Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit OralVaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Animals, Bacteria, and Vaccines Formulation
2.2. Experimental Design
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Splenocyte Proliferation Assay
2.5. Total and Recall SE Specific Lymphocyte Subsets Frequencies by Flow Cytometry
2.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
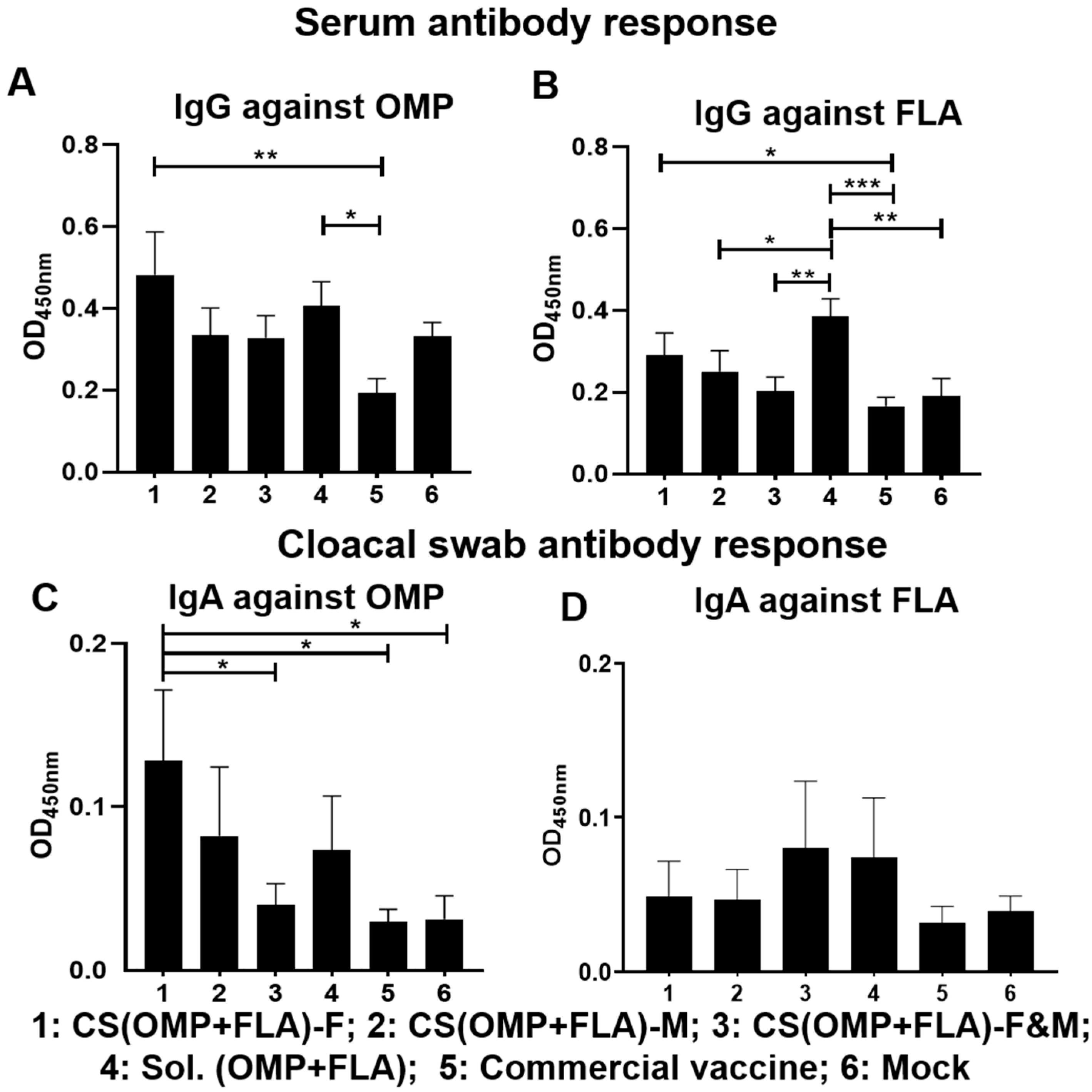
3.1. Pre-Challenge Antibody Response against Salmonella Antigens
3.2. Post-Challenge Antibody Response against Salmonella Antigens
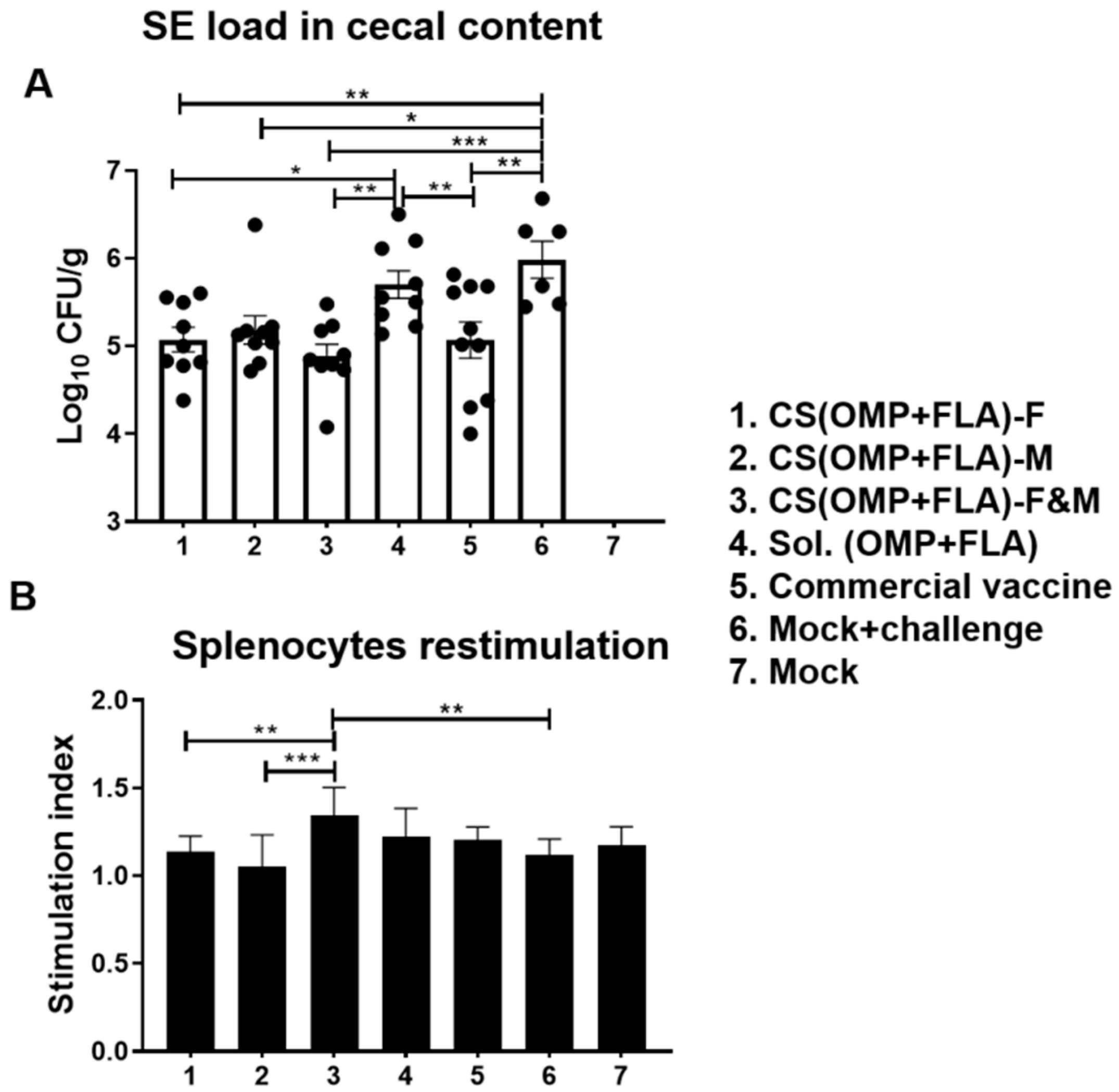
3.3. Live Vaccine and Challenge Bacterial Load in Vaccinated Birds
3.4. Salmonella Antigens (OMP+FLA) Specific Recall Lymphocyte Proliferation Response in Vaccinates
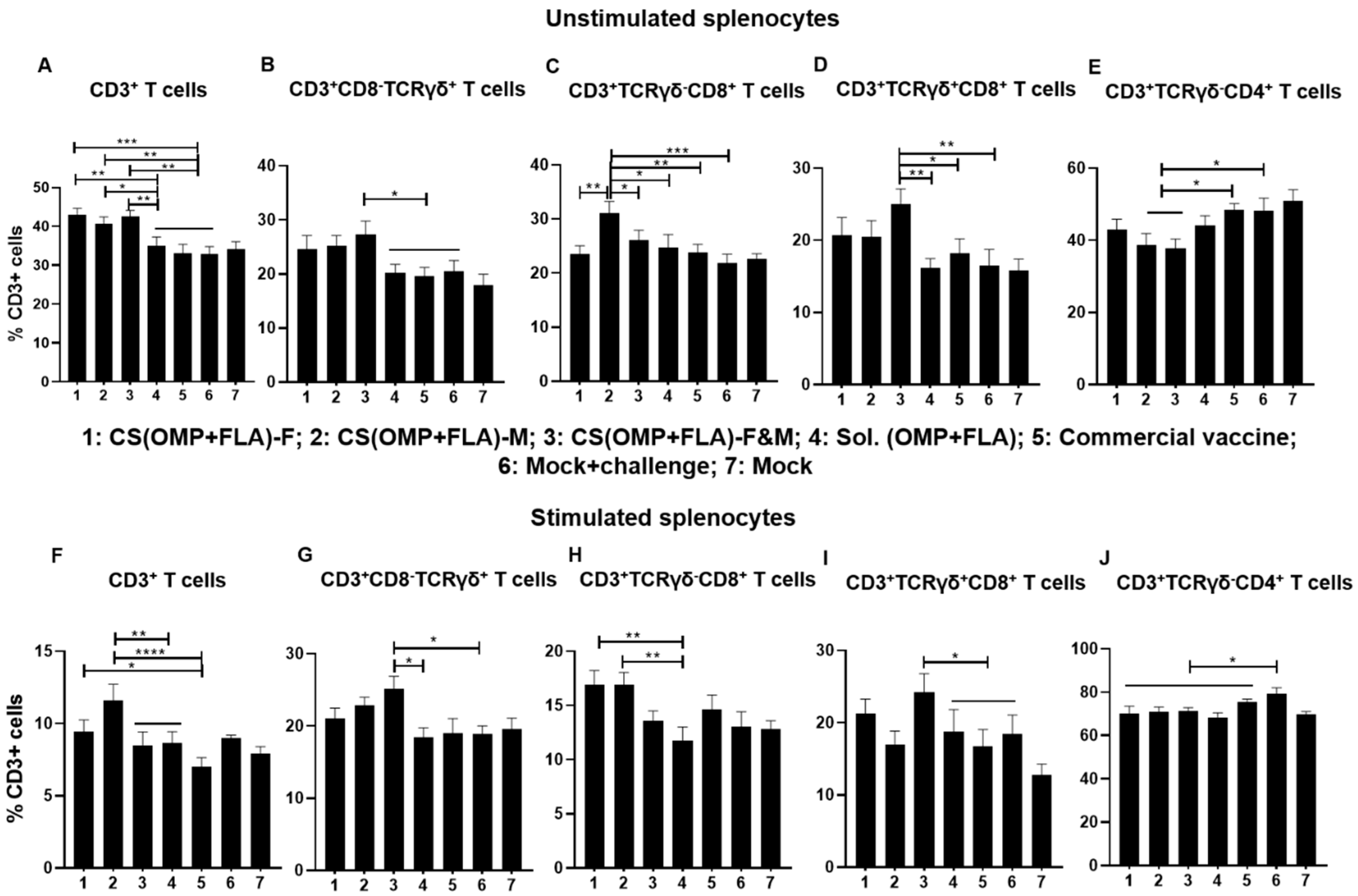
3.5. Both the Total and Antigen Specific Recall T Cell Subset Frequencies in CS(OMP+FLA) Vaccinates
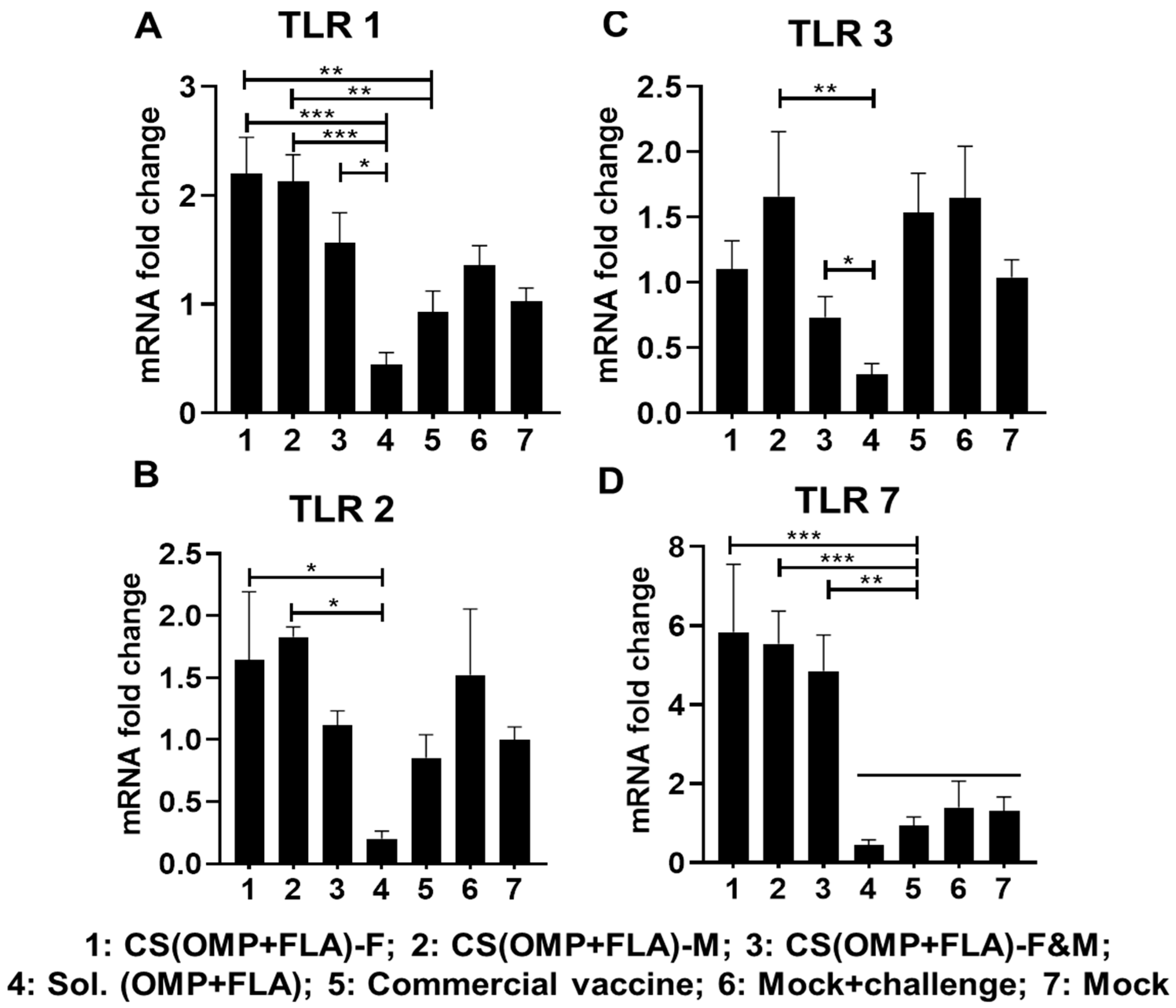
3.6. CS(OMP+FLA) Vaccine-Induced TLRs and Cytokine mRNA Expression in Cecal Tonsils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.S.; Stern, N.J.; Fedorka-Cray, P.; Craven, S.E.; Cox, N.A.; Cosby, D.E.; Ladely, S.; Musgrove, M.T. Sources and movement of Salmonella through integrated poultry operations: A multistate epidemiological investigation. J. Food Prot. 2001, 64, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Mayo, J.A.; Saville, D.; Wolyniak, C.; Klontz, K.C. Recalls of foods due to microbiological contamination classified by the U.S. Food and Drug Administration, fiscal years 2003 through 2011. J. Food Prot. 2013, 76, 932–938. [Google Scholar] [CrossRef]
- Frey, J. Biological safety concepts of genetically modified live bacterial vaccines. Vaccine 2007, 25, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Immune Response to Salmonella Enteritidis Infection in Broilers Immunized Orally With Chitosan-Based Salmonella Subunit Nanoparticle Vaccine. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Wang, J.; Zhang, W.; Xu, B.; Xu, X.; Zong, L. Targeted delivery of antigen to intestinal dendritic cells induces oral tolerance and prevents autoimmune diabetes in NOD mice. Diabetologia 2018, 61, 1384–1396. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Kang, M.L.; Quan, J.-S.; Kang, S.G.; Akaike, T.; Yoo, H.S.; Cho, C.-S. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials 2008, 29, 1931–1939. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Salman, H.H.; Irache, J.M.; Gamazo, C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine 2009, 27, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Alderton, M.R.; Fahey, K.J.; Coloe, P.J. Humoral responses and salmonellosis protection in chickens given a vitamin-dependent Salmonella typhimurium mutant. Avian Dis. 1991, 35, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Raehtz, S.; Hargis, B.M.; Kuttappan, V.A.; Pamukcu, R.; Bielke, L.R.; McCabe, L.R. High Molecular Weight Polymer Promotes Bone Health and Prevents Bone Loss Under Salmonella Challenge in Broiler Chickens. Front. Physiol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Markazi, A.D.; Dhakal, S.; Lakshmanappa, Y.S.; Gourapura, S.R.; Shanmugasundaram, R.; Senapati, S.; Narasimhan, B.; Selvaraj, R.K.; Renukaradhya, G.J. Surface engineered polyanhydride-based oral Salmonella subunit nanovaccine for poultry. Int. J. Nanomed. 2018, 13, 8195–8215. [Google Scholar] [CrossRef]
- Renu, S.; Markazi, A.D.; Dhakal, S.; Lakshmanappa, Y.S.; Shanmugasundaram, R.; Selvaraj, R.K.; Renukaradhya, G.J. Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens. Int. J. Nanomed. 2020, 15, 761–777. [Google Scholar] [CrossRef]
- Yalpani, M.; Hall, L.D. Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984, 17, 272–281. [Google Scholar] [CrossRef]
- Chaubey, P.; Mishra, B. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr. Polym. 2014, 101, 1101–1108. [Google Scholar] [CrossRef]
- Andersen, S.H.; Vervelde, L.; Sutton, K.; Norup, L.R.; Wattrang, E.; Juul-Madsen, H.R.; Dalgaard, T.S. Quantification and phenotypic characterisation of peripheral IFN-γ producing leucocytes in chickens vaccinated against Newcastle disease. Vet. Immunol. Immunopathol. 2017, 193, 18–28. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Desin, T.S.; Koster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Expert Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Fukutome, K.; Watarai, S.; Mukamoto, M.; Kodama, H. Intestinal mucosal immune response in chickens following intraocular immunization with liposome-associated Salmonella enterica serovar enteritidis antigen. Dev. Comp. Immunol. 2001, 25, 475–484. [Google Scholar] [CrossRef]
- Babu, U.; Dalloul, R.; Okamura, M.; Lillehoj, H.; Xie, H.; Raybourne, R.; Gaines, D.; Heckert, R. Salmonella enteritidis clearance and immune responses in chickens following Salmonella vaccination and challenge. Vet. Immunol. Immunopathol. 2004, 101, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Lillehoj, H.; Raybourne, R.; Babu, U.; Heckert, R. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: Lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-γ production. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Chung, K.S. Postnatal development of T-lymphocyte subpopulations in the intestinal intraepithelium and lamina propria in chickens. Vet. Immunol. Immunopathol. 1992, 31, 347–360. [Google Scholar] [CrossRef]
- Emoto, M.; Nishimura, H.; Sakai, T.; Hiromatsu, K.; Gomi, H.; Itohara, S.; Yoshikai, Y. Mice deficient in gamma delta T cells are resistant to lethal infection with Salmonella choleraesuis. Infect. Immun. 1995, 63, 3736–3738. [Google Scholar] [CrossRef] [PubMed]
- Mixter, P.F.; Camerini, V.; Stone, B.J.; Miller, V.L.; Kronenberg, M. Mouse T lymphocytes that express a gamma delta T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect. Immun. 1994, 62, 4618–4621. [Google Scholar] [CrossRef]
- McKenzie, E.J.; Taylor, P.R.; Stillion, R.J.; Lucas, A.D.; Harris, J.; Gordon, S.; Martinez-Pomares, L. Mannose Receptor Expression and Function Define a New Population of Murine Dendritic Cells. J. Immunol. 2007, 178, 4975. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Gordon, S.; Martinez-Pomares, L.; McKenzie, I.F.C. Aldehyde-mannan antigen complexes target the MHC class I antigen-presentation pathway. Eur. J. Immunol. 2000, 30, 1714–1723. [Google Scholar] [CrossRef]
- Toda, S.; Ishii, N.; Okada, E.; Kusakabe, K.I.; Arai, H.; Hamajima, K.; Gorai, I.; Nishioka, K.; Okuda, K. HIV-1-specific cell-mediated immune responses induced by DNA vaccination were enhanced by mannan-coated liposomes and inhibited by anti-interferon-gamma antibody. Immunology 1997, 92, 111–117. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Shayeganpour, A.; Samuel, J.; Lavasanifar, A. Activation of Antigen-Specific T Cell-Responses by Mannan-Decorated PLGA Nanoparticles. Pharm. Res. 2011, 28, 2288. [Google Scholar] [CrossRef]
- Jones, B.D.; Falkow, S. Salmonellosis: Host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996, 14, 533–561. [Google Scholar] [CrossRef] [PubMed]
- Mittrucker, H.W.; Kaufmann, S.H. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 2000, 67, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Sandor, F.; Latz, E.; Re, F.; Mandell, L.; Repik, G.; Golenbock, D.T.; Espevik, T.; Kurt-Jones, E.A.; Finberg, R.W. Importance of extra- and intracellular domains of TLR1 and TLR2 in NFkappa B signaling. J. Cell Biol. 2003, 162, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, K.; He, H.; Nerren, J.; Swaggerty, C.; Genovese, K.; Kogut, M. Expression profile of toll-like receptors within the gastrointestinal tract of 2-day-old Salmonella enteriditis-infected broiler chickens. Vet. Microbiol. 2009, 137, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Sato, S.; Horiuchi, T.; Hoshino, K.; Takeda, K.; Dong, Z.; Modlin, R.L.; Akira, S. Cutting edge: Role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002, 169, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Philbin, V.J.; Iqbal, M.; Boyd, Y.; Goodchild, M.J.; Beal, R.K.; Bumstead, N.; Young, J.; Smith, A.L. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology 2005, 114, 507–521. [Google Scholar] [CrossRef]
- Iqbal, M.; Philbin, V.J.; Withanage, G.S.; Wigley, P.; Beal, R.K.; Goodchild, M.J.; Barrow, P.; McConnell, I.; Maskell, D.J.; Young, J.; et al. Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar typhimurium. Infect. Immun. 2005, 73, 2344–2350. [Google Scholar] [CrossRef]
- Taylor, M.E.; Bezouska, K.; Drickamer, K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J. Biol. Chem. 1992, 267, 1719–1726. [Google Scholar]
- Doz, E.; Rose, S.; Nigou, J.; Gilleron, M.; Puzo, G.; Erard, F.; Ryffel, B.; Quesniaux, V.F. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 2007, 282, 26014–26025. [Google Scholar] [CrossRef]
- Ramakrishna, V.; Vasilakos, J.P.; Tario, J.D., Jr.; Berger, M.A.; Wallace, P.K.; Keler, T. Toll-like receptor activation enhances cell-mediated immunity induced by an antibody vaccine targeting human dendritic cells. J. Transl. Med. 2007, 5, 5. [Google Scholar] [CrossRef]
- Lee, J.H. Protection against salmonella typhimurium, salmonella gallinarum, and salmonella enteritidis infection in layer chickens conferred by a live attenuated salmonella typhimurium strain. Immune Netw. 2015, 15, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Tujimoto-Silva, A.; Muniz, E.; Verdi, R.; Santin, E. Salmonella typhimurium vaccine to control a brazilian Salmonella heidelberg strain in broiler chickens. Ars Vet. 2018, 34, 105–114. [Google Scholar] [CrossRef]


| Group | Vaccine Received | N | 1st Dose/Age | 2nd Dose/Age | Challenge/Age |
|---|---|---|---|---|---|
| 1 | 10 µg CS(OMP+FLA)-F | 9 | 3 day | 3 week | 5 week |
| 2 | 10 µg CS(OMP+FLA)-M | 9 | 3 day | 3 week | 5 week |
| 3 | 10 µg CS(OMP+FLA)-F&M | 9 | 3 day | 3 week | 5 week |
| 4 | 10 µg Sol.Ag (OMP+FLA) | 9 | 3 day | 3 week | 5 week |
| 5 | Commercial vaccine | 10 | 3 day | 3 week | 5 week |
| 6 | PBS (Mock-challenge) | 6 | 3 day | 3 week | 5 week |
| 7 | PBS (Mock) | 13 | 3 day | 3 week | NA |
| Antibody | Catalog Number and Company Name |
|---|---|
| Mouse anti-chicken CD3 AF700 | Cat# 8200-27; SouthernBiotech, Birmingham, AL, USA |
| Mouse anti-chicken CD4 FITC | Cat# 8210-02; SouthernBiotech, Birmingham, AL, USA |
| Mouse anti-chicken CD8α PE | Cat# 8220-09; SouthernBiotech, Birmingham, AL, USA |
| Mouse anti-chicken TCRγδ biotin | Cat# 8230-08; SouthernBiotech, Birmingham, AL, USA |
| Streptavidin PE | Cat# 557598; BD Pharmingen, San Jose, CA, USA |
| Streptavidin AF488 | Cat#405235, BioLegend, San Diego, CA, USA |
| Rabbit anti-chicken IFNγ | Cat# AHP945Z; Bio-Rad; Hercules, CA, USA |
| Goat anti-rabbit IgG AF647 | Cat# 4050-31; SouthernBiotech, Birmingham, AL, USA |
| Primers | Oligonucleotides (5′–3′) | Annealing Temperature |
|---|---|---|
| β-actin | Forward: ACCGGACTATTACCAACACC | 56 °C |
| Reverse: GACTGCTGCTGACACCTTCA | ||
| TLR 1 | Forward: GCTGTGTCAGCATCAGAGGA | 58 °C |
| Reverse: GTGGTACCTCGCAGGGATAA | ||
| TLR 2 | Forward: GCTCAACAGCTTCTCCAAGG | 57 °C |
| Reverse: CCACCAGGATGAGGATGAAC | ||
| TLR 3 | Forward: CCTCCTTGGGACACCTGAAA | 54 °C |
| Reverse: ATTCCGCAGTGGATGAAAAG | ||
| TLR 7 | Forward: AGAGACTGGCTTCCAGGACA | 58 °C |
| Reverse: CAGCTGAACATACCGGGACT | ||
| IL-1β | Forward: TGGGCATCAAGGGCTACA | 57 °C |
| Reverse: TCGGGTTGGTTGGTGATG | ||
| IL-10 | Forward: CATGCTGCTGGGCCTGAA | 57 °C |
| Reverse: CGTCTCCTTGATCTGCTTGATG | ||
| TNF-α | Forward: ATCCTCACCCCTACCCTGTC | 56 °C |
| Reverse: GGCGGTCATAGAACAGCACT | ||
| TGF-β1 | Forward: AGGATCTGCAGTGGAGTGGAT | 54 °C |
| Reverse: CCCCGGGTTGTGTTGGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit OralVaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers. Vaccines 2020, 8, 299. https://doi.org/10.3390/vaccines8020299
Han Y, Renu S, Patil V, Schrock J, Feliciano-Ruiz N, Selvaraj R, Renukaradhya GJ. Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit OralVaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers. Vaccines. 2020; 8(2):299. https://doi.org/10.3390/vaccines8020299
Chicago/Turabian StyleHan, Yi, Sankar Renu, Veerupaxagouda Patil, Jennifer Schrock, Ninoshkaly Feliciano-Ruiz, Ramesh Selvaraj, and Gourapura J. Renukaradhya. 2020. "Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit OralVaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers" Vaccines 8, no. 2: 299. https://doi.org/10.3390/vaccines8020299
APA StyleHan, Y., Renu, S., Patil, V., Schrock, J., Feliciano-Ruiz, N., Selvaraj, R., & Renukaradhya, G. J. (2020). Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit OralVaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers. Vaccines, 8(2), 299. https://doi.org/10.3390/vaccines8020299







