The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Plasmids
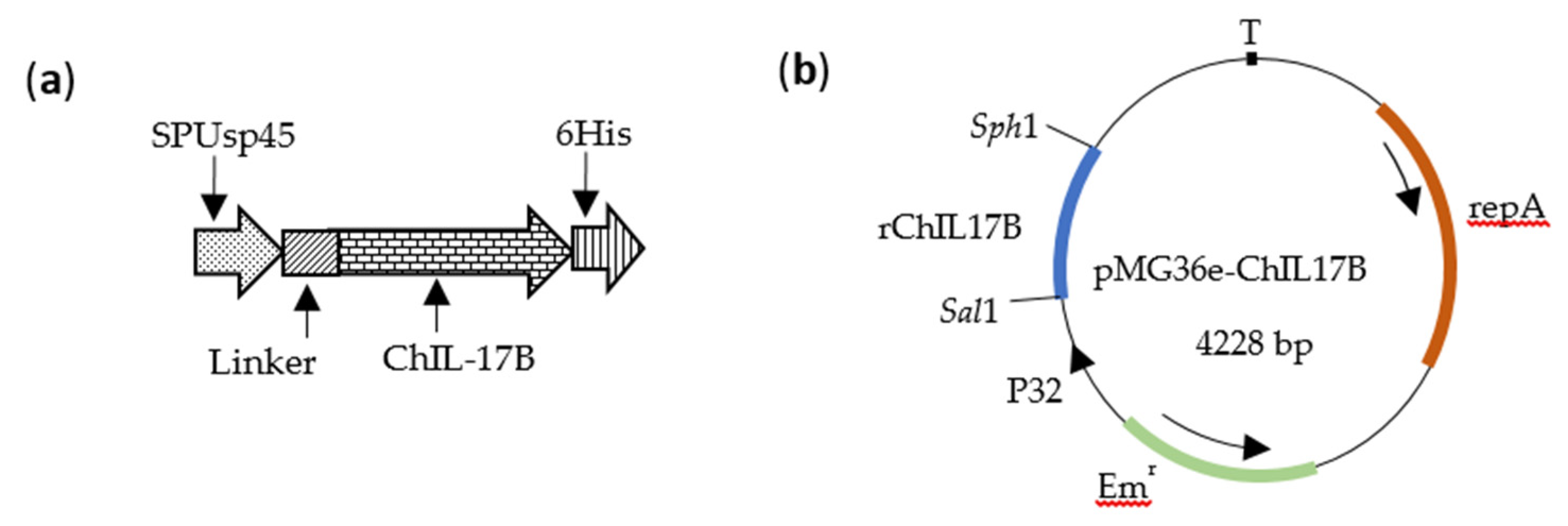
2.2. Construction of Recombinant L. Plantarum NC8-ChIL17B
2.3. Analysis of rChIL-17B Expression by Western Blot and ELISA
2.4. Analysis of the rChIL-17B Activity on the Proliferation of IBV in Vitro
2.5. Animal Experimental Protocol
2.6. Evaluation of the Immunoadjuvant Effect of NC8-ChIL17B In Vivo
2.6.1. Measures of IBV-Specific Antibodies and the Concentration of Cytokines in the Serum by ELISA
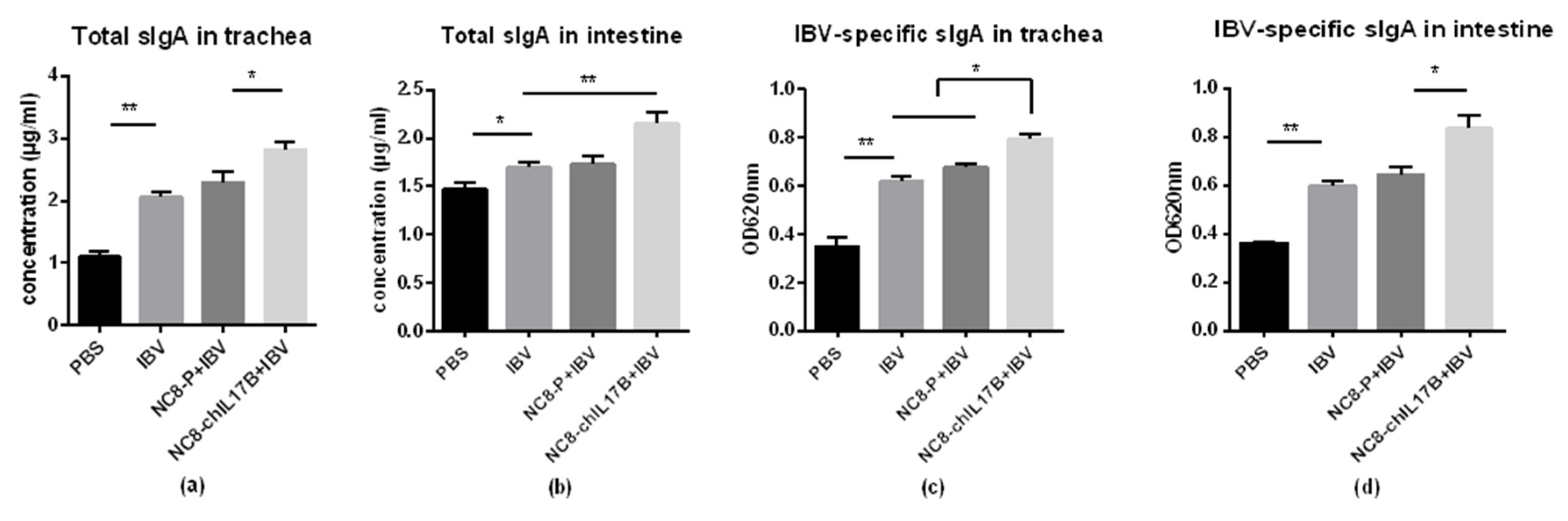
2.6.2. Measures of sIgA from the Tracheas and Small Intestines by ELISA
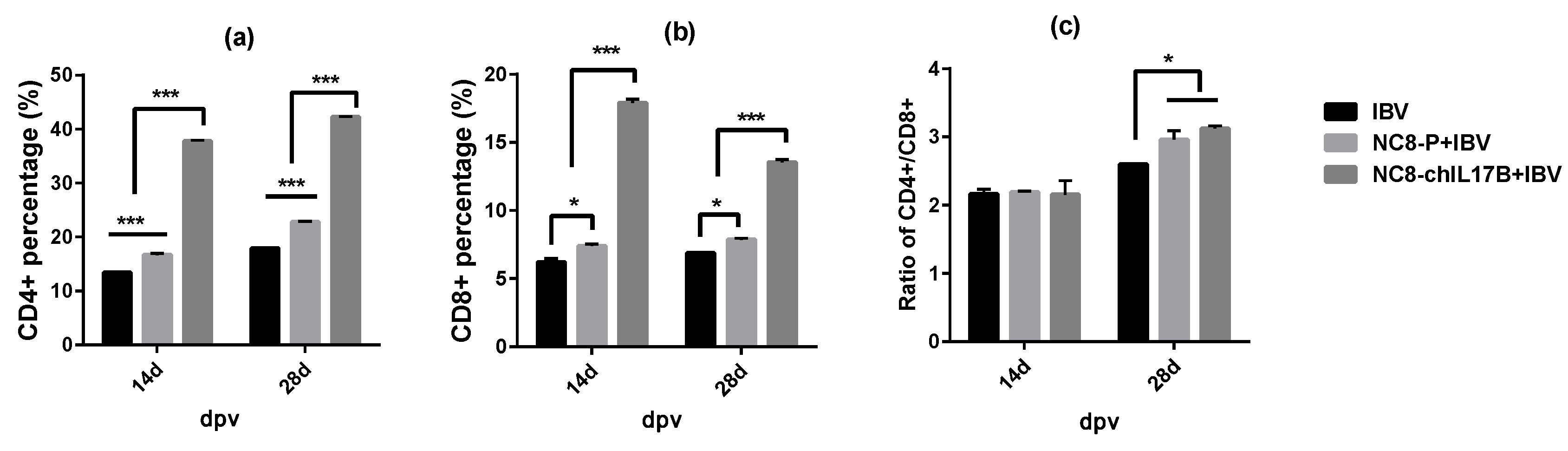
2.6.3. Analysis of CD4+ and CD8+ T Cells in the Peripheral Blood by Flow Cytometry (FCM)
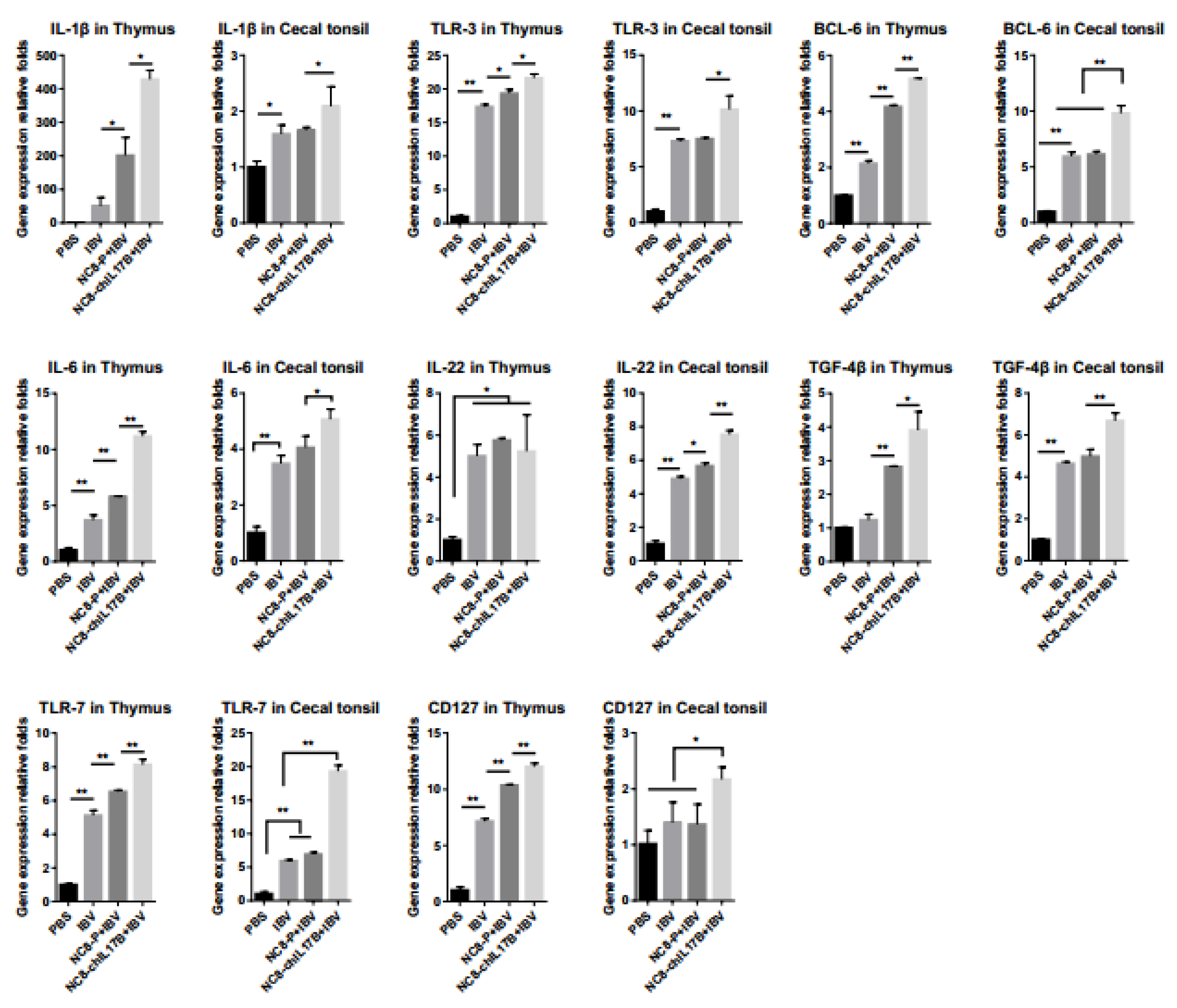
2.6.4. Analysis of Immune-Related Genes by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
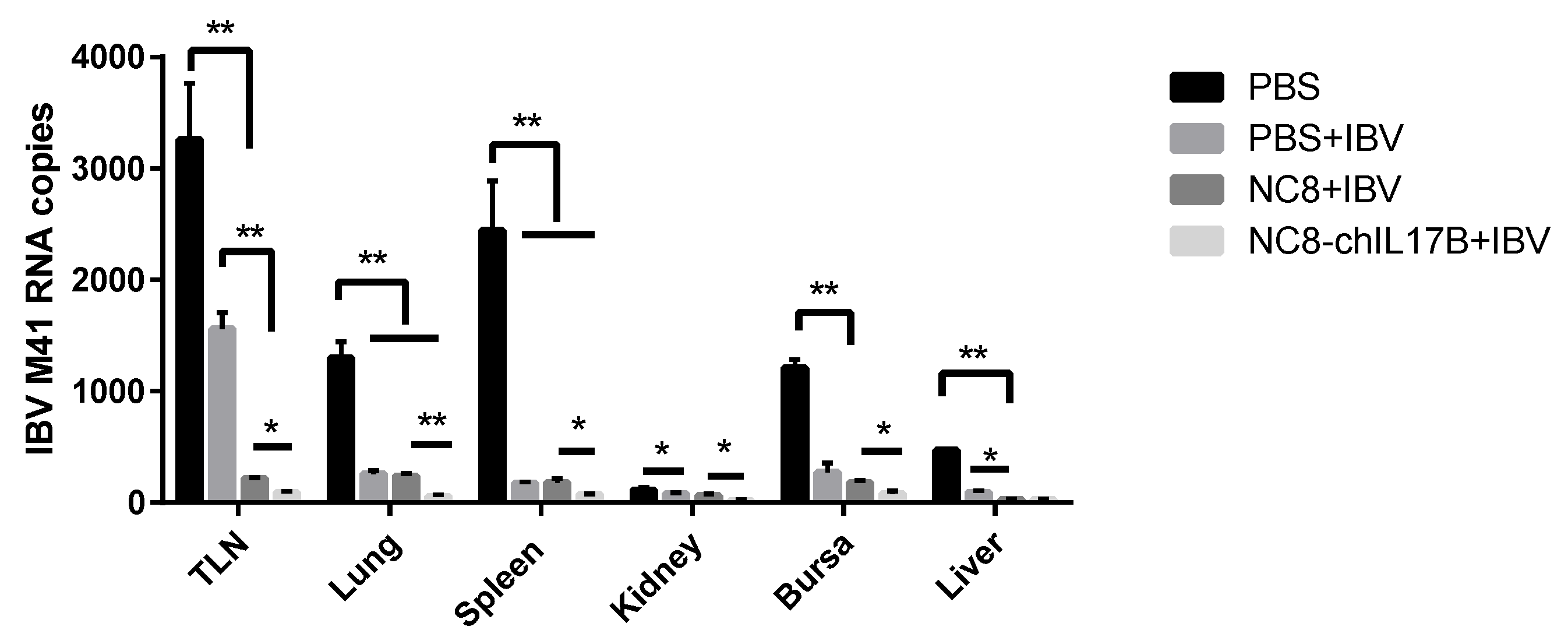
2.6.5. Measurement of IBV M41 Copies in Challenged Chickens by Absolute qRT-PCR
2.7. Statistical Analysis
3. Results
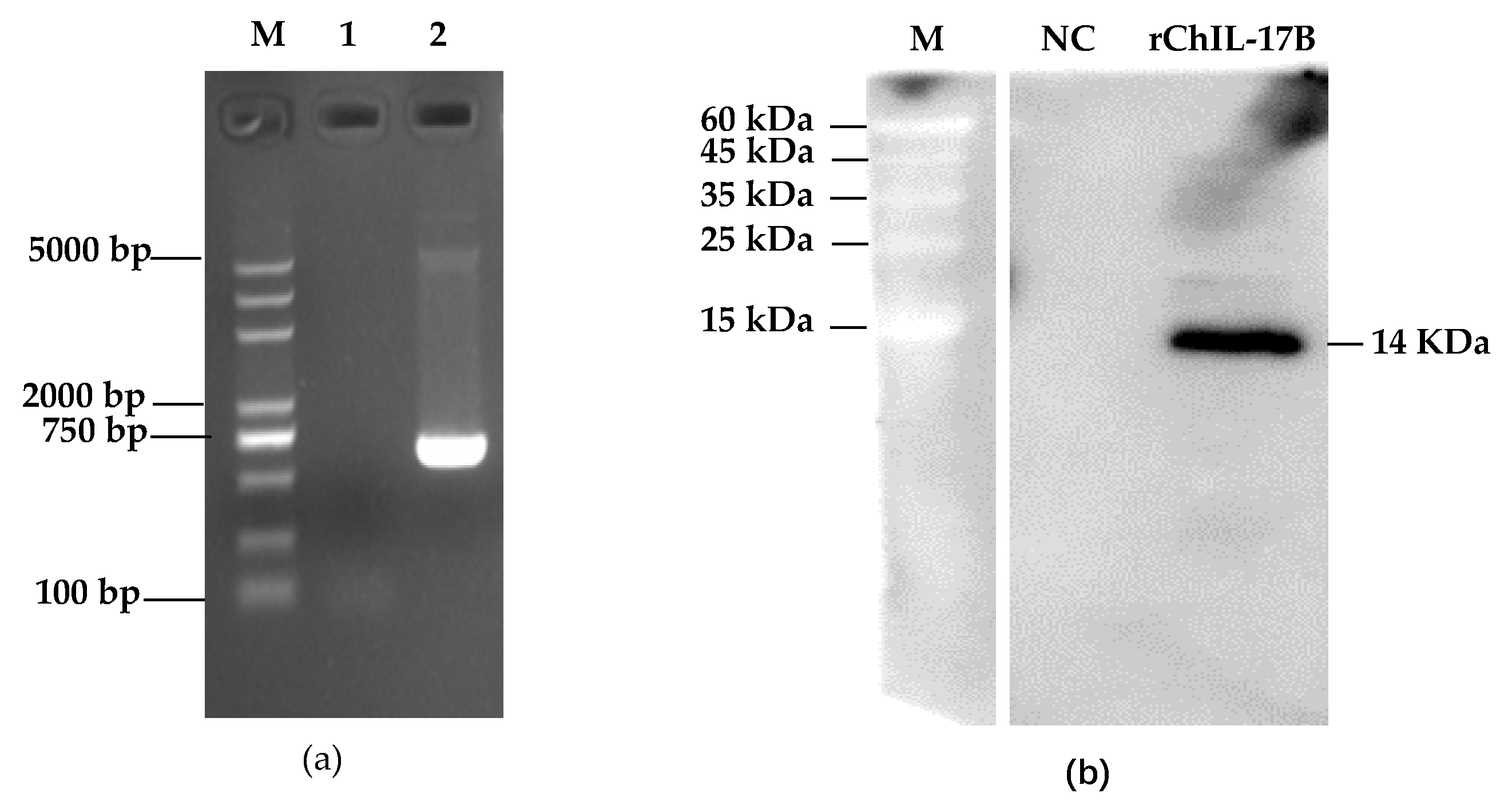
3.1. Construction of the NC8-ChIL17B Strain
3.2. Analysis of the rChIL17B Activity on the Proliferation of the IBV Beaudette Strain in HD11 Cells
3.3. Weight Change in the Experimental Chickens
3.4. Evaluation of the Immunoadjuvant Activities of NC8-ChIL17B in Chickens
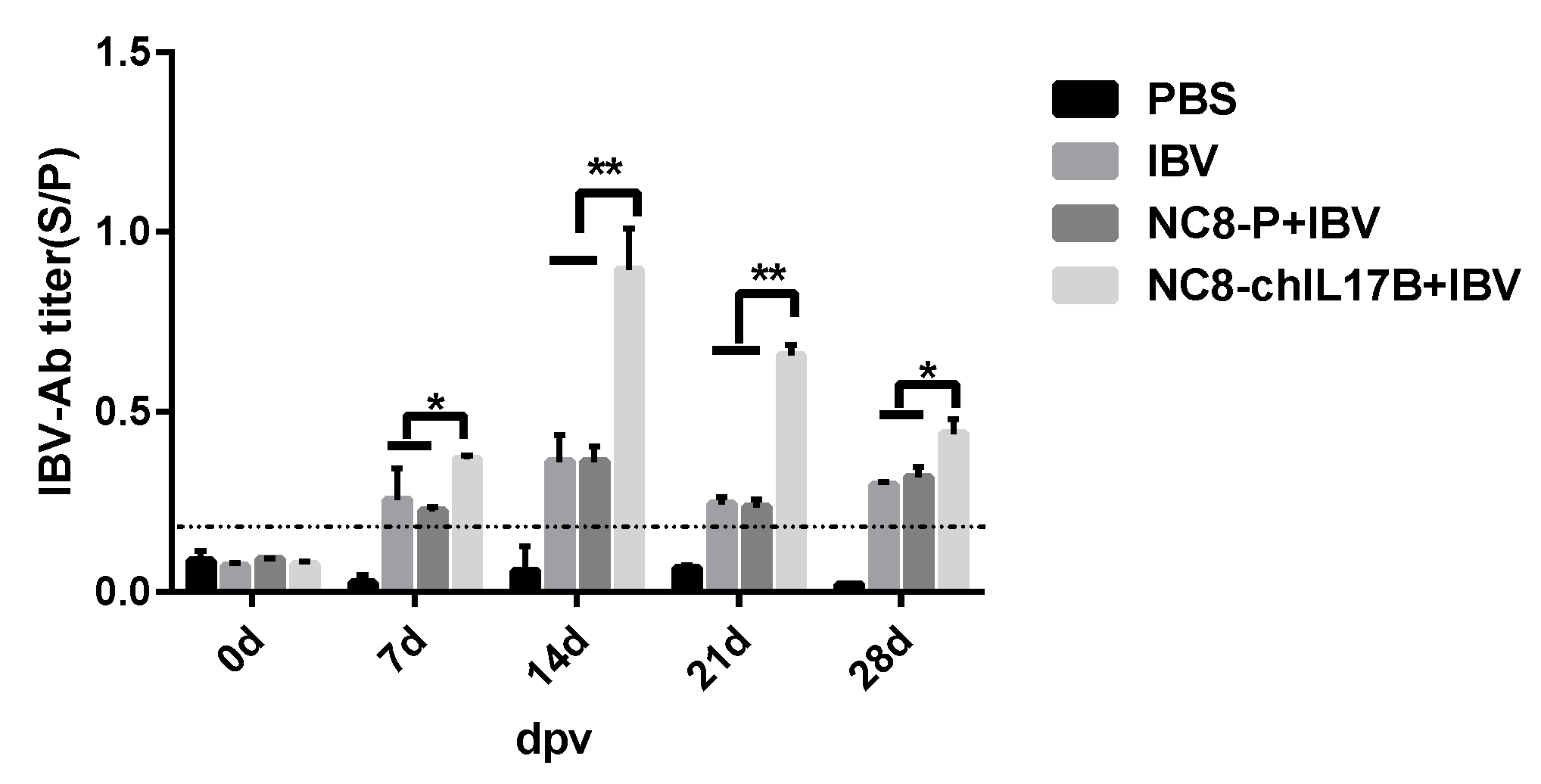
3.4.1. Analysis of the IBV-Specific Antibodies in the Serum
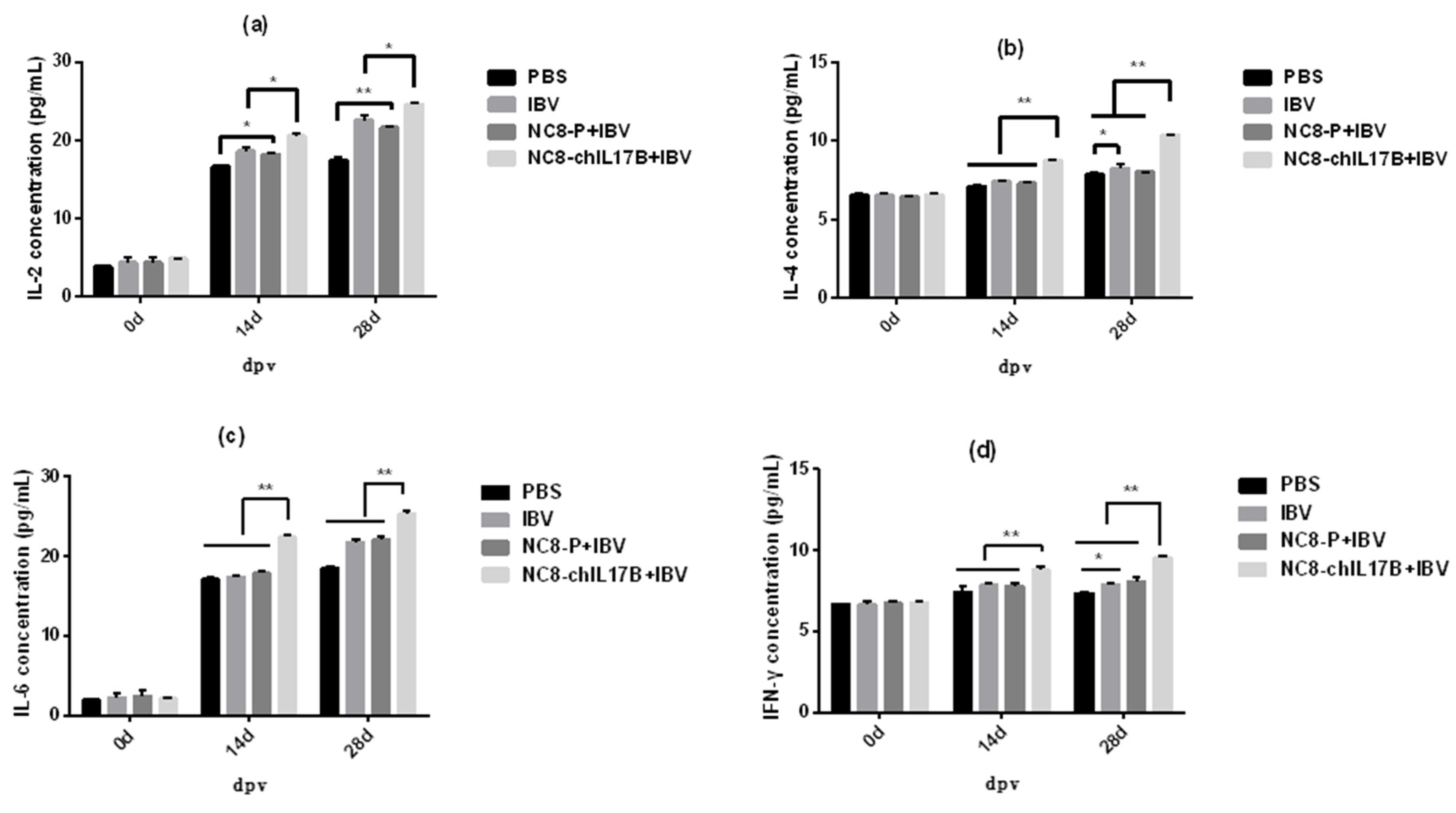
3.4.2. Changes in the Concentrations of Cytokines in the Serum
3.4.3. Changes in the CD4+ and CD8+ T-Cell Counts in the Peripheral Blood
3.4.4. Changes in Mucosal sIgA in the Tracheas and Small Intestines
3.4.5. Changes of the Expression of Immune-Related Genes
3.4.6. Evaluation of the Protection Against IBV M41 Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28 (Suppl. S3), C25–C36. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.C.; Coulter, A.R. Adjuvants—A classification and review of their modes of action. Vaccine 1997, 15, 248–256. [Google Scholar] [CrossRef]
- Gupta, R.K.; Relyveld, E.H.; Lindblad, E.B.; Bizzini, B.; Ben-Efraim, S.; Gupta, C.K. Adjuvants—A balance between toxicity and adjuvanticity. Vaccine 1993, 11, 293–306. [Google Scholar] [CrossRef]
- Lowenthal, J.W.; York, J.J.; O’Neil, T.E.; Rhodes, S.; Prowse, S.J.; Strom, D.G.; Digby, M.R. In vivo effects of chicken interferon-gamma during infection with Eimeria. J. Interferon. Cytokine. Res. 1997, 17, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Karaca, K.; Sharma, J.M.; Winslow, B.J.; Junker, D.E.; Reddy, S.; Cochran, M.; McMillen, J. Recombinant fowlpox viruses coexpressing chicken type I IFN and Newcastle disease virus HN and F genes: Influence of IFN on protective efficacy and humoral responses of chickens following in ovo or post-hatch administration of recombinant viruses. Vaccine 1998, 16, 1496–1503. [Google Scholar] [CrossRef]
- Schijns, V.E.; Weining, K.C.; Nuijten, P.; Rijke, E.O.; Staeheli, P. Immunoadjuvant activities of E. coli- and plasmid-expressed recombinant chicken IFN-alpha/beta, IFN-gamma and IL-1beta in 1-day- and 3-week-old chickens. Vaccine 2000, 18, 2147–2154. [Google Scholar] [CrossRef]
- Ding, X.; Lillehoj, H.S.; Quiroz, M.A.; Bevensee, E.; Lillehoj, E.P. Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect. Immun. 2004, 72, 6939–6944. [Google Scholar] [CrossRef]
- Park, J.H.; Sung, H.W.; Yoon, B.I.; Kwon, H.M. Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-gamma. J. Vet. Sci. 2009, 10, 131–139. [Google Scholar] [CrossRef]
- Degen, W.G.; van Zuilekom, H.I.; Scholtes, N.C.; van Daal, N.; Schijns, V.E. Potentiation of humoral immune responses to vaccine antigens by recombinant chicken IL-18 (rChIL-18). Vaccine 2005, 23, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Mingxiao, M.; Ningyi, J.; Zhenguo, W.; Ruilin, W.; Dongliang, F.; Min, Z.; Gefen, Y.; Chang, L.; Leili, J.; Kuoshi, J.; et al. Construction and immunogenicity of recombinant fowlpox vaccines coexpressing HA of AIV H5N1 and chicken IL18. Vaccine 2006, 24, 4304–4311. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.H.; Li, H.P.; Lien, Y.Y.; Wu, M.L.; Chaung, H.C. Adjuvant effects of chicken interleukin-18 in avian Newcastle disease vaccine. Vaccine 2010, 28, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, K.; Yang, G.; Shi, C.; Huang, H.; Wang, J.; Yang, W.; Liu, J.; Liu, Q.; Kang, Y.; et al. Construction of a novel DNA vaccine candidate targeting F gene of genotype VII Newcastle disease virus and chicken IL-18 delivered by Salmonella. J. Appl. Microbiol. 2019, 126, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Loewen, P.C.; Marquardt, R.R. A plasmid DNA encoding chicken interleukin-6 and Escherichia coli K88 fimbrial protein FaeG stimulates the production of anti-K88 fimbrial antibodies in chickens. Poult. Sci. 2004, 83, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Kim, W.H.; Lillehoj, H.S. Interleukin-4 (IL-4) may regulate alternative activation of macrophage-like cells in chickens: A sequential study using novel and specific neutralizing monoclonal antibodies against chicken IL-4. Vet Immunol. Immunopathol. 2018, 205, 72–82. [Google Scholar] [CrossRef]
- Ma, D.; Ma, C.; Pan, L.; Li, G.; Yang, J.; Hong, J.; Cai, H.; Ren, X. Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp. Parasitol. 2011, 127, 208–214. [Google Scholar] [CrossRef]
- Song, X.; He, X.; Li, X.; Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol. Immunol. 2016, 13, 418–431. [Google Scholar] [CrossRef]
- Song, X.; Qian, Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal 2013, 25, 2335–2347. [Google Scholar] [CrossRef]
- Lee, J.; Ho, W.H.; Maruoka, M.; Corpuz, R.T.; Baldwin, D.T.; Foster, J.S.; Goddard, A.D.; Yansura, D.G.; Vandlen, R.L.; Wood, W.I.; et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 2001, 276, 1660–1664. [Google Scholar] [CrossRef]
- Shi, Y.; Ullrich, S.J.; Zhang, J.; Connolly, K.; Grzegorzewski, K.J.; Barber, M.C.; Wang, W.; Wathen, K.; Hodge, V.; Fisher, C.L.; et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J. Biol. Chem. 2000, 275, 19167–19176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Urban, J.F., Jr.; Sun, R.; Stiltz, J.; Morimoto, M.; Notari, L.; Madden, K.B.; Yang, Z.; Grinchuk, V.; Ramalingam, T.R.; et al. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J. Immunol. 2010, 185, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.M.; Lee, Y.H.; Shi, Y.; Wang, X.; Angkasekwinai, P.; Nallaparaju, K.C.; Flaherty, S.; Chang, S.H.; Watarai, H.; Dong, C. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 2015, 42, 692–703. [Google Scholar] [CrossRef]
- Hoang, C.T.; Hong, Y.; Truong, A.D.; Lee, J.; Lee, K.; Hong, Y.H. Molecular cloning of chicken interleukin-17B, which induces proinflammatory cytokines through activation of the NF-kappaB signaling pathway. Dev. Comp. Immunol. 2017, 74, 40–48. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, G.; Wang, Q.; Wang, Z.; Yang, W.; Gu, W.; Shi, C.; Wang, J.; Huang, H.; Wang, C. Molecular mechanisms underlying protection against H9N2 influenza virus challenge in mice by recombinant Lactobacillus plantarum with surface displayed HA2-LTB. J. Biotechnol. 2017, 259, 6–14. [Google Scholar] [CrossRef]
- Yang, W.T.; Yang, G.L.; Wang, Q.; Huang, H.B.; Jiang, Y.L.; Shi, C.W.; Wang, J.Z.; Huang, K.Y.; Jin, Y.B.; Wang, C.F. Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antivir. Res. 2017, 138, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, J.; Guo, Y.; Yang, W.; Ye, L.; Shi, C.; Liu, Y.; Yang, G.; Wang, C. Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing HN of Newcastle disease virus and DC- targeting peptide fusion protein. J. Biotechnol. 2015, 216, 82–89. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Zhang, X.K.; Liu, Y.Y.; Zhang, L.J.; Ye, L.P.; Hu, J.T.; Xing, X.; Qi, C.; et al. Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res. 2016, 211, 46–57. [Google Scholar] [CrossRef]
- Van de Guchte, M.; van der Vossen, J.M.; Kok, J.; Venema, G. Construction of a lactococcal expression vector: Expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 1989, 55, 224–228. [Google Scholar] [CrossRef]
- Lin, K.H.; Hsu, A.P.; Shien, J.H.; Chang, T.J.; Liao, J.W.; Chen, J.R.; Lin, C.F.; Hsu, W.L. Avian reovirus sigma C enhances the mucosal and systemic immune responses elicited by antigen-conjugated lactic acid bacteria. Vaccine 2012, 30, 5019–5029. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Norton, P.M.; Brown, H.W.; Wells, J.M.; Macpherson, A.M.; Wilson, P.W.; Le Page, R.W. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 1996, 14, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Gaerthe, B.; Leer, R.J.; Van Der Stap, J.G.; Smittenaar, C.; Heijne Den Bak-Glashouwer, M.; Thole, J.E.; Tielen, F.J.; Pouwels, P.H.; Havenith, C.E. Engineering the microflora to vaccinate the mucosa: Serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 2000, 100, 510–518. [Google Scholar] [CrossRef]
- Bermudez-Humaran, L.G.; Cortes-Perez, N.G.; Le Loir, Y.; Alcocer-Gonzalez, J.M.; Tamez-Guerra, R.S.; de Oca-Luna, R.M.; Langella, P. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J. Med. Microbiol. 2004, 53, 427–433. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Han, X.; Tian, Y.; Guan, R.; Gao, W.; Yang, X.; Zhou, L.; Wang, H. Infectious Bronchitis Virus Infection Induces Apoptosis during Replication in Chicken Macrophage HD11 Cells. Viruses 2017, 9, 198. [Google Scholar] [CrossRef]
- Huang, C.K.; Yang, C.Y.; Jeng, Y.M.; Chen, C.L.; Wu, H.H.; Chang, Y.C.; Ma, C.; Kuo, W.H.; Chang, K.J.; Shew, J.Y.; et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-kappaB-mediated antiapoptotic pathway. Oncogene 2014, 33, 2968–2977. [Google Scholar] [CrossRef]
- Sarma, J.B.; Marshall, B.; Cleeve, V.; Tate, D.; Oswald, T. Impact of universal screening on MRSA bacteremias in a single acute NHS organisation (2006-12): Interrupted time-series analysis. Antimicrob. Resist. Infect. Control 2013, 2, 2. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef]
- Chen, Y.; Song, T.; Xiao, Y.L.; Wan, X.; Yang, L.; Li, J.; Zeng, G.; Fang, P.; Wang, Z.Z.; Gao, R. Enhancement of immune response of piglets to PCV-2 vaccine by porcine IL-2 and fusion IL-4/6 gene entrapped in chitosan nanoparticles. Res. Vet. Sci. 2018, 117, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Muhl, H.; Scheiermann, P.; Bachmann, M.; Hardle, L.; Heinrichs, A.; Pfeilschifter, J. IL-22 in tissue-protective therapy. Br. J. Pharmacol. 2013, 169, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Perales-Linares, R.; Navas-Martin, S. Toll-like receptor 3 in viral pathogenesis: Friend or foe? Immunology 2013, 140, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, X.; Li, D.; Sun, L.; He, Y.; Wei, H.; Jin, F.; Cao, Y. Toll-like receptor 7 and Toll-like receptor 9 agonists effectively enhance immunological memory in Plasmodium chabaudi infected BALB/c mice. Int. Immunopharmacol. 2020, 81, 106248. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massague, J. Transforming Growth Factor-Beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Boettler, T.; Panther, E.; Bengsch, B.; Nazarova, N.; Spangenberg, H.C.; Blum, H.E.; Thimme, R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 2006, 80, 3532–3540. [Google Scholar] [CrossRef]
- Susta, L.; Diel, D.G.; Courtney, S.; Cardenas-Garcia, S.; Sundick, R.S.; Miller, P.J.; Brown, C.C.; Afonso, C.L. Expression of chicken interleukin-2 by a highly virulent strain of Newcastle disease virus leads to decreased systemic viral load but does not significantly affect mortality in chickens. Virol. J. 2015, 12, 122. [Google Scholar] [CrossRef]
- Wu, X.; Zhai, X.; Lai, Y.; Zuo, L.; Zhang, Y.; Mei, X.; Xiang, R.; Kang, Z.; Zhou, L.; Wang, H. Construction and Immunogenicity of Novel Chimeric Virus-Like Particles Bearing Antigens of Infectious Bronchitis Virus and Newcastle Disease Virus. Viruses 2019, 11, 254. [Google Scholar] [CrossRef]
| Primers | F/R | Oligonucleotide Sequences (5′–3′) | GenBank Accession No. |
|---|---|---|---|
| β-actin | F | TGCTGTGTTCCCATCTATCG | X00182 |
| β-actin | R | TTGGTGACAATACCGTGTTCA | - |
| TLR-3 | F | GTGCTTGCTAGCTCTCGACT | FJ915480.1 |
| TLR-3 | R | GCTTTCTGTGTGCTCCAAGC | - |
| CD127 | F | TGGCATTCAAGCAAAAGCCG | EF116487.1 |
| CD127 | R | AATCCTTGCAGGACTTCGCT | - |
| IL-1β | F | TCGGGTTGGTTGGTGATG | NM_204524 |
| IL-1β | R | TGGGCATCAAGGGCTACA | - |
| TGF-β4 | F | CGTGCCCGTACATCTGGAG | AF459839.1 |
| TGF-β4 | R | GAGGGGGTCGAGGGTCTG | - |
| TLR-7 | F | ACGGTGTTGGATCTTGGGAC | NM_001011688.2 |
| TLR-7 | R | TGGACTTGCAACTTCGACCA | - |
| IL-22 | F | CAATGCCCATCAAGCCTGCA | AJ617782.1 |
| IL-22 | R | CTGTGCCACATCCTCAGCAT | - |
| BCL-6 | F | CTCATCTTCAGACGGCAAAGG | BQ038697.2 |
| BCL-6 | R | ATGTCTGTGCAGTGGAGTGTT | - |
| IL-6 | F | CAAGGTGACGGAGGAGGAC | JQ897539 |
| IL-6 | R | TGGCGAGGAGGGATTTCT | - |
| Treatment | Lg TCID50/mL |
|---|---|
| PBS | 0 |
| IBV | 6.58 ± 0.11 c |
| NC8-P + IBV | 6.08 ± 0.11 B |
| NC8-ChIL17B + IBV | 4.65 ± 0.05 A |
| Group | Initial Weight (g) | End Weight (g) | Net Gain (g) |
|---|---|---|---|
| PBS | 62.0 ± 0.76 | 360.0 ± 5.79 | 298.0 ± 4.63 |
| IBV | 61.0 ± 0.96 | 350.0 ± 5.92 | 294.0 ± 5.17 |
| NC8-P + IBV | 67.0 ± 0.49 | 375.0 ± 4.29 | 309.0 ± 5.52 |
| NC8-ChIL17B + IBV | 66.0 ± 0.47 | 380.0 ± 7.26 | 314.0 ± 2.98 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Peng, J.; Xiao, Y.; Liu, Y.; Hao, W.; Yang, X.; Wang, H.; Gao, R. The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens. Vaccines 2020, 8, 282. https://doi.org/10.3390/vaccines8020282
Guo S, Peng J, Xiao Y, Liu Y, Hao W, Yang X, Wang H, Gao R. The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens. Vaccines. 2020; 8(2):282. https://doi.org/10.3390/vaccines8020282
Chicago/Turabian StyleGuo, Shaohua, Junjie Peng, Yongle Xiao, Yanyan Liu, Weiwei Hao, Xin Yang, Hongning Wang, and Rong Gao. 2020. "The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens" Vaccines 8, no. 2: 282. https://doi.org/10.3390/vaccines8020282
APA StyleGuo, S., Peng, J., Xiao, Y., Liu, Y., Hao, W., Yang, X., Wang, H., & Gao, R. (2020). The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens. Vaccines, 8(2), 282. https://doi.org/10.3390/vaccines8020282









