Pre-Erythrocytic Vaccines against Malaria
Abstract
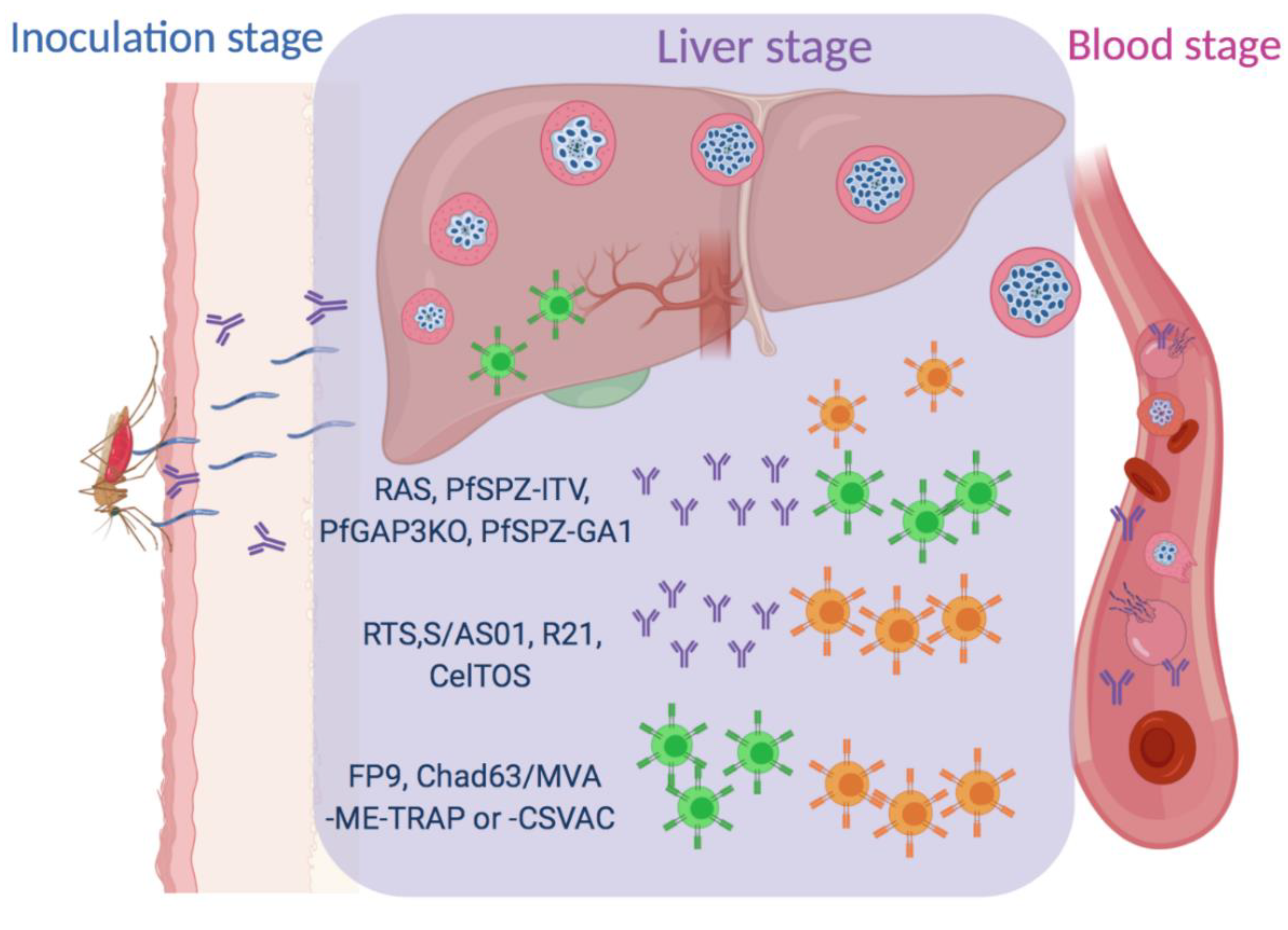
:1. Introduction
2. Subunit Vaccines
2.1. RTS, S/AS01 Vaccine
2.2. R21
2.3. Viral-Vectored Vaccines
2.4. CelTOS
3. Whole Sporozoite Vaccines
3.1. PfSPZ with Chemoprophylaxis (PfSPZ ITV)
3.2. Radiation Attenuated Sporozoites (RAS)
3.3. Genetically-Attenuated Parasites (GAPs)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Malaria Eradication: Benefits, Future Scenarios & Feasibility. In A Report of the Strategic Advisory Group on Malaria Eradication; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Crompton, P.D.; Moebius, J.; Portugal, S.; Waisberg, M.; Hart, G.; Garver, L.S.; Miller, L.H.; Barillas-Mury, C.; Pierce, S.K. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu. Rev. Immunol. 2014, 32, 157–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.M.; Li, S.; Doumbo, S.; Doumtabe, D.; Huang, C.Y.; Dia, S.; Bathily, A.; Sangala, J.; Kone, Y.; Traore, A.; et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin. Infect. Dis. 2013, 57, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Odongo-Aginya, E.; Ssegwanyi, G.; Kategere, P.; Vuzi, P.C. Relationship between malaria infection intensity and rainfall pattern in Entebbe peninsula, Uganda. Afr. Health Sci. 2005, 5, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Midekisa, A.; Beyene, B.; Mihretie, A.; Bayabil, E.; Wimberly, M.C. Seasonal associations of climatic drivers and malaria in the highlands of Ethiopia. Parasites Vectors 2015, 8, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saute, F.; Aponte, J.; Almeda, J.; Ascaso, C.; Vaz, N.; Dgedge, M.; Alonso, P. Malaria in southern Mozambique: Incidence of clinical malaria in children living in a rural community in Manhica district. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 655–660. [Google Scholar] [CrossRef]
- Kurup, S.P.; Butler, N.S.; Harty, J.T. T cell-mediated immunity to malaria. Nat. Rev. Immunol. 2019, 19, 457–471. [Google Scholar] [CrossRef]
- Liehl, P.; Zuzarte-Luis, V.; Chan, J.; Zillinger, T.; Baptista, F.; Carapau, D.; Konert, M.; Hanson, K.K.; Carret, C.; Lassnig, C.; et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat. Med. 2014, 20, 47–53. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B.; Nico, D. The Delay in the Licensing of Protozoal Vaccines: A Comparative History. Front. Immunol. 2020, 11, 204. [Google Scholar] [CrossRef]
- Aide, P.; Bassat, Q.; Alonso, P.L. Towards an effective malaria vaccine. Arch. Dis. Child. 2007, 92, 476–479. [Google Scholar] [CrossRef] [Green Version]
- Holz, L.E.; Fernandez-Ruiz, D.; Heath, W.R. Protective immunity to liver-stage malaria. Clin. Transl. Immunol. 2016, 5, e105. [Google Scholar] [CrossRef]
- Garcia, J.E.; Puentes, A.; Patarroyo, M.E. Developmental biology of sporozoite-host interactions in Plasmodium falciparum malaria: Implications for vaccine design. Clin. Microbiol. Rev. 2006, 19, 686–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menard, R. The journey of the malaria sporozoite through its hosts: Two parasite proteins lead the way. Microbes Infect. 2000, 2, 633–642. [Google Scholar] [CrossRef]
- Pradel, G.; Garapaty, S.; Frevert, U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol. Microbiol. 2002, 45, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Rathore, D.; McCutchan, T.F.; Garboczi, D.N.; Toida, T.; Hernaiz, M.J.; LeBrun, L.A.; Lang, S.C.; Linhardt, R.J. Direct measurement of the interactions of glycosaminoglycans and a heparin decasaccharide with the malaria circumsporozoite protein. Biochemistry 2001, 40, 11518–11524. [Google Scholar] [CrossRef] [PubMed]
- McCutchan, T.F.; Kissinger, J.C.; Touray, M.G.; Rogers, M.J.; Li, J.; Sullivan, M.; Braga, E.M.; Krettli, A.U.; Miller, L.H. Comparison of circumsporozoite proteins from avian and mammalian malarias: Biological and phylogenetic implications. Proc. Natl. Acad. Sci. USA 1996, 93, 11889–11894. [Google Scholar] [CrossRef] [Green Version]
- Good, M.F.; Pombo, D.; Quakyi, I.A.; Riley, E.M.; Houghten, R.A.; Menon, A.; Alling, D.W.; Berzofsky, J.A.; Miller, L.H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: Immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc. Natl. Acad. Sci. USA 1988, 85, 1199–1203. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, A.; Cifuentes, G.; Guzman, F.; Salazar, L.M.; Patarroyo, M.E. Immunogenicity and protectivity of Plasmodium falciparum EBA-175 peptide and its analog is associated with alpha-helical region shortening and displacement. Biol. Chem. 2003, 384, 1443–1450. [Google Scholar] [CrossRef]
- Cohen, J.; Nussenzweig, V.; Nussenzweig, R.; Vekemans, J.; Leach, A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccines 2010, 6, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.M.; McGovern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; LaChance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 1995, 171, 1576–1585. [Google Scholar] [CrossRef]
- White, M.T.; Bejon, P.; Olotu, A.; Griffin, J.T.; Riley, E.M.; Kester, K.E.; Ockenhouse, C.F.; Ghani, A.C. The relationship between RTS, S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS ONE 2013, 8, e61395. [Google Scholar] [CrossRef] [Green Version]
- Casares, S.; Brumeanu, T.D.; Richie, T.L. The RTS, S malaria vaccine. Vaccine 2010, 28, 4880–4894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Schwenk, R.; White, K.; Stoute, J.A.; Cohen, J.; Ballou, W.R.; Voss, G.; Kester, K.E.; Heppner, D.G.; Krzych, U. Protective immunity induced with malaria vaccine, RTS, S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J. Immunol. 2003, 171, 6961–6967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valea, I.; Adjei, S.; Usuf, E.; Traore, O.; Ansong, D.; Tinto, H.; Owusu Boateng, H.; Some, A.M.; Buabeng, P.; Vekemans, J.; et al. Long-term immunogenicity and immune memory response to the hepatitis B antigen in the RTS, S/AS01E malaria vaccine in African children: A randomized trial. Hum. Vaccines Immunother. 2020, 16, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassijns, J.; Bollaerts, K.; Baay, M.; Verstraeten, T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine 2016, 34, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tejada, A.; Chea, E.K.; George, C.; Gardner, J.R.; Livingston, P.O.; Ragupathi, G.; Tan, D.S.; Gin, D.Y. Design, synthesis, and immunologic evaluation of vaccine adjuvant conjugates based on QS-21 and tucaresol. Bioorganic Med. Chem. 2014, 22, 5917–5923. [Google Scholar] [CrossRef] [Green Version]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroux-Roels, G.; Leroux-Roels, I.; Clement, F.; Ofori-Anyinam, O.; Lievens, M.; Jongert, E.; Moris, P.; Ballou, W.R.; Cohen, J. Evaluation of the immune response to RTS, S/AS01 and RTS, S/AS02 adjuvanted vaccines: Randomized, double-blind study in malaria-naive adults. Hum. Vaccines Immunother. 2014, 10, 2211–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asante, K.P.; Abdulla, S.; Agnandji, S.; Lyimo, J.; Vekemans, J.; Soulanoudjingar, S.; Owusu, R.; Shomari, M.; Leach, A.; Jongert, E.; et al. Safety and efficacy of the RTS, S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect. Dis. 2011, 11, 741–749. [Google Scholar] [CrossRef]
- Molina-Franky, J.; Cuy-Chaparro, L.; Camargo, A.; Reyes, C.; Gomez, M.; Salamanca, D.R.; Patarroyo, M.A.; Patarroyo, M.E. Plasmodium falciparum pre-erythrocytic stage vaccine development. Malar. J. 2020, 19, 56. [Google Scholar] [CrossRef]
- The RTS, S Clinical Trials Partnership. Efficacy and Safety of the RTS, S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014, 11. [Google Scholar] [CrossRef]
- Bejon, P.; Lusingu, J.; Olotu, A.; Leach, A.; Lievens, M.; Vekemans, J.; Mshamu, S.; Lang, T.; Gould, J.; Dubois, M.C.; et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 2008, 359, 2521–2532. [Google Scholar] [CrossRef] [Green Version]
- Otieno, L.; Guerra Mendoza, Y.; Adjei, S.; Agbenyega, T.; Agnandji, S.T.; Aide, P.; Akoo, P.; Ansong, D.; Asante, K.P.; Berkley, J.A.; et al. Safety and immunogenicity of the RTS, S/AS01 malaria vaccine in infants and children identified as HIV-infected during a randomized trial in sub-Saharan Africa. Vaccine 2020, 38, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Agnandji, S.T.; Asante, K.P.; Lyimo, J.; Vekemans, J.; Soulanoudjingar, S.S.; Owusu, R.; Shomari, M.; Leach, A.; Fernandes, J.; Dosoo, D.; et al. Evaluation of the safety and immunogenicity of the RTS, S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J. Infect. Dis. 2010, 202, 1076–1087. [Google Scholar] [CrossRef]
- Mota, M.M.; Pradel, G.; Vanderberg, J.P.; Hafalla, J.C.; Frevert, U.; Nussenzweig, R.S.; Nussenzweig, V.; Rodriguez, A. Migration of Plasmodium sporozoites through cells before infection. Science 2001, 291, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, L.M.; Coppi, A.; Snounou, G.; Sinnis, P. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 2007, 9, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Kimani, D.; Jagne, Y.J.; Cox, M.; Kimani, E.; Bliss, C.M.; Gitau, E.; Ogwang, C.; Afolabi, M.O.; Bowyer, G.; Collins, K.A.; et al. Translating the immunogenicity of prime-boost immunization with ChAd63 and MVA ME-TRAP from malaria naive to malaria-endemic populations. Mol. Ther. 2014, 22, 1992–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthy, V.S.; McConkey, S.; Roberts, M.; Gothard, P.; Arulanantham, N.; Degano, P.; Schneider, J.; Hannan, C.; Roy, M.; Gilbert, S.C.; et al. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine 2003, 21, 1995–2002. [Google Scholar] [CrossRef]
- Rathore, D.; Sacci, J.B.; de la Vega, P.; McCutchan, T.F. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J. Biol. Chem. 2002, 277, 7092–7098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongfen, S.E.; Ntsama, P.M.; Offner, S.; Smith, T.; Felger, I.; Tanner, M.; Alonso, P.; Nebie, I.; Romero, J.F.; Silvie, O.; et al. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine 2009, 27, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.; Weilenman, C.; Audran, R.; Roggero, M.A.; Bonelo, A.; Tiercy, J.M.; Spertini, F.; Corradin, G. A synthetic malaria vaccine elicits a potent CD8(+) and CD4(+) T lymphocyte immune response in humans. Implications for vaccination strategies. Eur. J. Immunol. 2001, 31, 1989–1998. [Google Scholar] [CrossRef]
- Audran, R.; Lurati-Ruiz, F.; Genton, B.; Blythman, H.E.; Ofori-Anyinam, O.; Reymond, C.; Corradin, G.; Spertini, F. The synthetic Plasmodium falciparum circumsporozoite peptide PfCS102 as a malaria vaccine candidate: A randomized controlled phase I trial. PLoS ONE 2009, 4, e7304. [Google Scholar] [CrossRef]
- Langowski, M.D.; Khan, F.A.; Bitzer, A.A.; Genito, C.J.; Schrader, A.J.; Martin, M.L.; Soto, K.; Zou, X.; Hadiwidjojo, S.; Beck, Z.; et al. Optimization of a Plasmodium falciparum circumsporozoite protein repeat vaccine using the tobacco mosaic virus platform. Proc. Natl. Acad. Sci. USA 2020, 117, 3114–3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schijns, V.E.; Lavelle, E.C. Trends in vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Heppner, D.G., Jr.; Kester, K.E.; Ockenhouse, C.F.; Tornieporth, N.; Ofori, O.; Lyon, J.A.; Stewart, V.A.; Dubois, P.; Lanar, D.E.; Krzych, U.; et al. Towards an RTS, S-based, multi-stage, multi-antigen vaccine against falciparum malaria: Progress at the Walter Reed Army Institute of Research. Vaccine 2005, 23, 2243–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caro-Aguilar, I.; Rodriguez, A.; Calvo-Calle, J.M.; Guzman, F.; De la Vega, P.; Patarroyo, M.E.; Galinski, M.R.; Moreno, A. Plasmodium vivax promiscuous T-helper epitopes defined and evaluated as linear peptide chimera immunogens. Infect. Immun. 2002, 70, 3479–3492. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef] [Green Version]
- Safety, Immunogenicity and Efficacy of R21 Matrix-M in 5–17 Month Old Children in Nanoro, Burkina Faso. Available online: https://clinicaltrials.gov/ct2/show/NCT03896724 (accessed on 20 July 2020).
- Schneider, J.; Gilbert, S.C.; Blanchard, T.J.; Hanke, T.; Robson, K.J.; Hannan, C.M.; Becker, M.; Sinden, R.; Smith, G.L.; Hill, A.V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 1998, 4, 397–402. [Google Scholar] [CrossRef]
- Webster, D.P.; Dunachie, S.; McConkey, S.; Poulton, I.; Moore, A.C.; Walther, M.; Laidlaw, S.M.; Peto, T.; Skinner, M.A.; Gilbert, S.C.; et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine 2006, 24, 3026–3034. [Google Scholar] [CrossRef]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef] [Green Version]
- Degano, P.; Schneider, J.; Hannan, C.M.; Gilbert, S.C.; Hill, A.V. Gene gun intradermal DNA immunization followed by boosting with modified vaccinia virus Ankara: Enhanced CD8+ T cell immunogenicity and protective efficacy in the influenza and malaria models. Vaccine 1999, 18, 623–632. [Google Scholar] [CrossRef]
- Tiono, A.B.; Nebie, I.; Anagnostou, N.; Coulibaly, A.S.; Bowyer, G.; Lam, E.; Bougouma, E.C.; Ouedraogo, A.; Yaro, J.B.B.; Barry, A.; et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5-17 months old infants and children. PLoS ONE 2018, 13, e0208328. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; O’Hara, G.A.; Duncan, C.J.; Collins, K.A.; Sheehy, S.H.; Reyes-Sandoval, A.; Goodman, A.L.; Edwards, N.J.; Elias, S.C.; Halstead, F.D.; et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat. Commun. 2013, 4, 2836. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, S.H.; Ewer, K.J.; Bliss, C.M.; Edwards, N.J.; Rampling, T.; Anagnostou, N.A.; de Barra, E.; Havelock, T.; Bowyer, G.; Poulton, I.D.; et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J. Infect. Dis. 2015, 211, 1076–1086. [Google Scholar] [CrossRef]
- Rampling, T.; Ewer, K.J.; Bowyer, G.; Edwards, N.J.; Wright, D.; Sridhar, S.; Payne, R.; Powlson, J.; Bliss, C.; Venkatraman, N.; et al. Safety and efficacy of novel malaria vaccine regimens of RTS, S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP. NPJ Vaccines 2018, 3, 49. [Google Scholar] [CrossRef]
- de Barra, E.; Hodgson, S.H.; Ewer, K.J.; Bliss, C.M.; Hennigan, K.; Collins, A.; Berrie, E.; Lawrie, A.M.; Gilbert, S.C.; Nicosia, A.; et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLoS ONE 2014, 9, e115161. [Google Scholar] [CrossRef] [PubMed]
- Halbroth, B.R.; Sebastian, S.; Salman, A.M.; Ulaszewska, M.; Gola, A.; Longley, R.J.; Janse, C.J.; Khan, S.M.; Hill, A.V.S.; Spencer, A.J. Preclinical Development and Assessment of Viral Vectors Expressing a Fusion Antigen of Plasmodium falciparum LSA1 and LSAP2 for Efficacy against Liver-Stage Malaria. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, C.J.; Ware, L.A.; Barbosa, A.; Angov, E.; Lyon, J.A.; Heppner, D.G.; Lanar, D.E. Process development and analysis of liver-stage antigen 1, a preerythrocyte-stage protein-based vaccine for Plasmodium falciparum. Infect. Immun. 2005, 73, 2109–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikolajczak, S.A.; Sacci, J.B., Jr.; De La Vega, P.; Camargo, N.; VanBuskirk, K.; Krzych, U.; Cao, J.; Jacobs-Lorena, M.; Cowman, A.F.; Kappe, S.H. Disruption of the Plasmodium falciparum liver-stage antigen-1 locus causes a differentiation defect in late liver-stage parasites. Cell Microbiol. 2011, 13, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Kariu, T.; Ishino, T.; Yano, K.; Chinzei, Y.; Yuda, M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol. Microbiol. 2006, 59, 1369–1379. [Google Scholar] [CrossRef]
- Jimah, J.R.; Salinas, N.D.; Sala-Rabanal, M.; Jones, N.G.; Sibley, L.D.; Nichols, C.G.; Schlesinger, P.H.; Tolia, N.H. Malaria parasite CelTOS targets the inner leaflet of cell membranes for pore-dependent disruption. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamriz, S.; Ofoghi, H. Expression of Recombinant PfCelTOS Antigen in the Chloroplast of Chlamydomonas reinhardtii and its Potential Use in Detection of Malaria. Mol. Biotechnol. 2019, 61, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Legler, P.M.; Savranskaya, T.; Ockenhouse, C.F.; Angov, E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine 2011, 29, 5940–5949. [Google Scholar] [CrossRef] [PubMed]
- Pirahmadi, S.; Zakeri, S.; Mehrizi, A.A.; Djadid, N.D.; Raz, A.A.; Sani, J.J. Combining Monophosphoryl Lipid A (MPL), CpG Oligodeoxynucleotide (ODN), and QS-21 Adjuvants Induces Strong and Persistent Functional Antibodies and T Cell Responses against Cell-Traversal Protein for Ookinetes and Sporozoites (CelTOS) of Plasmodium falciparum in BALB/c Mice. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, C.L.; Birkett, A.J.; Moore, A.C.; Hill, A.V. Combination of protein and viral vaccines induces potent cellular and humoral immune responses and enhanced protection from murine malaria challenge. Infect. Immun. 2007, 75, 5819–5826. [Google Scholar] [CrossRef] [Green Version]
- Nussenzweig, R.S.; Krettli, A.U. Breakthroughs towards a malaria vaccine. Hist Cienc Saude Manguinhos 2011, 18, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Orjih, A.U.; Cochrane, A.H.; Nussenzweig, R.S. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 57–61. [Google Scholar] [CrossRef]
- Belnoue, E.; Costa, F.T.; Frankenberg, T.; Vigario, A.M.; Voza, T.; Leroy, N.; Rodrigues, M.M.; Landau, I.; Snounou, G.; Renia, L. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 2004, 172, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Mordmuller, B.; Surat, G.; Lagler, H.; Chakravarty, S.; Ishizuka, A.S.; Lalremruata, A.; Gmeiner, M.; Campo, J.J.; Esen, M.; Ruben, A.J.; et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017, 542, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, M.; Teirlinck, A.C.; McCall, M.B.; Teelen, K.; Makamdop, K.N.; Wiersma, J.; Arens, T.; Beckers, P.; van Gemert, G.; van de Vegte-Bolmer, M.; et al. Long-term protection against malaria after experimental sporozoite inoculation: An open-label follow-up study. Lancet 2011, 377, 1770–1776. [Google Scholar] [CrossRef]
- Walk, J.; Reuling, I.J.; Behet, M.C.; Meerstein-Kessel, L.; Graumans, W.; van Gemert, G.J.; Siebelink-Stoter, R.; van de Vegte-Bolmer, M.; Janssen, T.; Teelen, K.; et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: A double-blind randomized controlled clinical trial. BMC Med. 2017, 15, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richie, T.L.; Billingsley, P.F.; Sim, B.K.; James, E.R.; Chakravarty, S.; Epstein, J.E.; Lyke, K.E.; Mordmuller, B.; Alonso, P.; Duffy, P.E.; et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 2015, 33, 7452–7461. [Google Scholar] [CrossRef]
- White, N.J. Antimalarial drug resistance. J. Clin. Invest. 2004, 113, 1084–1092. [Google Scholar] [CrossRef]
- Nussenzweig, R.S.; Vanderberg, J.; Most, H.; Orton, C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 1967, 216, 160–162. [Google Scholar] [CrossRef]
- Kurup, S.P.; Anthony, S.M.; Hancox, L.S.; Vijay, R.; Pewe, L.L.; Moioffer, S.J.; Sompallae, R.; Janse, C.J.; Khan, S.M.; Harty, J.T. Monocyte-Derived CD11c(+) Cells Acquire Plasmodium from Hepatocytes to Prime CD8 T Cell Immunity to Liver-Stage Malaria. Cell Host Microbe 2019, 25, 565–577. [Google Scholar] [CrossRef]
- Hoffman, S.L.; Goh, L.M.; Luke, T.C.; Schneider, I.; Le, T.P.; Doolan, D.L.; Sacci, J.; de la Vega, P.; Dowler, M.; Paul, C.; et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 2002, 185, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Doolan, D.L.; Hoffman, S.L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 1999, 163, 884–892. [Google Scholar] [PubMed]
- Sissoko, M.S.; Healy, S.A.; Katile, A.; Omaswa, F.; Zaidi, I.; Gabriel, E.E.; Kamate, B.; Samake, Y.; Guindo, M.A.; Dolo, A.; et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect. Dis. 2017, 17, 498–509. [Google Scholar] [CrossRef]
- Epstein, J.E.; Richie, T.L. The whole parasite, pre-erythrocytic stage approach to malaria vaccine development: A review. Curr. Opin. Infect. Dis. 2013, 26, 420–428. [Google Scholar] [CrossRef]
- Seder, R.A.; Chang, L.J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef] [Green Version]
- McColm, A.A.; Dalton, L. Heterologous immunity in rodent malaria: Comparison of the degree of cross-immunity generated by vaccination with that produced by exposure to live infection. Ann. Trop. Med. Parasitol. 1983, 77, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Vanderberg, J.P.; Frevert, U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 2004, 34, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, M.; McCall, M.; Hopman, J.; Wiersma, J.; Luty, A.J.; van Gemert, G.J.; van de Vegte-Bolmer, M.; van Schaijk, B.; Teelen, K.; Arens, T.; et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009, 361, 468–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, S.L.; Billingsley, P.F.; James, E.; Richman, A.; Loyevsky, M.; Li, T.; Chakravarty, S.; Gunasekera, A.; Chattopadhyay, R.; Li, M.; et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccines 2010, 6, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, A.K.; Labaied, M.; Kappe, S.H.; Matuschewski, K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 2005, 433, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.M.; Wang, R.; Kappe, S.H. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum. Vaccines 2010, 6, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Kublin, J.G.; Mikolajczak, S.A.; Sack, B.K.; Fishbaugher, M.E.; Seilie, A.; Shelton, L.; VonGoedert, T.; Firat, M.; Magee, S.; Fritzen, E.; et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Butler, N.S.; Schmidt, N.W.; Vaughan, A.M.; Aly, A.S.; Kappe, S.H.; Harty, J.T. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 2011, 9, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczak, S.A.; Silva-Rivera, H.; Peng, X.; Tarun, A.S.; Camargo, N.; Jacobs-Lorena, V.; Daly, T.M.; Bergman, L.W.; de la Vega, P.; Williams, J.; et al. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol. Cell Biol. 2008, 28, 6196–6207. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, K.; Matuschewski, K.; Camargo, N.; Ross, J.; Kappe, S.H. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol. Microbiol. 2004, 51, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Tarun, A.S.; Dumpit, R.F.; Camargo, N.; Labaied, M.; Liu, P.; Takagi, A.; Wang, R.; Kappe, S.H.I. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J. Infect. Dis. 2007, 196, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobe, O.; Donofrio, G.; Sun, G.; Liepinsh, D.; Schwenk, R.; Krzych, U. Immunization with radiation-attenuated Plasmodium berghei sporozoites induces liver cCD8alpha+DC that activate CD8+T cells against liver-stage malaria. PLoS ONE 2009, 4, e5075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annoura, T.; van Schaijk, B.C.; Ploemen, I.H.; Sajid, M.; Lin, J.W.; Vos, M.W.; Dinmohamed, A.G.; Inaoka, D.K.; Rijpma, S.R.; van Gemert, G.J.; et al. Two Plasmodium 6-Cys family-related proteins have distinct and critical roles in liver-stage development. FASEB J. 2014, 28, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, S.A.; Swearingen, K.E.; Martinson, T.; Steel, R.; Dankwa, D.A.; Harupa, A.; Camargo, N.; Betz, W.; Vigdorovich, V.; Oliver, B.G.; et al. The Micronemal Plasmodium Proteins P36 and P52 Act in Concert to Establish the Replication-Permissive Compartment Within Infected Hepatocytes. Front. Cell. Infect. Microbiol. 2018, 8, 413. [Google Scholar] [CrossRef] [Green Version]
- van Schaijk, B.C.; Ploemen, I.H.; Annoura, T.; Vos, M.W.; Foquet, L.; van Gemert, G.J.; Chevalley-Maurel, S.; van de Vegte-Bolmer, M.; Sajid, M.; Franetich, J.F.; et al. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. eLife 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Labaied, M.; Harupa, A.; Dumpit, R.F.; Coppens, I.; Mikolajczak, S.A.; Kappe, S.H. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 2007, 75, 3758–3768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annoura, T.; Ploemen, I.H.; van Schaijk, B.C.; Sajid, M.; Vos, M.W.; van Gemert, G.J.; Chevalley-Maurel, S.; Franke-Fayard, B.M.; Hermsen, C.C.; Gego, A.; et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine 2012, 30, 2662–2670. [Google Scholar] [CrossRef]
- Aly, A.S.; Mikolajczak, S.A.; Rivera, H.S.; Camargo, N.; Jacobs-Lorena, V.; Labaied, M.; Coppens, I.; Kappe, S.H. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol. Microbiol. 2008, 69, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Spring, M.; Murphy, J.; Nielsen, R.; Dowler, M.; Bennett, J.W.; Zarling, S.; Williams, J.; de la Vega, P.; Ware, L.; Komisar, J.; et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 2013, 31, 4975–4983. [Google Scholar] [CrossRef]
- Roestenberg, M.; Walk, J.; van der Boor, S.C.; Langenberg, M.C.C.; Hoogerwerf, M.A.; Janse, J.J.; Manurung, M.; Yap, X.Z.; Garcia, A.F.; Koopman, J.P.R.; et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Kappe, S.H.; Vaughan, A.M.; Boddey, J.A.; Cowman, A.F. That was then but this is now: Malaria research in the time of an eradication agenda. Science 2010, 328, 862–866. [Google Scholar] [CrossRef]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, E6736–E6743. [Google Scholar] [CrossRef] [Green Version]
- Kudyba, H.M.; Cobb, D.W.; Florentin, A.; Krakowiak, M.; Muralidharan, V. CRISPR/Cas9 Gene Editing to Make Conditional Mutants of Human Malaria Parasite, P. falciparum. J. Vis. Exp. 2018, 139, 57747. [Google Scholar] [CrossRef]
- Lee, M.C.S.; Lindner, S.E.; Lopez-Rubio, J.J.; Llinas, M. Cutting back malaria: CRISPR/Cas9 genome editing of Plasmodium. Brief. Funct. Genom. 2019, 18, 281–289. [Google Scholar] [CrossRef]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S.; et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Kurup, S.P.; Tarleton, R.L. Perpetual expression of PAMPs necessary for optimal immune control and clearance of a persistent pathogen. Nat. Commun. 2013, 4, 2616. [Google Scholar] [CrossRef] [Green Version]
- Doolan, D.L.; Dobano, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques-da-Silva, C.; Peissig, K.; Kurup, S.P. Pre-Erythrocytic Vaccines against Malaria. Vaccines 2020, 8, 400. https://doi.org/10.3390/vaccines8030400
Marques-da-Silva C, Peissig K, Kurup SP. Pre-Erythrocytic Vaccines against Malaria. Vaccines. 2020; 8(3):400. https://doi.org/10.3390/vaccines8030400
Chicago/Turabian StyleMarques-da-Silva, Camila, Kristen Peissig, and Samarchith P. Kurup. 2020. "Pre-Erythrocytic Vaccines against Malaria" Vaccines 8, no. 3: 400. https://doi.org/10.3390/vaccines8030400
APA StyleMarques-da-Silva, C., Peissig, K., & Kurup, S. P. (2020). Pre-Erythrocytic Vaccines against Malaria. Vaccines, 8(3), 400. https://doi.org/10.3390/vaccines8030400




