A Comparison of Intramuscular and Subcutaneous Administration of LigA Subunit Vaccine Adjuvanted with Neutral Liposomal Formulation Containing Monophosphoryl Lipid A and QS21
Abstract
1. Introduction
2. Materials and Methods
2.1. Hamsters
2.2. Leptospira Culture
2.3. LMQ Preparation
2.4. Recombinant LigAc Protein Production
2.5. SDS-PAGE and Western Blotting
2.6. Immunization and Challenge
2.7. Histopathology Determination
2.8. Detection of Viable Leptospires
2.9. Quantitative Real-Time PCR (qPCR)
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results
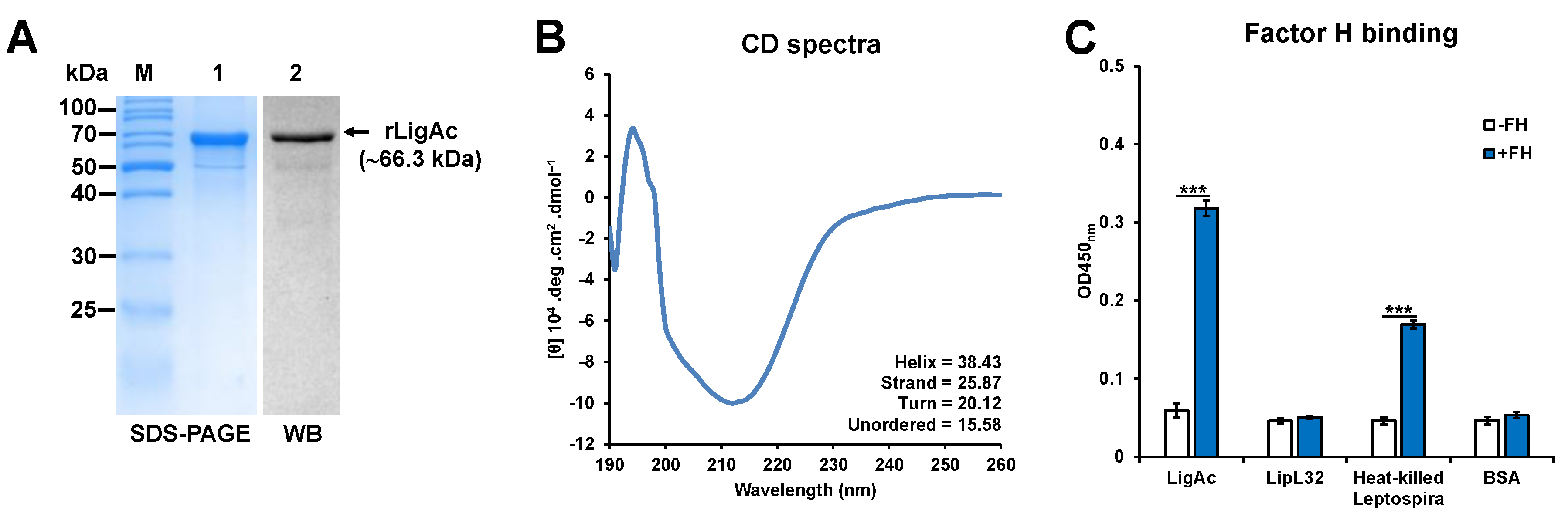
3.1. Preparation and Characterization of rLigAc
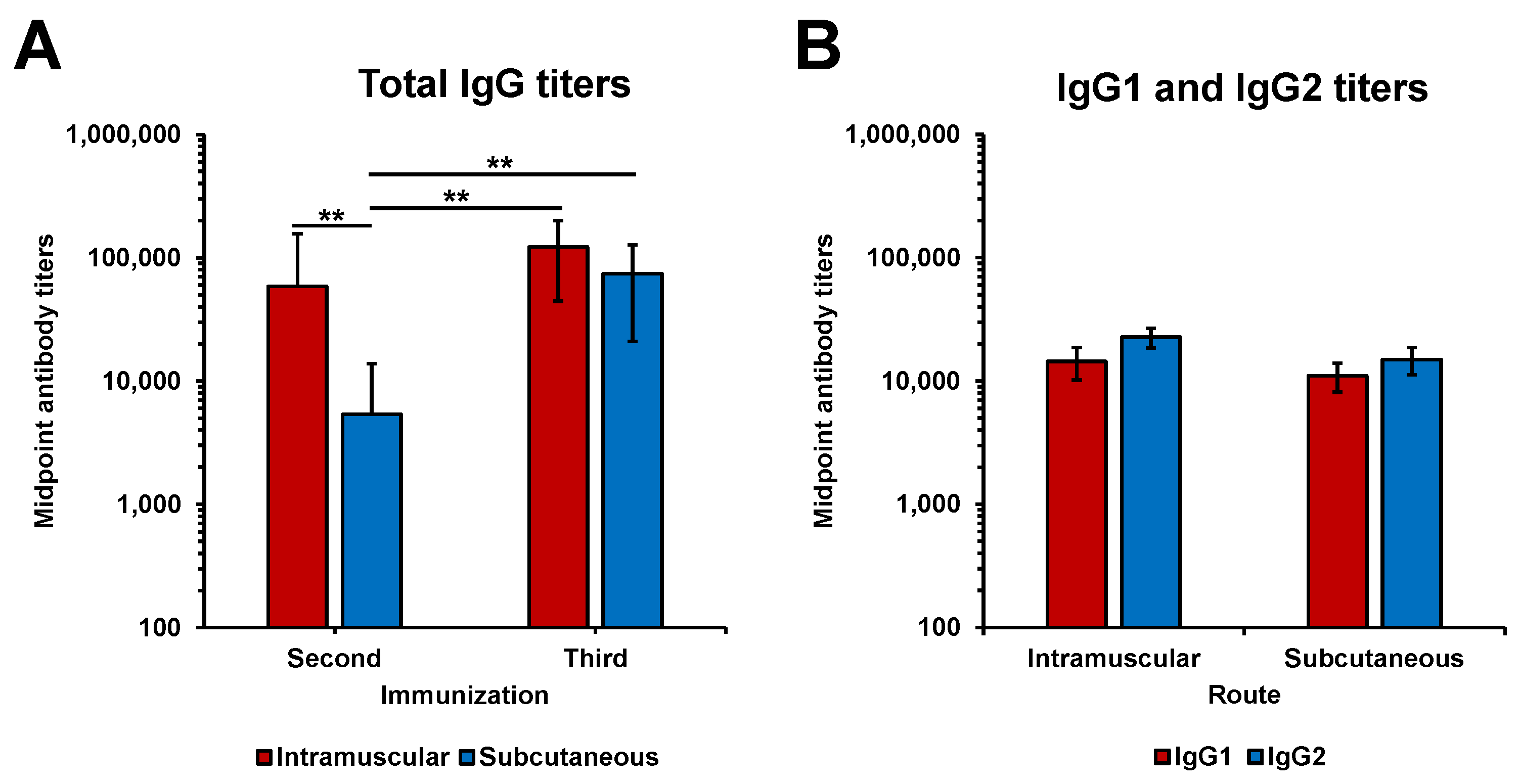
3.2. Immunogenicity of rLigAc-LMQ in Hamsters
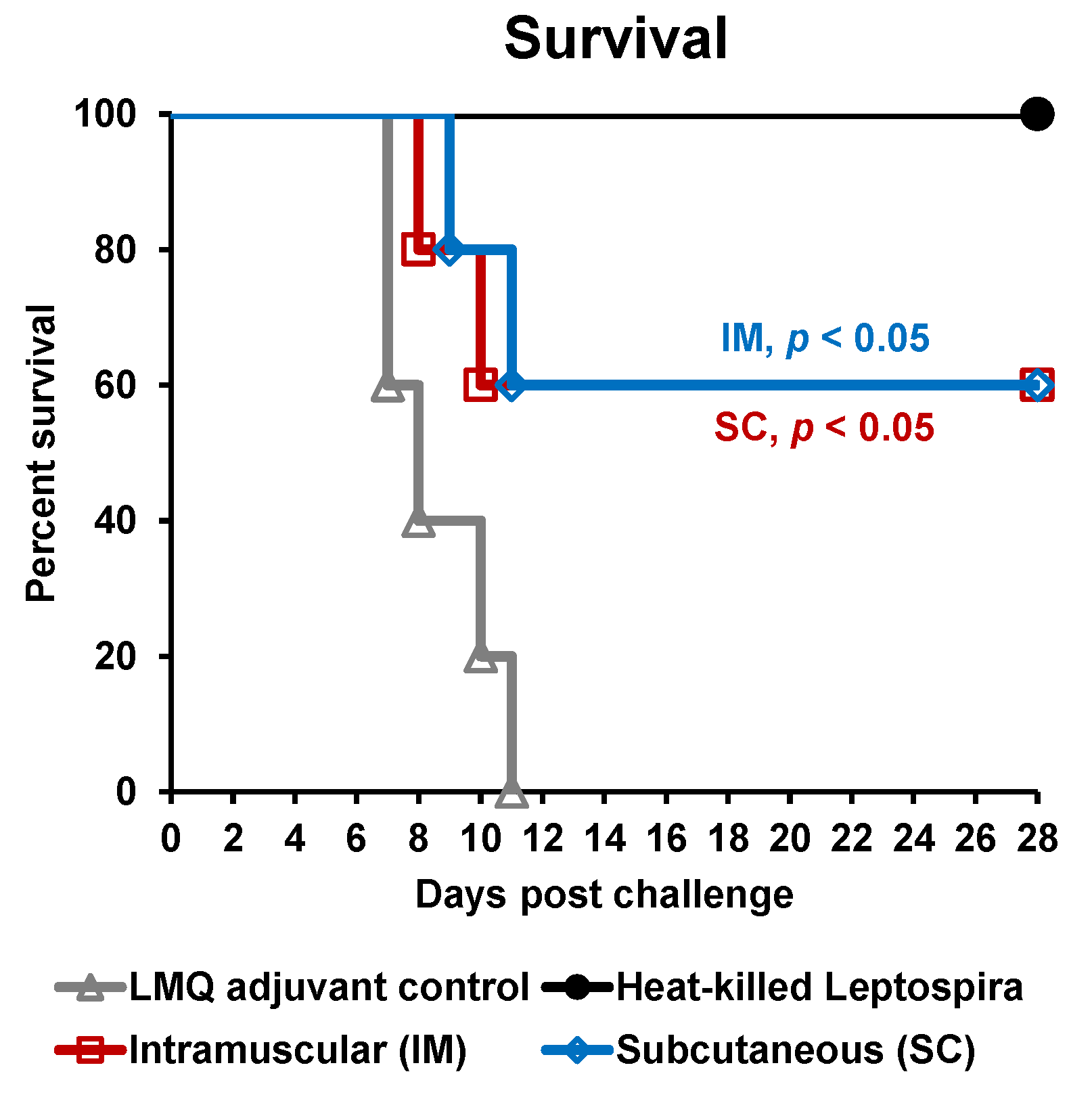
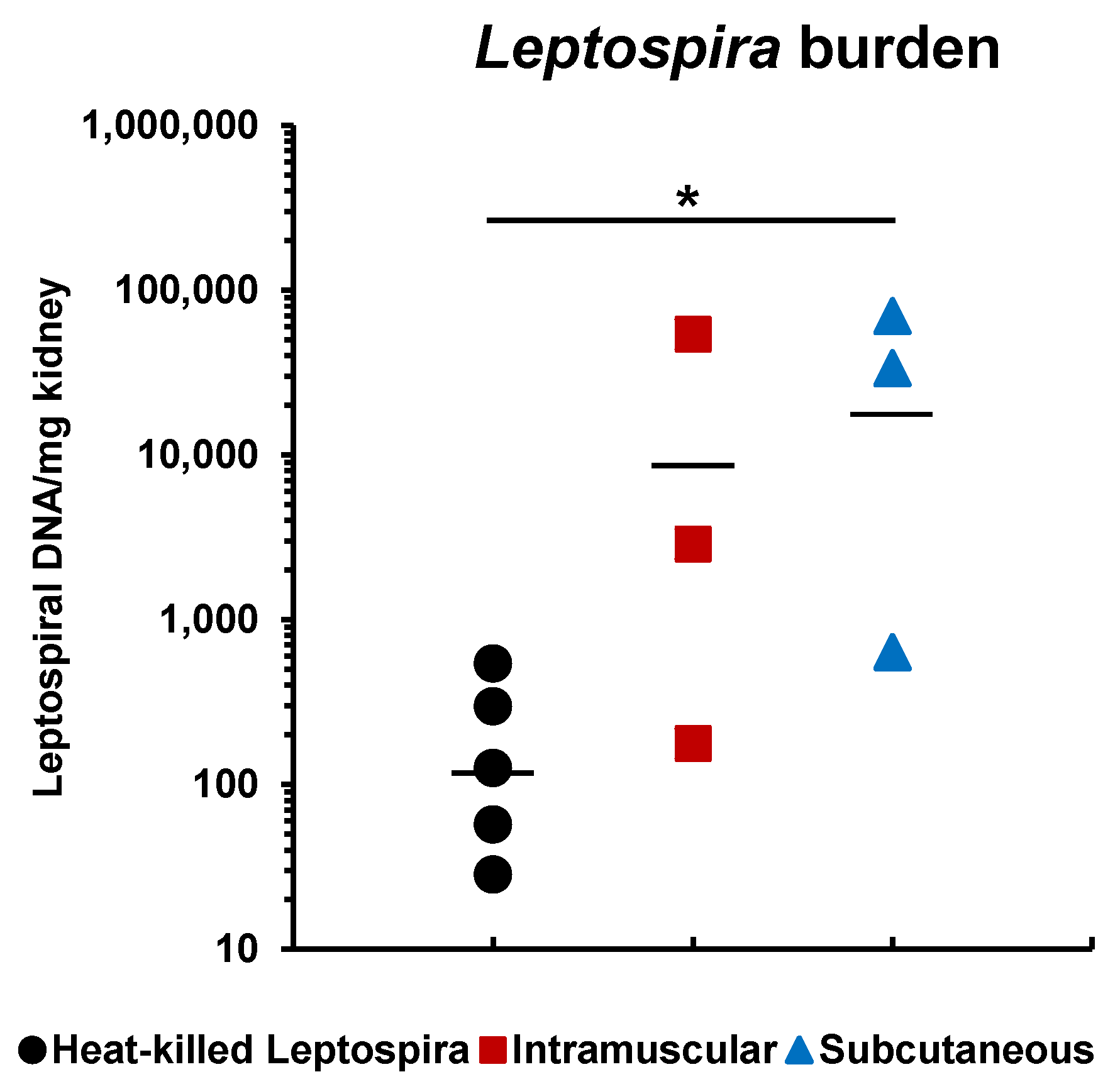
3.3. Protective Efficacy of rLigAc-LMQ in Hamsters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Adler, B.; de la Pena Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Evangelista, K.V.; Coburn, J. Leptospira as an emerging pathogen: A review of its biology, pathogenesis and host immune responses. Future Microbiol. 2010, 5, 1413–1425. [Google Scholar] [CrossRef]
- Adler, B. Vaccines Against Leptospirosis. In Leptospira and Leptospirosis; Adler, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 251–272. [Google Scholar] [CrossRef]
- Coutinho, M.L.; Choy, H.A.; Kelley, M.M.; Matsunaga, J.; Babbitt, J.T.; Lewis, M.S.; Aleixo, J.A.; Haake, D.A. A LigA three-domain region protects hamsters from lethal infection by Leptospira interrogans. PLoS Neglect. Trop. Dis. 2011, 5, e1422. [Google Scholar] [CrossRef]
- Evangelista, K.V.; Lourdault, K.; Matsunaga, J.; Haake, D.A. Immunoprotective properties of recombinant LigA and LigB in a hamster model of acute leptospirosis. PLoS ONE 2017, 12, e0180004. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.F.; Medeiros, M.A.; McBride, A.J.; Matsunaga, J.; Esteves, G.S.; Ramos, J.G.; Santos, C.S.; Croda, J.; Homma, A.; Dellagostin, O.A.; et al. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine 2007, 25, 6277–6286. [Google Scholar] [CrossRef] [PubMed]
- Forster, K.M.; Hartwig, D.D.; Seixas, F.K.; Bacelo, K.L.; Amaral, M.; Hartleben, C.P.; Dellagostin, O.A. A conserved region of leptospiral immunoglobulin-like A and B proteins as a DNA vaccine elicits a prophylactic immune response against leptospirosis. Clin. Vaccine Immunol. 2013, 20, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.M.; Yan, W.; Chen, C.S.; Palaniappan, R.U.; McDonough, S.P.; Chang, Y.F. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine 2008, 26, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Monaris, D.; Sbrogio-Almeida, M.E.; Dib, C.C.; Canhamero, T.A.; Souza, G.O.; Vasconcellos, S.A.; Ferreira, L.C.; Abreu, P.A. Protective Immunity and Reduced Renal Colonization Induced by Vaccines Containing Recombinant Leptospira interrogans Outer Membrane Proteins and Flagellin Adjuvant. Clin. Vaccine Immunol. 2015, 22, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Techawiwattanaboon, T.; Barnier-Quer, C.; Palaga, T.; Jacquet, A.; Collin, N.; Sangjun, N.; Komanee, P.; Piboonpocanun, S.; Patarakul, K. Reduced Renal Colonization and Enhanced Protection by Leptospiral Factor H Binding Proteins as a Multisubunit Vaccine Against Leptospirosis in Hamsters. Vaccines 2019, 7, 95. [Google Scholar] [CrossRef]
- Fernandes, L.G.V.; Teixeira, A.F.; Filho, A.F.S.; Souza, G.O.; Vasconcellos, S.A.; Heinemann, M.B.; Romero, E.C.; Nascimento, A. Immune response and protective profile elicited by a multi-epitope chimeric protein derived from Leptospira interrogans. Int. J. Infect. Dis. 2017, 57, 61–69. [Google Scholar] [CrossRef]
- Lucas, D.S.; Cullen, P.A.; Lo, M.; Srikram, A.; Sermswan, R.W.; Adler, B. Recombinant LipL32 and LigA from Leptospira are unable to stimulate protective immunity against leptospirosis in the hamster model. Vaccine 2011, 29, 3413–3418. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, C.E.P.; Bettin, E.B.; Bakry, A.; Seixas Neto, A.C.P.; Amaral, M.G.; Dellagostin, O.A. Evaluation of different strategies to promote a protective immune response against leptospirosis using a recombinant LigA and LigB chimera. Vaccine 2019, 37, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Lourdault, K.; Wang, L.C.; Vieira, A.; Matsunaga, J.; Melo, R.; Lewis, M.S.; Haake, D.A.; Gomes-Solecki, M. Oral immunization with Escherichia coli expressing a lipidated form of LigA protects hamsters against challenge with Leptospira interrogans serovar Copenhageni. Infect. Immun. 2014, 82, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.M.; Yan, W.; McDonough, S.P.; Chang, Y.F. Leptospira immunoglobulin-like protein A variable region (LigAvar) incorporated in liposomes and PLGA microspheres produces a robust immune response correlating to protective immunity. Vaccine 2009, 27, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bacelo, K.L.; Hartwig, D.D.; Seixas, F.K.; Schuch, R.; Moreira Ada, S.; Amaral, M.; Collares, T.; Vendrusculo, C.T.; McBride, A.J.; Dellagostin, O.A. Xanthan gum as an adjuvant in a subunit vaccine preparation against leptospirosis. Biomed. Res. Int. 2014, 2014, 636491. [Google Scholar] [CrossRef]
- Cook, I.F. Evidence based route of administration of vaccines. Hum. Vaccines 2008, 4, 67–73. [Google Scholar] [CrossRef]
- Cook, I.F.; Barr, I.; Hartel, G.; Pond, D.; Hampson, A.W. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 2006, 24, 2395–2402. [Google Scholar] [CrossRef]
- Laurichesse, H.; Gourdon, F.; Smits, H.L.; Abdoe, T.H.; Estavoyer, J.M.; Rebika, H.; Pouliquen, P.; Catalina, P.; Dubray, C.; Beytout, J. Safety and immunogenicity of subcutaneous or intramuscular administration of a monovalent inactivated vaccine against Leptospira interrogans serogroup Icterohaemorrhagiae in healthy volunteers. Clin. Microbiol. Infect. 2007, 13, 395–403. [Google Scholar] [CrossRef]
- Diez-Domingo, J.; Weinke, T.; Garcia de Lomas, J.; Meyer, C.U.; Bertrand, I.; Eymin, C.; Thomas, S.; Sadorge, C. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged >/=50 years: A randomised non-inferiority clinical trial. Vaccine 2015, 33, 789–795. [Google Scholar] [CrossRef]
- Tiner, B.L.; Sha, J.; Ponnusamy, D.; Baze, W.B.; Fitts, E.C.; Popov, V.L.; van Lier, C.J.; Erova, T.E.; Chopra, A.K. Intramuscular Immunization of Mice with a Live-Attenuated Triple Mutant of Yersinia pestis CO92 Induces Robust Humoral and Cell-Mediated Immunity To Completely Protect Animals against Pneumonic Plague. Clin. Vaccine Immunol. 2015, 22, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Liu, Q.; Jia, J.; Liu, Y.; Zhang, W.; Ma, G.; Su, Z. Polycation-decorated PLA microspheres induce robust immune responses via commonly used parenteral administration routes. Int. Immunopharmacol. 2014, 23, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kang, J.; Ning, H.; Wang, L.; Xu, Y.; Xue, Y.; Xu, Z.; Wu, X.; Bai, Y. Immunological characteristics of Mycobacterium tuberculosis subunit vaccines immunized through different routes. Microb. Pathog. 2018, 125, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; McDonough, S.P.; Sharma, Y.; Chang, Y.F. The terminal immunoglobulin-like repeats of LigA and LigB of Leptospira enhance their binding to gelatin binding domain of fibronectin and host cells. PLoS ONE 2010, 5, e11301. [Google Scholar] [CrossRef] [PubMed]
- Srikram, A.; Zhang, K.; Bartpho, T.; Lo, M.; Hoke, D.E.; Sermswan, R.W.; Adler, B.; Murray, G.L. Cross-protective immunity against leptospirosis elicited by a live, attenuated lipopolysaccharide mutant. J. Infect. Dis. 2011, 203, 870–879. [Google Scholar] [CrossRef]
- Zuerner, R.L. Laboratory maintenance of pathogenic Leptospira. Curr. Protocols Microbiol. 2005. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Felix, C.R.; Siedler, B.S.; Barbosa, L.N.; Timm, G.R.; McFadden, J.; McBride, A.J.A. An overview of human leptospirosis vaccine design and future perspectives. Expert Opin. Drug Discov. 2020, 15, 179–188. [Google Scholar] [CrossRef]
- Oliveira, T.L.; Bacelo, K.L.; Schuch, R.A.; Seixas, F.K.; Collares, T.; Rodrigues, O.E.; Vargas, J.; Nascimento, R.O.; Dellagostin, O.A.; Hartwig, D.D. Immune response in hamsters immunised with a recombinant fragment of LigA from Leptospira interrogans, associated with carrier molecules. Memorias Inst. Oswaldo Cruz 2016, 111, 712–716. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef]
- Namvarpour, M.; Tebianian, M.; Mansouri, R.; Ebrahimi, S.M.; Kashkooli, S. Comparison of different immunization routes on the immune responses induced by Mycobacterium tuberculosis ESAT-6/CFP-10 recombinant protein. Biologicals 2019, 59, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Loré, K. Local innate immune responses in the vaccine adjuvant-injected muscle. Clin. Transl. Immunol. 2016, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Tamoutounour, S.; Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.M. The Capillaries of the Skin: A Review. J. Investig. Dermatol. 1938, 1, 295–311. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Cheng, G.; Wei, H.Y.; Zeng, Q. Encapsulation, pharmacokinetics and tissue distribution of interferon alpha-2b liposomes after intramuscular injection to rats. Arch. Pharm. Res. 2011, 34, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, D.; Slutter, B.; Henriksen-Lacey, M.; Jiskoot, W.; Bouwstra, J.A.; Perrie, Y.; Kundig, T.M.; Gander, B.; Johansen, P. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J. Control Release 2010, 147, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.W.; Marshall, J.S.; Ulrich, J.T. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int. Arch. Allergy Immunol. 2001, 126, 135–139. [Google Scholar] [CrossRef]
- Moore, A.; McCarthy, L.; Mills, K.H. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 1999, 17, 2517–2527. [Google Scholar] [CrossRef]
- Hartwig, D.D.; Bacelo, K.L.; Oliveira, P.D.; Oliveira, T.L.; Seixas, F.K.; Amaral, M.G.; Hartleben, C.P.; McBride, A.J.; Dellagostin, O.A. Mannosylated LigANI produced in Pichia pastoris protects hamsters against leptospirosis. Curr. Microbiol. 2014, 68, 524–530. [Google Scholar] [CrossRef]
- Lambert, P.-H.; Liu, M.; Siegrist, C.-A. Can successful vaccines teach us how to induce efficient protective immune responses? Nat. Med. 2005, 11, S54–S62. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Lloyd, M. Conduct of Minor Procedures. In Handbook of Laboratory Animal Management and Welfare, 3rd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2003; pp. 150–181. [Google Scholar]
| Group | Antigen | Dose | Adjuvant | Volume | Route |
|---|---|---|---|---|---|
| Control | Tris buffer | – | LMQ | 250 µL | Subcutaneous |
| HK | Heat-killed Leptospira | 108 cells | Freund’s | 250 µL | Subcutaneous |
| IM | rLigAc | 20 µg | LMQ | 75 µL in each hind leg | Intramuscular |
| SC | rLigAc | 20 µg | LMQ | 250 µL | Subcutaneous |
| Group a | Protection b | Endpoint Days | Positive Culture c | Pathology Score d | |||
|---|---|---|---|---|---|---|---|
| Blood | Kidney | Lung | Liver | Kidney | |||
| Control | 0% | 7, 7, 8, 10, 11 | ND | ND | ND | ND | ND |
| HK | 100% ** | 28, 28, 28, 28, 28 | 0/5 | 1/5 | 1, 1, 1, 1, 1 | 0, 0, 0, 1, 2 | 0, 0, 0, 0, 0 |
| IM | 60% * | 8, 8, 28, 28, 28 | 0/3 | 2/3 | 1, 2, 2 * | 0, 1, 2 | 0, 0, 0 |
| SC | 60% * | 9, 11, 28, 28, 28 | 0/3 | 2/3 | 2, 2, 2 ** | 1, 1, 1 | 1, 1, 1 **,# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Techawiwattanaboon, T.; Barnier-Quer, C.; Palaga, T.; Jacquet, A.; Collin, N.; Sangjun, N.; Komanee, P.; Patarakul, K. A Comparison of Intramuscular and Subcutaneous Administration of LigA Subunit Vaccine Adjuvanted with Neutral Liposomal Formulation Containing Monophosphoryl Lipid A and QS21. Vaccines 2020, 8, 494. https://doi.org/10.3390/vaccines8030494
Techawiwattanaboon T, Barnier-Quer C, Palaga T, Jacquet A, Collin N, Sangjun N, Komanee P, Patarakul K. A Comparison of Intramuscular and Subcutaneous Administration of LigA Subunit Vaccine Adjuvanted with Neutral Liposomal Formulation Containing Monophosphoryl Lipid A and QS21. Vaccines. 2020; 8(3):494. https://doi.org/10.3390/vaccines8030494
Chicago/Turabian StyleTechawiwattanaboon, Teerasit, Christophe Barnier-Quer, Tanapat Palaga, Alain Jacquet, Nicolas Collin, Noppadon Sangjun, Pat Komanee, and Kanitha Patarakul. 2020. "A Comparison of Intramuscular and Subcutaneous Administration of LigA Subunit Vaccine Adjuvanted with Neutral Liposomal Formulation Containing Monophosphoryl Lipid A and QS21" Vaccines 8, no. 3: 494. https://doi.org/10.3390/vaccines8030494
APA StyleTechawiwattanaboon, T., Barnier-Quer, C., Palaga, T., Jacquet, A., Collin, N., Sangjun, N., Komanee, P., & Patarakul, K. (2020). A Comparison of Intramuscular and Subcutaneous Administration of LigA Subunit Vaccine Adjuvanted with Neutral Liposomal Formulation Containing Monophosphoryl Lipid A and QS21. Vaccines, 8(3), 494. https://doi.org/10.3390/vaccines8030494





