
- Abolins, A.; Vanags, A.; Trofimovics, G.; Miklasevics, E.; Gardovskis, J.; Strumfa, I. Molecular subtype shift in breast cancer upon trastuzumab treatment: A case report. Pol. J. Pathol. 2011, 62, 65–68. [Google Scholar]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Zvereva, M.I.; Shcherbakova, D.M.; Dontsova, O.A. Telomerase: Structure, functions, and activity regulation. Biochemistry (Mosc) 2010, 75, 1563–1583. [Google Scholar] [CrossRef]
- Rubtsova, M.P.; Vasilkova, D.P.; Malyavko, A.N.; Naraikina, Y.V.; Zvereva, M.I.; Dontsova, O.A. Telomere lengthening and other functions of telomerase. Acta Nat. 2012, 4, 44–61. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Liu, N.; Wei, Q.; Jiang, L.; Tong, X.; Ye, X. Polo-like Kinase 1 (Plk1) Up-regulates Telomerase Activity by Affecting Human Telomerase Reverse Transcriptase (hTERT) Stability. J. Biol. Chem. 2015, 290, 18865–18873. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.M.; Kang, M.R.; Oh, S.Y.; Lee, T.H.; Muller, M.T.; Chung, I.K. Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT. Genes Dev. 2005, 19, 776–781. [Google Scholar] [CrossRef]
- Jung, H.Y.; Wang, X.; Jun, S.; Park, J.I. Dyrk2-associated EDD-DDB1-VprBP E3 ligase inhibits telomerase by TERT degradation. J. Biol. Chem. 2013, 288, 7252–7262. [Google Scholar] [CrossRef]
- Georgoulis, A.; Vorgias, C.E.; Chrousos, G.P.; Rogakou, E.P. Genome Instability and gammaH2AX. Int. J. Mol. Sci. 2017, 18, 1979. [Google Scholar] [CrossRef]
- Dai, C.; Sun, F.; Zhu, C.; Hu, X. Tumor environmental factors glucose deprivation and lactic acidosis induce mitotic chromosomal instability--an implication in aneuploid human tumors. PLoS ONE 2013, 8, e63054. [Google Scholar] [CrossRef]
- Matouk, I.J.; Mezan, S.; Mizrahi, A.; Ohana, P.; Abu-Lail, R.; Fellig, Y.; Degroot, N.; Galun, E.; Hochberg, A. The oncofetal H19 RNA connection: Hypoxia, p53 and cancer. Biochim. Biophys. Acta 2010, 1803, 443–451. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef]
- Ribeyre, C.; Shore, D. Regulation of telomere addition at DNA double-strand breaks. Chromosoma 2013, 122, 159–173. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Baklaushev, V.P.; Kilpelainen, A.; Petkov, S.; Abakumov, M.A.; Grinenko, N.F.; Yusubalieva, G.M.; Latanova, A.A.; Gubskiy, I.L.; Zabozlaev, F.G.; Starodubova, E.S.; et al. Luciferase Expression Allows Bioluminescence Imaging But Imposes Limitations on the Orthotopic Mouse (4T1) Model of Breast Cancer. Sci. Rep. 2017, 7, 7715. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, S.; Donia, M.; Thor Straten, P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron 2013, 6, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Impellizeri, J.A.; Gavazza, A.; Greissworth, E.; Crispo, A.; Montella, M.; Ciliberto, G.; Lubas, G.; Aurisicchio, L. Tel-eVax: A genetic vaccine targeting telomerase for treatment of canine lymphoma. J. Transl. Med. 2018, 16, 349. [Google Scholar] [CrossRef] [PubMed]
- Lue, N.F.; Lin, Y.C.; Mian, I.S. A conserved telomerase motif within the catalytic domain of telomerase reverse transcriptase is specifically required for repeat addition processivity. Mol. Cell Biol. 2003, 23, 8440–8449. [Google Scholar] [CrossRef]
- Aznar, M.A.; Tinari, N.; Rullan, A.J.; Sanchez-Paulete, A.R.; Rodriguez-Ruiz, M.E.; Melero, I. Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally. J. Immunol. 2017, 198, 31–39. [Google Scholar] [CrossRef]
- Hidema, S.; Fukuda, T.; Date, S.; Tokitake, Y.; Matsui, Y.; Sasaki, H.; Nishimori, K. Transgenic expression of Telomerase reverse transcriptase (Tert) improves cell proliferation of primary cells and enhances reprogramming efficiency into the induced pluripotent stem cell. Biosci. Biotechnol. Biochem. 2016, 80, 1925–1933. [Google Scholar] [CrossRef]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Lai, C.K.; Collins, K. Two purified domains of telomerase reverse transcriptase reconstitute sequence-specific interactions with RNA. J. Biol. Chem. 2005, 280, 17533–17539. [Google Scholar] [CrossRef]
- Brown, A.F.; Podlevsky, J.D.; Qi, X.; Chen, Y.; Xie, M.; Chen, J.J.-L. A self-regulating template in human telomerase. Proc. Natl. Acad. Sci. USA 2014, 111, 11311–11316. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural biology of telomeres and telomerase. Cell Mol. Life Sci. 2020, 77, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Segal-Bendirdjian, E.; Geli, V. Non-canonical Roles of Telomerase: Unraveling the Imbroglio. Front. Cell Dev. Biol. 2019, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef]
- Shkreli, M.; Sarin, K.Y.; Pech, M.F.; Papeta, N.; Chang, W.; Brockman, S.A.; Cheung, P.; Lee, E.; Kuhnert, F.; Olson, J.L. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat. Med. 2012, 18, 111. [Google Scholar] [CrossRef]
- Diala, I.; Wagner, N.; Magdinier, F.; Shkreli, M.; Sirakov, M.; Bauwens, S.; Schluth-Bolard, C.; Simonet, T.; Renault, V.M.; Ye, J. Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep. 2013, 14, 356–363. [Google Scholar] [CrossRef]
- Ibata, M.; Takahashi, T.; Shimizu, T.; Inoue, Y.; Maeda, S.; Tashiro-Yamaji, J.; Okada, M.; Ueda, K.; Kubota, T.; Yoshida, R. Spontaneous rejection of intradermally transplanted non-engineered tumor cells by neutrophils and macrophages from syngeneic strains of mice. Microbiol. Immunol. 2011, 55, 726–735. [Google Scholar]
- Musiani, P.; Allione, A.; Modica, A.; Lollini, P.L.; Giovarelli, M.; Cavallo, F.; Belardelli, F.; Forni, G.; Modesti, A. Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-alpha, IFN-gamma, and TNF-alpha. Lab. Investig. J. Tech. Methods Pathol. 1996, 74, 146–157. [Google Scholar]
- Hoo, W.S.; Lundeen, K.A.; Kohrumel, J.R.; Pham, N.-L.; Brostoff, S.W.; Bartholomew, R.M.; Carlo, D.J. Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J. Immunol. 1999, 162, 7343–7349. [Google Scholar]
- Ibe, S.; Qin, Z.; Schuler, T.; Preiss, S.; Blankenstein, T. Tumor rejection by disturbing tumor stroma cell interactions. J. Exp. Med. 2001, 194, 1549–1559. [Google Scholar] [CrossRef]
- Tsung, K.; Dolan, J.P.; Tsung, Y.L.; Norton, J.A. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 2002, 62, 5069–5075. [Google Scholar] [PubMed]
- Inoue, Y.; Tashiro-Yamaji, J.; Hayashi, M.; Kiyonari, H.; Shimizu, T.; Ibata, M.; Yamana, H.; Kubota, T.; Tanigawa, N.; Yoshida, R. Transgene number-dependent, gene expression rate-independent rejection of Dd-, Kd-, or DdKd-transgened mouse skin or tumor cells from C57BL/6 (DbKb) mice. Microbiol. Immunol. 2011, 55, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Briesemeister, D.; Sommermeyer, D.; Loddenkemper, C.; Loew, R.; Uckert, W.; Blankenstein, T.; Kammertoens, T. Tumor rejection by local interferon gamma induction in established tumors is associated with blood vessel destruction and necrosis. Int. J. Cancer 2011, 128, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.V.; Regnier, F.; Feuillet, V.; Vimeux, L.; Weiss, J.M.; Bismuth, G.; Altan-Bonnet, G.; Guilbert, T.; Thoreau, M.; Finisguerra, V. TGFβ blocks IFNα/β release and tumor rejection in spontaneous mammary tumors. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Sheehan, K.C.; Shankaran, V.; Uppaluri, R.; Bui, J.D.; Diamond, M.S.; Koebel, C.M.; Arthur, C.; White, J.M. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005, 6, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef]
- Tatematsu, M.; Funami, K.; Seya, T.; Matsumoto, M. Extracellular RNA Sensing by Pattern Recognition Receptors. J. Innate Immun. 2018, 10, 398–406. [Google Scholar] [CrossRef]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Bek, S.; Stritzke, F.; Wintges, A.; Nedelko, T.; Bohmer, D.F.R.; Fischer, J.C.; Haas, T.; Poeck, H.; Heidegger, S. Targeting intrinsic RIG-I signaling turns melanoma cells into type I interferon-releasing cellular antitumor vaccines. Oncoimmunology 2019, 8, e1570779. [Google Scholar] [CrossRef]
- Ruzicka, M.; Koenig, L.M.; Formisano, S.; Boehmer, D.F.; Vick, B.; Heuer, E.-M.; Meinl, H.; Kocheise, L.; Zeitlhöfler, M.; Ahlfeld, J. RIG-I-based immunotherapy enhances survival in preclinical AML models and sensitizes AML cells to checkpoint blockade. Leukemia 2020, 34, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Rigby, R.E.; Webb, L.M.; Mackenzie, K.J.; Li, Y.; Leitch, A.; Reijns, M.A.; Lundie, R.J.; Revuelta, A.; Davidson, D.J.; Diebold, S.; et al. RNA: DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 2014, 33, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Shen, Y.-L.; Hsia, H.-Y.; Tiang, Y.-P.; Sung, T.-L.; Chen, L.-Y. Extrachromosomal telomere repeat DNA is linked to ALT development via cGAS-STING DNA sensing pathway. Nat. Struct. Mol. Biol. 2017, 24, 1124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, Z.; Dahmane, N.; Tsai, K.; Wang, P.; Williams, D.R.; Kossenkov, A.V.; Showe, L.C.; Zhang, R.; Huang, Q. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6293–E6300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lieberman, P.M. The crosstalk of telomere dysfunction and inflammation through cell-free TERRA containing exosomes. RNA Biol. 2016, 13, 690–695. [Google Scholar] [CrossRef]
- Toubiana, S.; Selig, S. DNA: RNA hybrids at telomeres—When it is better to be out of the (R) loop. FEBS J. 2018, 285, 2552–2566. [Google Scholar] [CrossRef]
- Matsuo, M.; Wada, H.; Honda, S.; Tawara, I.; Uenaka, A.; Kanematsu, T.; Nakayama, E. Expression of multiple unique rejection antigens on murine leukemia BALB/c RLmale symbol1 and the role of dominant Akt antigen for tumor escape. J. Immunol. 1999, 162, 6420–6425. [Google Scholar]
- Bremnes, R.M.; Al-Shibli, K.; Donnem, T.; Sirera, R.; Al-Saad, S.; Andersen, S.; Stenvold, H.; Camps, C.; Busund, L.T. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 824–833. [Google Scholar] [CrossRef]
- Marshall, E.A.; Ng, K.W.; Kung, S.H.; Conway, E.M.; Martinez, V.D.; Halvorsen, E.C.; Rowbotham, D.A.; Vucic, E.A.; Plumb, A.W.; Becker-Santos, D.D.; et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol. Cancer 2016, 15, 67. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Jorritsma, S.; Gowans, E.; Grubor-Bauk, B.; Wijesundara, D. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Thalmensi, J.; Pliquet, E.; Liard, C.; Chamel, G.; Kreuz, C.; Bestetti, T.; Escande, M.; Kostrzak, A.; Pailhes-Jimenez, A.S.; Bourges, E.; et al. A DNA telomerase vaccine for canine cancer immunotherapy. Oncotarget 2019, 10, 3361–3372. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Southworth, L.K.; Sarin, K.Y.; Venteicher, A.S.; Ma, W.; Chang, W.; Cheung, P.; Jun, S.; Artandi, M.K.; Shah, N.; et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008, 4, e10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tergaonkar, V. Noncanonical functions of telomerase: Implications in telomerase-targeted cancer therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Huang, C.; He, X.; Tian, Y.Y.; Zhou, D.X.; He, Y.; Liu, X.H.; Li, J. Feedback regulation of telomerase reverse transcriptase: New insight into the evolving field of telomerase in cancer. Cell Signal. 2013, 25, 2462–2468. [Google Scholar] [CrossRef]
- Ding, D.; Xi, P.; Zhou, J.; Wang, M.; Cong, Y.S. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-kappaB-dependent transcription. FASEB J. 2013, 27, 4375–4383. [Google Scholar] [CrossRef]
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.K.; et al. Telomerase directly regulates NF-kappaB-dependent transcription. Nat. Cell Biol. 2012, 14, 1270–1281. [Google Scholar] [CrossRef]
- Koh, C.M.; Khattar, E.; Leow, S.C.; Liu, C.Y.; Muller, J.; Ang, W.X.; Li, Y.; Franzoso, G.; Li, S.; Guccione, E.; et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J. Clin. Investig. 2015, 125, 2109–2122. [Google Scholar] [CrossRef]
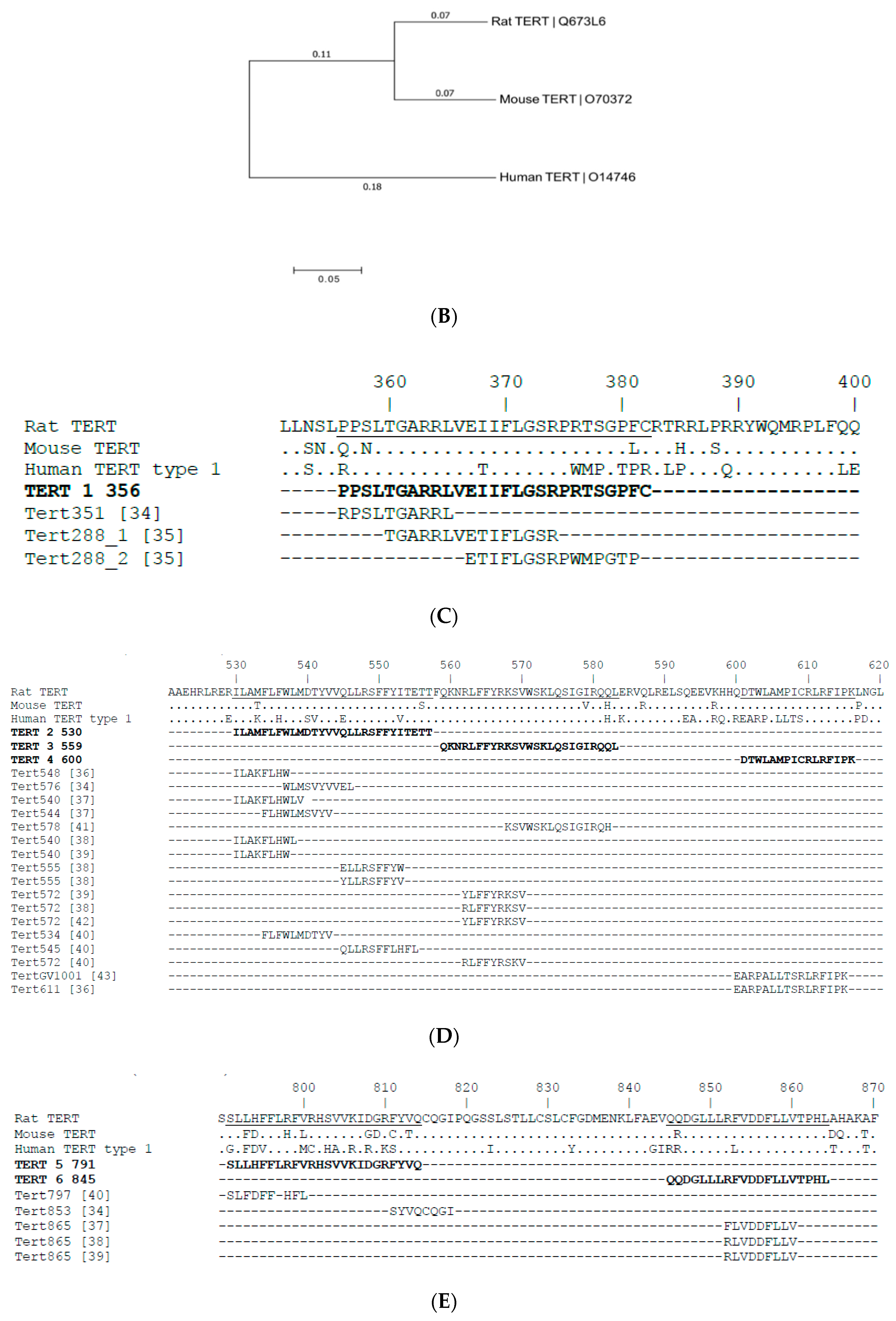






| Name | Amino Acid (aa) Sequence | Position in Rat TERT, 1st and Last aa Residue | Identity to Mouse TERT, Common aa/Total aa (%) | Identity to Human TERT, Unique aa/Total aa (%) | Class I Score for H2 Db Mice (IEDB) | Class I Score for H2 Dd Mice (IEDB) | The Highest Class II Scores (IEDB) | T Cell Epitopes Localized in the Region |
|---|---|---|---|---|---|---|---|---|
| TERT 1 | PPSLTGARRLVEIIFLGSRPRTSGPFC | 356–382 | 24/27 (89) | 19/27 (70) | 0.47 | 0.58 | 1.28–1.33 | [30,31] |
| TERT 2 | ILAMFLFWLMDTYVVQLLRSFFYITETT | 530–558 | 26/28 (93) | 22/28 (79) | 0.3727 | 0.349 | 6.48–9.01 | [30,32,33,34,35,36] |
| TERT 3 | QKNRLFFYRKSVWSKLQSIGIRQQL | 559–584 | 24/25 (96) | 24/25 (96) | −0.185 | −0.268 | 2.65–7.13 | [30,34,35,36,37,38] |
| TERT 4 | DTWLAMPICRLRFIPK | 600–616 | 16/16 (100) | 7/16 (44) | 0.31 | 0.19 | 9.44 | [32,39] |
| TERT 5 | SLLHFFLRFVRHSVVKIDGRFYVQ | 791–815 | 16/24 (67) | 12/24 (50) | 0.379 | 0.4973 | 9.4 | [30,36] |
| TERT 6 | QQDGLLLRFVDDFLLVTPHL | 845–865 | 19/20 (95) | 17/20 (85) | 0.32 | 0.438 | >10 | [33,34,35] |
| TERT 7 | KTVVNFPVETGALGGAAPHQLPAHCLFPW | 888–917 | 26/29 (90) | 22/29 (76) | 0.2196 | 0.5080 | 7.75–10.73 | [30,31,37] |
| TERT 8 | LGGAAPHQLPAHCLFPWCGLLLDTRTLE | 901–929 | 26/28 (93) | 23/28 (82) | 0.355 | 0.333 | 11.25 | [30,31,33,37] |
| TERT 9 | FLDLQVNSLQTVCINIYKIFLLQAYRFHACVI | 973–1001 | 32/32 (100) | 29/32 (90) | 0.176 | 0.1579 | 7.81–10.77 | [30,31,33,34,35,36,38] |
| Clones of 4T1luc2 Cells with rtTERT Inserts, Full Name | Abbreviated Name | Nn of Genomic Copies of rtTERT by Actb | Nn of Genomic Copies of rtTERT by Mstn | rtTERT mRNA Expression Relative to HPRT1 |
|---|---|---|---|---|
| 4T1luc2_rtTERT_B5 | B5 | 0.57 ± 0.08 | 0.45 ± 0.02 | 1.00 ± 0.00 |
| 4T1luc2_rtTERT_C6 | C6 | 1.33 ± 0.14 | 1.05 ± 0.09 | 6.56 ± 1.63 |
| 4T1luc2_rtTERT_H9 * | H9 | 2.96 ± 0.24 | 1.66 ± 0.19 | 15.28 ± 1.72 |
| 4T1luc2_rtTERT_F1 | F1 | 0.70 ± 0.06 | 0.70 ± 0.06 | 5.95 ± 0.70 |
| 4T1luc2 | 4T1luc2 | N/A | N/A | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansons, J.; Bayurova, E.; Skrastina, D.; Kurlanda, A.; Fridrihsone, I.; Kostyushev, D.; Kostyusheva, A.; Artyuhov, A.; Dashinimaev, E.; Avdoshina, D.; et al. Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells. Vaccines 2020, 8, 318. https://doi.org/10.3390/vaccines8020318
Jansons J, Bayurova E, Skrastina D, Kurlanda A, Fridrihsone I, Kostyushev D, Kostyusheva A, Artyuhov A, Dashinimaev E, Avdoshina D, et al. Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells. Vaccines. 2020; 8(2):318. https://doi.org/10.3390/vaccines8020318
Chicago/Turabian StyleJansons, Juris, Ekaterina Bayurova, Dace Skrastina, Alisa Kurlanda, Ilze Fridrihsone, Dmitry Kostyushev, Anastasia Kostyusheva, Alexander Artyuhov, Erdem Dashinimaev, Darya Avdoshina, and et al. 2020. "Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells" Vaccines 8, no. 2: 318. https://doi.org/10.3390/vaccines8020318
APA StyleJansons, J., Bayurova, E., Skrastina, D., Kurlanda, A., Fridrihsone, I., Kostyushev, D., Kostyusheva, A., Artyuhov, A., Dashinimaev, E., Avdoshina, D., Kondrashova, A., Valuev-Elliston, V., Latyshev, O., Eliseeva, O., Petkov, S., Abakumov, M., Hippe, L., Kholodnyuk, I., Starodubova, E., ... Isaguliants, M. (2020). Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells. Vaccines, 8(2), 318. https://doi.org/10.3390/vaccines8020318








