Immunological Analysis of the Hepatitis B Virus “a” Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of Baculovirus Stock
2.3. Expression of Nc-aD VLPs
2.4. Purification of Nc-aD VLPs
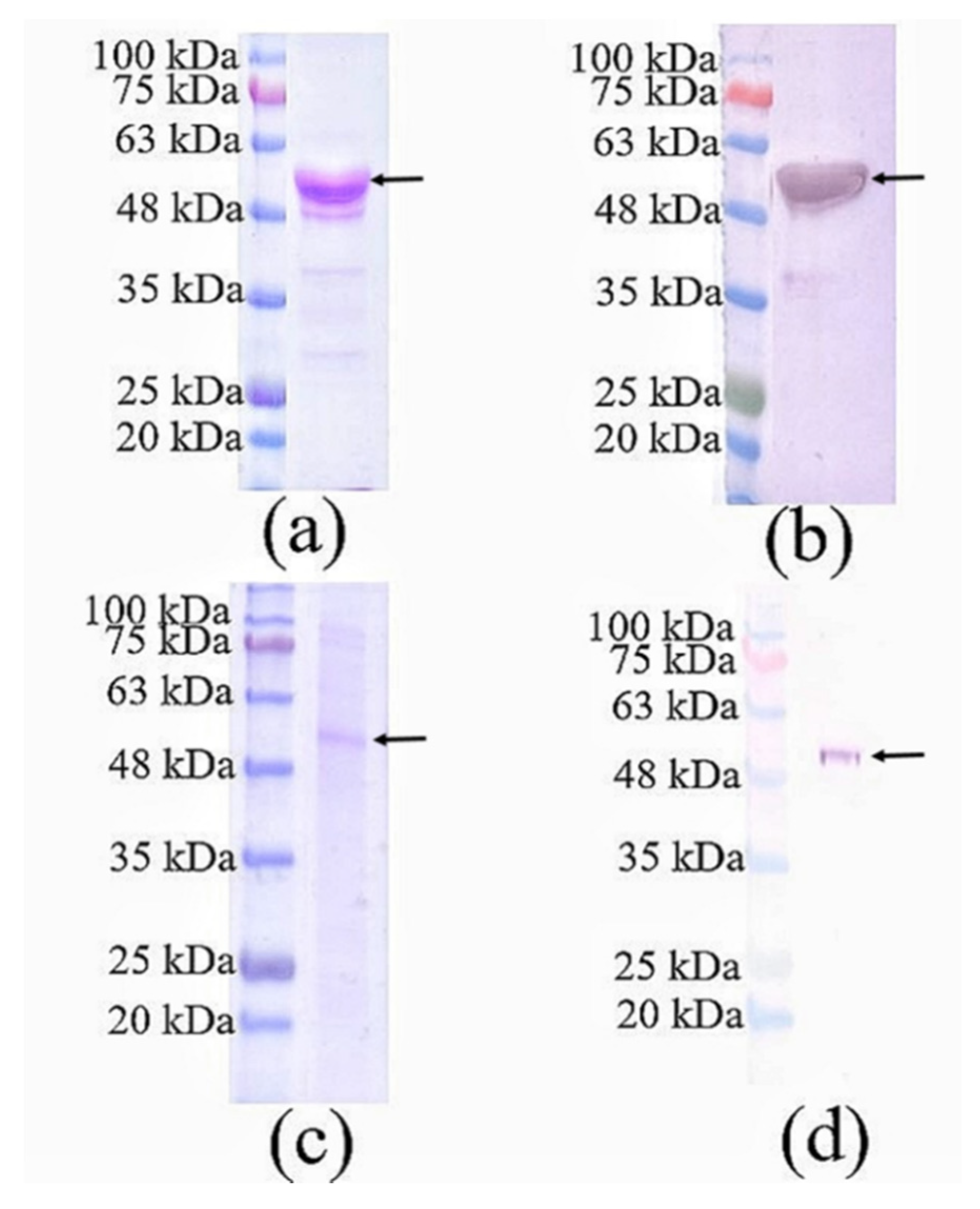
2.5. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
2.6. Transmission Electron Microscopy
2.7. Immunisation of BALB/c Mice
2.8. Immunogenicity of Nc-aD VLPs
2.9. Immunophenotyping of Mouse Splenocytes
2.10. Memory B-cell ELISPOT Assay
2.11. Statistical Analysis
3. Results
3.1. SDS-PAGE and Western Blotting of Purified Nc-aD VLPs Produced in Sf9 Cells
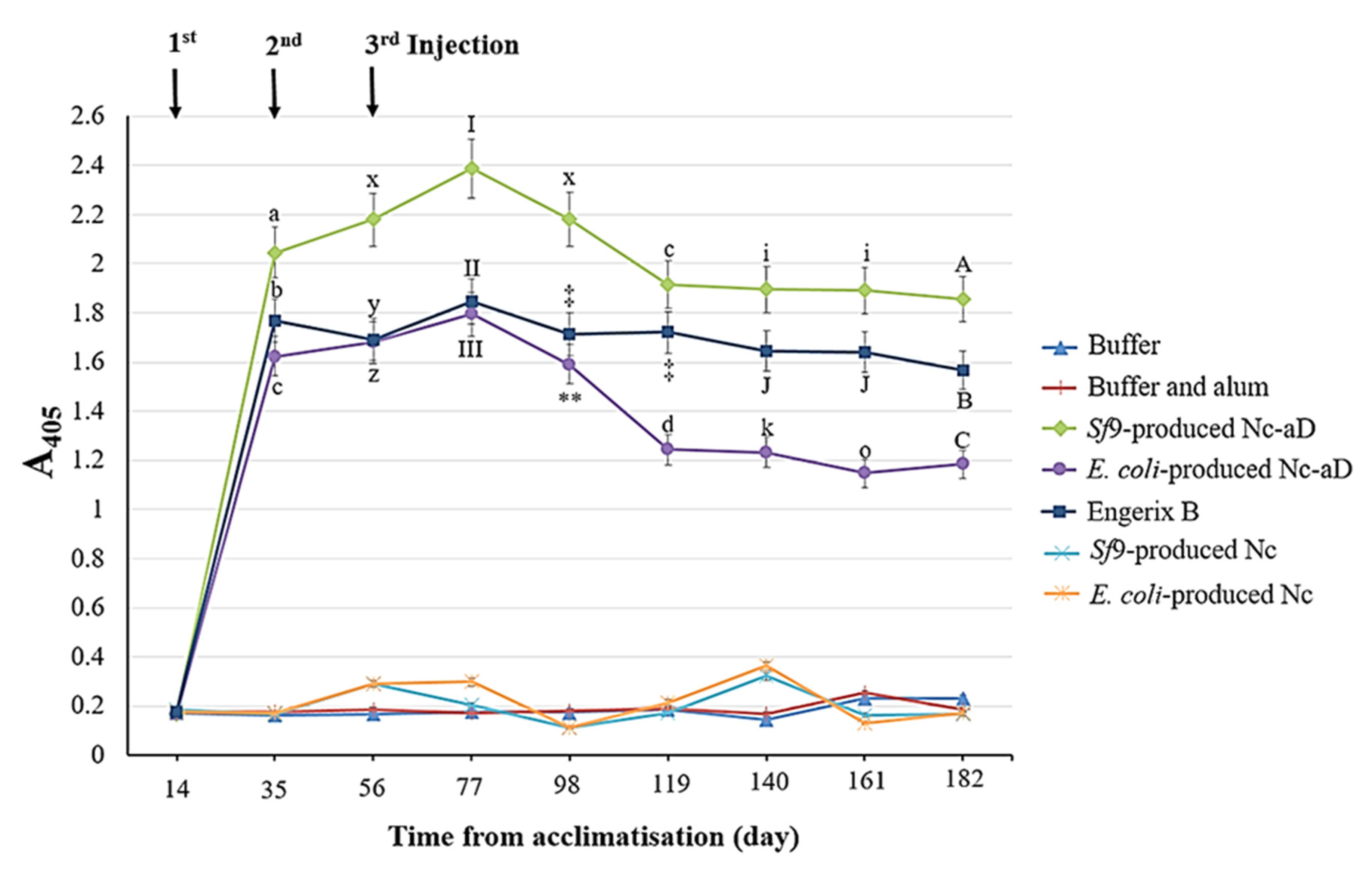
3.2. Immunogenicity of the Nc-aD VLPs in BALB/c Mice
3.3. Immunophenotyping of Mouse Splenocytes
3.4. ELISPOT of Memory B Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locarnini, S. Molecular virology of hepatitis B virus. Semin. Liver Dis. 2004, 24, 3–10. [Google Scholar] [CrossRef]
- Anikhindi, S.A.; Kumar, A.; Sharma, P.; Singla, V.; Bansal, N.; Arora, A. Ideal cure for hepatitis B infection: The target is in sight. J. Clin. Exp. Hepatol. 2018, 8, 188–194. [Google Scholar] [CrossRef]
- Tsai, K.; Kuo, C.; Ou, J.J. Mechanisms of hepatitis B virus persistence. Trends Microbiol. 2018, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, W.H. Do we need better hepatitis B vaccines? Indian J. Med. Res. 2017, 145, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.; Wilson, R.; Patrick, G.; André, F.; Watson, V. Hepatitis B vaccines: Assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin. Ther. 2001, 23, 392–403. [Google Scholar] [CrossRef]
- Bengsch, B.; Chang, K.M. Evolution in our understanding of hepatitis B virus virology and immunology. Clin. Liver Dis. 2016, 20, 629–644. [Google Scholar] [CrossRef]
- Lerous-Roels, G.; Cao, T.; De Knibber, A.; Meuleman, P.; Roobrouck, A.; Farhoudi, A.; Vanlandschoot, P.; Desombere, I. Prevention of hepatitis B infections: Vaccination and its limitations. Acta Clin. Belg. 2001, 56, 209–219. [Google Scholar] [CrossRef]
- Shouval, D. Hepatitis B vaccines. J. Hepatol. 2003, 39, 70–76. [Google Scholar] [CrossRef]
- Hassemer, M.; Finkernagel, M.; Peiffer, K.H.; Glebe, D.; Akhras, S.; Reuter, A.; Scheiblauer, H.; Sommer, L.; Chudy, M.; Nübling, M.; et al. Comparative characterization of hepatitis B virus surface antigen derived from different hepatitis B virus genotypes. Virology 2017, 502, 1–12. [Google Scholar] [CrossRef]
- Howard, C.R.; Allison, L.M.C. Hepatitis B surface antigen variation and protective immunity. Intervirology 1995, 38, 35–40. [Google Scholar] [CrossRef]
- Youm, J.W.; Won, Y.S.; Jeon, J.H.; Ryu, C.J.; Choi, Y.K.; Kim, H.C.; Kim, B.; Joung, H.; Kim, H.S. Oral immunogenicity of potato-derived HBsAg middle protein in BALB/c mice. Vaccine 2007, 25, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Netter, H.J.; Woo, W.; Tindle, R.; Macfarlan, R.I.; Gowans, E.J. Immunogenicity of recombinant HBsAg/HCV particles in mice pre-immunised with hepatitis B virus-specific vaccine. Vaccine 2003, 21, 2692–2697. [Google Scholar] [CrossRef]
- Ong, H.K.; Yong, C.Y.; Tan, W.S.; Yeap, S.K.; Omar, A.R.; Razak, M.A.; Ho, K.L. An influenza A vaccine based on the extracellular domain of matrix 2 protein protects BALB/c mice against H1N1 and H3N2. Vaccines 2019, 7, 91. [Google Scholar] [CrossRef]
- Ho, K.L.; Kueh, C.L.; Beh, P.L.; Tan, W.S.; Bhella, D. Cryo-electron microscopy structure of the Macrobrachium rosenbergii nodavirus capsid at 7 angstroms resolution. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Y.; Yeap, S.K.; Ho, K.L.; Omar, A.R.; Tan, W.S. Potential recombinant vaccine against influenza A virus based on M2e displayed on nodaviral capsid nanoparticles. Int. J. Nanomed. 2015, 10, 2751–2763. [Google Scholar] [CrossRef]
- Yong, C.Y.; Yeap, S.K.; Goh, Z.H.; Ho, K.L.; Omar, A.R.; Tan, W.S. Induction of humoral and cell-mediated immune responses by hepatitis B virus epitope displayed on the virus-like particles of prawn nodavirus. Appl. Environ. Microbiol. 2015, 81, 882–889. [Google Scholar] [CrossRef]
- Kueh, C.L.; Yong, C.Y.; Masoomi Dezfooli, S.; Bhassu, S.; Tan, S.G.; Tan, W.S. Virus-like particle of Macrobrachium rosenbergii nodavirus produced in Spodoptera frugiperda (Sf9) cells is distinctive from that produced in Escherichia coli. Biotechnol. Prog. 2016, 33, 548–557. [Google Scholar] [CrossRef]
- Goh, Z.H.; Tan, S.G.; Bhassu, S.; Tan, W.S. Virus-like particles of Macrobrachium rosenbergii nodavirus produced in bacteria. J. Virol. Methods 2011, 175, 74–79. [Google Scholar] [CrossRef]
- Tan, W.S.; Ho, K.L. Phage display creates innovative applications to combat hepatitis B virus. World J. Gastroenterol. 2014, 20, 11650–11670. [Google Scholar] [CrossRef]
- Hossain, M.G.; Ueda, K. Investigation of a novel hepatitis B virus surface antigen (HBsAg) escape mutant affecting immunogenicity. PLoS ONE 2017, 12, e0167871. [Google Scholar] [CrossRef]
- Hyakumura, M.; Walsh, R.; Thaysen-Andersen, M.; Kingston, N.J.; La, M.; Lu, L.; Lovrecz, G.; Packer, N.H.; Locarnini, S.; Netter, H.J. Modification of asparagine-linked glycan density for the design of hepatitis B virus virus-like particles with enhanced immunogenicity. J. Virol. 2015, 89, 11312–11322. [Google Scholar] [CrossRef] [PubMed]
- Lünsdorf, H.; Gurramkonda, C.; Adnan, A.; Khanna, N.; Rinas, U. Virus-like particle production with yeast: Ultrastructural and immunocytochemical insights into Pichia pastoris producing high levels of the hepatitis B surface antigen. Microb. Cell Fact. 2011, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hadiji-Abbes, N.; Martin, M.; Benzina, W.; Karray-Hakim, H.; Gergely, C.; Gargouri, A.; Mokdad-Gargouri, R. Extraction and purification of hepatitis B virus-like M particles from a recombinant Saccharomyces cerevisiae strain using alumina powder. J. Virol. Methods 2013, 187, 132–137. [Google Scholar] [CrossRef]
- Guan, Z.; Guo, B.; Huo, Y.; Guan, Z.; Wei, Y. Overview of expression of hepatitis B surface antigen in transgenic plants. Vaccine 2010, 28, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Shchelkunova, G.A. Plant-based vaccines against human hepatitis B virus. Expert Rev. Vaccines 2010, 9, 947–955. [Google Scholar] [CrossRef]
- Landford, R.E.; Luckow, V.; Kennedy, R.C.; Dreesman, G.R.; Notvall, L.; Summers, M.D. Expression and characterization of hepatitis B virus surface antigen polypeptides in insect cells with a baculovirus expression system. J. Virol. 1989, 63, 1549–1557. [Google Scholar] [CrossRef]
- Shi, X.; Jarvis, D.L. Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets 2007, 8, 1116–1125. [Google Scholar] [CrossRef]
- Via, S.T.; Meyer Zu Altenschildesche, G.; Doerfler, W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology 1983, 125, 107–117. [Google Scholar] [CrossRef]
- Kim, E.J.; Yoo, S.K. Cell surface display of hepatitis B virus surface antigen by using Pseudomonas syringae ice nucleation protein. Lett. Appl. Microbiol. 1999, 29, 292–297. [Google Scholar] [CrossRef]
- Ho, K.L.; Gabrielsen, M.; Beh, P.L.; Kueh, C.L.; Thong, Q.X.; Streetley, J.; Tan, W.S.; Bhella, D. Structure of the Macrobrachium rosenbergii nodavirus: A new genus within the Nodaviridae? PLoS Biol. 2018, 16, 3000038. [Google Scholar] [CrossRef]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Yoshimura, M.; Miyazaki, N.; Guan, H.H.; Chuankhayan, P.; Lin, C.C.; Chen, S.K.; Lin, P.J.; Huang, Y.C.; Iwasaki, K.; et al. The atomic structures of shrimp nodaviruses reveal new dimeric spike structures and particle polymorphism. Commun. Biol. 2019, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, K.; Serti, E.; Tsitoura, P.; Lavdas, A.A.; Varaklioti, A.; Pickl-Herk, A.M.; Blaas, D.; Oz-Arslan, D.; Zhu, R.; Hinterdorfer, P.; et al. Green fluorescent protein-tagged HCV non-enveloped capsid like particles: Development of a new tool for tracking HCV core uptake. Biochimie 2009, 91, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.; Gonzalez, J.M.; Gomez-Blanco, J.; Marabini, R.; Chichon, J.; Mena, I.; Angulo, I.; Carrascosa, J.L.; Verdague, N.; Trus, B.L.; et al. Epitope insertion at the N-terminal molecular switch of the rabbit hemorrhagic disease virus T = 3 capsid protein leads to larger T = 4 capsids. J. Virol. 2012, 86, 6470–6480. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Zhang, Z. Humoral immunity, the underestimated player in hepatitis B. Cell. Mol. Immunol. 2018, 15, 645–648. [Google Scholar] [CrossRef]
- Lindemann, M.; Koldehoff, M.; Fiedler, M.; Schumann, A.; Ottinger, H.D.; Heinemann, F.M.; Roggendorf, M.; Horn, P.A.; Beelen, D.W. Control of hepatitis B virus infection in hematopoietic stem cell recipients after receiving grafts from vaccinated donors. Bone Marrow Transplant. 2016, 51, 428–431. [Google Scholar] [CrossRef]
- Ryu, C.J.; Gripon, P.; Park, H.R.; Park, S.S.; Kim, Y.K.; Guguen-Guillouzo, C.; Yoo, O.J.; Hyo, J.H. In Vitro neutralization of hepatitis B virus by monoclonal antibodies against the viral surface antigen. J. Med. Virol. 1997, 52, 226–233. [Google Scholar] [CrossRef]
- Pride, M.W.; Shi, H.; Anchin, J.M.; Linthicum, D.S.; LoVerde, P.T.; Thakur, A.; Thanavala, Y. Molecular mimicry of hepatitis B surface antigen by an anti-idiotype-derived synthetic peptide. Proc. Natl. Acad. Sci. USA 1992, 89, 11900–11904. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Ishikawa, T.; Hobbs, M.V.; Matzke, B.; Schreiber, R.; Chisari, F.V. Intracellular inactivation of the hepatitis B virus by inflammatory cytokines. Immunity 1996, 39, 25–36. [Google Scholar] [CrossRef]
- Schuch, A.; Hoh, A.; Thimme, R. The role of natural killer cells and CD8+ T cells in hepatitis B virus infection. Front. Immunol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Pan, W.; Bayer, W.; Thoens, C.; Heim, K.; Dittmer, U.; Timm, J.; Wang, Q.; Yu, Q.; et al. MMP2/MMP9-mediated CD100 shedding is crucial for inducing intrahepatic anti-HBV CD8 T cell responses and HBV clearance. J. Hepatol. 2019, 71, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, T.; Gao, L.; Ma, C.; Yang, X.; Liang, X. Prostaglandin E2 facilitates hepatitis B virus replication by impairing CTL function. Mol. Immunol. 2018, 103, 243–250. [Google Scholar] [CrossRef]
- Liang, S.; Du, J.; Yan, H.; Zhou, Q.; Zhou, Y.; Yuan, Z.; Yan, S.; Fu, Q.; Wang, X.; Jia, S.; et al. A mouse model for studying the clearance of hepatitis B virus In Vivo using a luciferase reporter. PLoS ONE 2013, 8, e60005. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, P.; Dembek, C.; Kuklick, L.; Jäger, C.; Tedjokusumo, R.; John von Freyend, M.; Drebber, U.; Janowicz, Z.; Melber, K.; Protzer, U. A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice. Vaccine 2013, 31, 1197–1203. [Google Scholar] [CrossRef]
- Gamvrellis, A.; Leong, D.; Hanley, J.C.; Xiang, S.D.; Mottram, P.; Plebanski, M. Vaccines that facilitate antigen entry into dendritic cells. Immunol. Cell Biol. 2004, 82, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Grgacic, E.V.L.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Rochford, R.; Chung, J.; Shapiro, M.; Purcell, R.; Chisari, F.V. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999, 284, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chen, Y.; Liu, Y.; Wang, S.; Li, Y.; Wang, G.; Xia, J.; Zhao, X.; Huang, R.; Lu, S.; et al. Use of ELISpot assay to study HBs-specific B cell responses in vaccinated and HBV infected humans. Emerg. Infect. Dis. 2018, 7, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Tuaillon, E.; Al Tabaa, Y.; Petitjean, G.; Huguet, M.F.; Pajeaux, G.; Fondere, J.M.; Ponseille, B.; Ducos, J.; Blanc, P.; Vendrell, J.P. Detection of memory B lymphocytes specific to hepatitis B virus (HBV) surface antigen (HBsAg) from HBsAg-vaccinated or HBV-Immunized subjects by ELISPOT assay. J. Immunol. Methods 2006, 315, 144–152. [Google Scholar] [CrossRef]


| Immunisation Groups | Frequency of Gated Splenocytes (%) | ||
|---|---|---|---|
| CD3+ CD4+ | CD3+ CD8+ | CD8+/CD4+ Ratio | |
| Buffer only | 21.62 ± 0.95 a | 11.42 ± 0.51 g | 0.53 ± 0.01 v |
| Buffer and alum | 19.70 ± 0.30 b | 11.51 ± 0.02 g | 0.58 ± 0.01 v |
| Sf9-produced Nc-aD VLPs | 13.95 ± 0.15 c | 9.07 ± 0.23 h | 0.65 ± 0.02 w |
| E. coli-produced Nc-aD VLPS | 11.81 ± 0.32 d | 8.17 ± 0.08 i | 0.69 ± 0.01 w |
| Engerix B | 17.95 + 0.42 e | 11.92 + 0.12 k | 0.66 + 0.02 w |
| Sf9-produced Nc VLPs | 13.48 ± 0.45 c | 7.63 ± 0.09 j | 0.57 ± 0.02v |
| E. coli-produced Nc VLPs | 17.63 ± 0.37 e | 9.69 ± 0.36 h | 0.54 ± 0.03 v |
| Immunisation Groups | Spot Count 1 |
|---|---|
| Buffer only | 0.67 ± 0.58 a |
| Buffer and alum | 0.00 ± 0.00 a |
| Sf9-produced Nc-aD VLPs | 26.67 ± 0.57 b |
| E. coli-produced Nc-aD VLPs | 6.50 ± 0.45 c |
| Engerix B | 11.30 + 0.71 d |
| Sf9-produced Nc VLPs | 0.33 ± 0.58 a |
| E. coli-produced Nc VLPs | 0.33 ± 0.58 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninyio, N.N.; Ho, K.L.; Ong, H.K.; Yong, C.Y.; Chee, H.Y.; Hamid, M.; Tan, W.S. Immunological Analysis of the Hepatitis B Virus “a” Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells. Vaccines 2020, 8, 275. https://doi.org/10.3390/vaccines8020275
Ninyio NN, Ho KL, Ong HK, Yong CY, Chee HY, Hamid M, Tan WS. Immunological Analysis of the Hepatitis B Virus “a” Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells. Vaccines. 2020; 8(2):275. https://doi.org/10.3390/vaccines8020275
Chicago/Turabian StyleNinyio, Nathaniel Nyakaat, Kok Lian Ho, Hui Kian Ong, Chean Yeah Yong, Hui Yee Chee, Muhajir Hamid, and Wen Siang Tan. 2020. "Immunological Analysis of the Hepatitis B Virus “a” Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells" Vaccines 8, no. 2: 275. https://doi.org/10.3390/vaccines8020275
APA StyleNinyio, N. N., Ho, K. L., Ong, H. K., Yong, C. Y., Chee, H. Y., Hamid, M., & Tan, W. S. (2020). Immunological Analysis of the Hepatitis B Virus “a” Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells. Vaccines, 8(2), 275. https://doi.org/10.3390/vaccines8020275






