Risk Assessment and Virological Monitoring Following an Accidental Exposure to Concentrated Sabin Poliovirus Type 3 in France, November 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. RNA Extraction and rRT-PCR for Detection and Identification of Enterovirus RNA
2.3. Virus Culture for Detection of Infectious Polioviruses
2.4. ITD/VDPV RT-PCR for Identification of Poliovirus
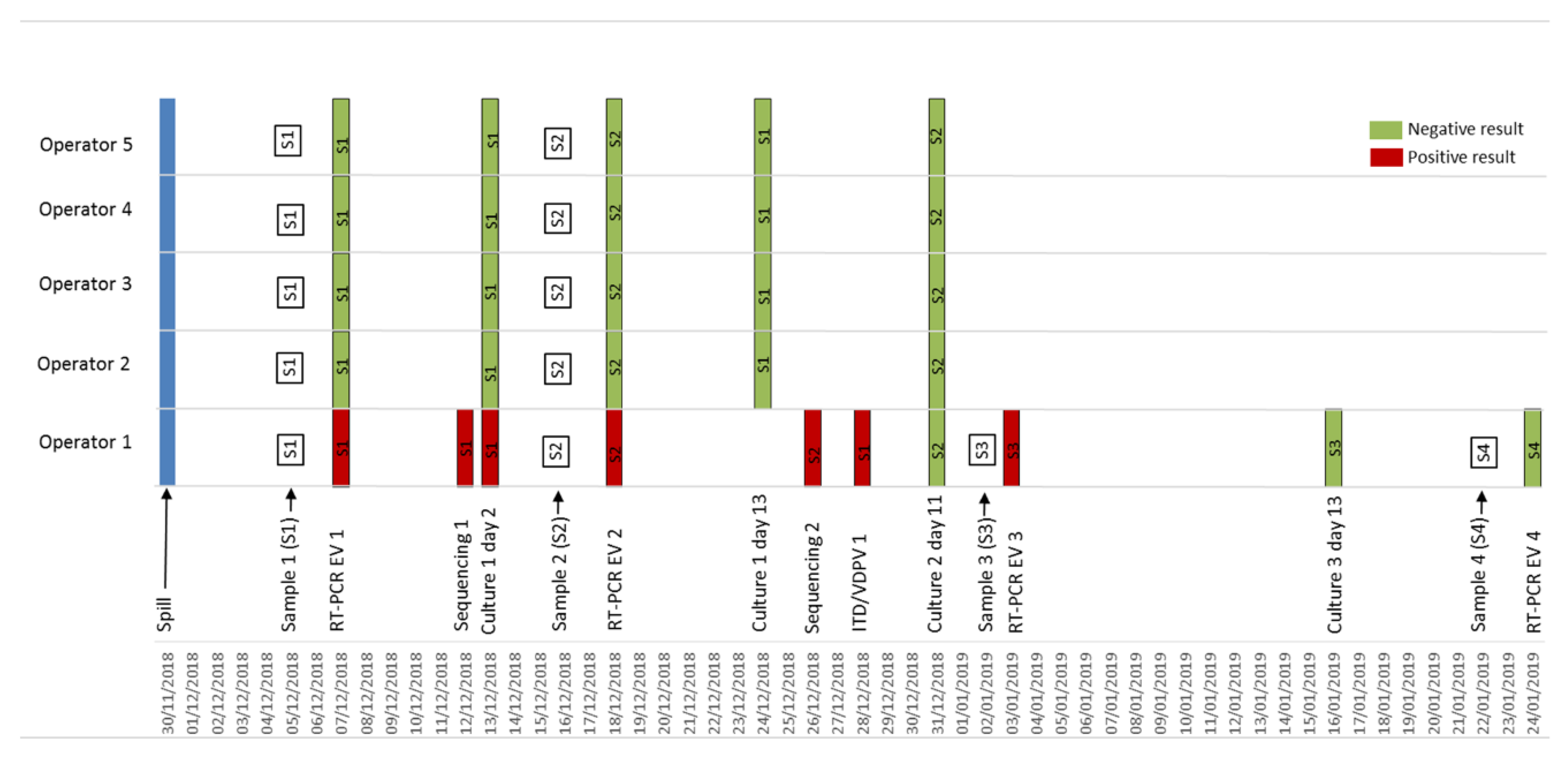
3. Results
3.1. Incident
3.2. Risk Assessment
3.3. Lab Investigations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poliomyelitis. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 8 June 2020).
- GPEI-This Week. Available online: http://polioeradication.org/polio-today/polio-now/this-week/ (accessed on 10 June 2020).
- World Health Organization. WHO Global Action Plan to Minimize Poliovirus Facility-Associated Risk after Type-Specific Eradication of Wild Polioviruses and Sequential Cessation of Oral Polio Vaccine Use: GAPIII; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Mulders, M.N.; Reimerink, J.H.; Koopmans, M.P.; van Loon, A.M.; van der Avoort, H.G. Genetic Analysis of Wild-Type Poliovirus Importation into The Netherlands (1979–1995). J. Infect. Dis. 1997, 176, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, J.M.; Nadkarni, S.S.; Siddiqui, Z.A. Detection of MEF-1 Laboratory Reference Strain of Poliovirus Type 2 in Children with Poliomyelitis in India in 2002 & 2003. Indian J. Med. Res. 2003, 118, 217–223. [Google Scholar] [PubMed]
- Duizer, E.; Rutjes, S.; de Roda Husman, A.M.; Schijven, J. Risk Assessment, Risk Management and Risk-Based Monitoring Following a Reported Accidental Release of Poliovirus in Belgium, September to November 2014. Euro Surveill. 2016, 21, 30169. [Google Scholar] [CrossRef] [PubMed]
- Duizer, E.; Ruijs, W.L.; van der Weijden, C.P.; Timen, A. Response to a Wild Poliovirus Type 2 (WPV2)-Shedding Event Following Accidental Exposure to WPV2, the Netherlands, April 2017. Euro Surveill. 2017, 22, 30542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, A.S.; Singh, H.; Fournier-Caruana, J.; Modlin, J.F.; Wenger, J.; Partridge, J.; Sutter, R.W.; Zaffran, M.J. Facility-Associated Release of Polioviruses into Communities-Risks for the Posteradication Era. Emerg. Infect. Dis. 2019, 25, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, Seminested PCR Amplification of VP1 Sequences for Direct Identification of All Enterovirus Serotypes from Original Clinical Specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Kilpatrick, D.R.; Ching, K.; Iber, J.; Chen, Q.; Yang, S.-J.; De, L.; Williams, A.J.; Mandelbaum, M.; Sun, H.; Oberste, M.S.; et al. Identification of Vaccine-Derived Polioviruses Using Dual-Stage Real-Time RT-PCR. J. Virol. Methods 2014, 197, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Données de Couverture Vaccinale Diphtérie-tétanos, Poliomyélite, Coqueluche Par Groupe d’âge. Available online: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/donnees-de-couverture-vaccinale-diphterie-tetanos-poliomyelite-coqueluche-par-groupe-d-age (accessed on 8 June 2020).
- Wassilak, S.; Pate, M.A.; Wannemuehler, K.; Jenks, J.; Burns, C.; Chenoweth, P.; Abanida, E.A.; Adu, F.; Baba, M.; Gasasira, A.; et al. Outbreak of Type 2 Vaccine-Derived Poliovirus in Nigeria: Emergence and Widespread Circulation in an Underimmunized Population. J. Infect. Dis. 2011, 203, 898–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Public Health Management of Facility Related Exposure to Live Polioviruses; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeannoël, M.; Antona, D.; Lazarus, C.; Lina, B.; Schuffenecker, I. Risk Assessment and Virological Monitoring Following an Accidental Exposure to Concentrated Sabin Poliovirus Type 3 in France, November 2018. Vaccines 2020, 8, 331. https://doi.org/10.3390/vaccines8020331
Jeannoël M, Antona D, Lazarus C, Lina B, Schuffenecker I. Risk Assessment and Virological Monitoring Following an Accidental Exposure to Concentrated Sabin Poliovirus Type 3 in France, November 2018. Vaccines. 2020; 8(2):331. https://doi.org/10.3390/vaccines8020331
Chicago/Turabian StyleJeannoël, Marion, Denise Antona, Clément Lazarus, Bruno Lina, and Isabelle Schuffenecker. 2020. "Risk Assessment and Virological Monitoring Following an Accidental Exposure to Concentrated Sabin Poliovirus Type 3 in France, November 2018" Vaccines 8, no. 2: 331. https://doi.org/10.3390/vaccines8020331
APA StyleJeannoël, M., Antona, D., Lazarus, C., Lina, B., & Schuffenecker, I. (2020). Risk Assessment and Virological Monitoring Following an Accidental Exposure to Concentrated Sabin Poliovirus Type 3 in France, November 2018. Vaccines, 8(2), 331. https://doi.org/10.3390/vaccines8020331




