HSV-1-Specific IgG3 Titers Correlate with Brain Cortical Thinning in Individuals with Mild Cognitive Impairment and Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. HSV-1 IgG Analyses
2.3. Apolipoprotein E (ApoE) Genotyping
2.4. Morphometrical Analyses—MRI
2.5. Statistical Analysis
2.5.1. Demographical and HSV-1-IgG Analyses
2.5.2. Morphometrical Analyses—MRI
3. Results
3.1. Demographical and Clinical Characteristics of the Subjects
3.2. HSV-1-Specific Antibody Titers
3.3. MRI Analyses
3.3.1. Assessment of Downstream Neuronal Degeneration
3.3.2. Correlations between Cortical Thickness and HSV-1-Specific Igg3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Report 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia. (accessed on 19 September 2019).
- Kumar, A.; Singh, A.; Ekavali, N. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Bourgade, K.; Khalil, A.; Zerif, E.; Larbi, A.; Hirokawa, K.; Pawelec, G.; Bocti, C.; Lacombe, G.; et al. Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer’s Disease? Front. Aging Neurosci. 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, R.F.; Lathe, R.; Balin, B.J.; Ball, M.J.; Bearer, E.L.; Braak, H.; Bullido, M.J.; Carter, C.; Clerici, M.; Cosby, S.L.; et al. Microbes and Alzheimer’s disease. J. Alzheimers Dis. 2016, 51, 979–984. [Google Scholar] [PubMed]
- Mancuso, R.; Sicurella, M.; Agostini, S.; Marconi, P.; Clerici, M. Herpes simplex virus type 1 and Alzheimer’s disease: Link and potential impact on treatment. Expert Rev. Anti-Infect. Ther. 2019, 17, 715–731. [Google Scholar] [CrossRef]
- Jamieson, G.A.; Maitland, N.J.; Wilcock, G.K.; Craske, J.; Itzhaki, R.F. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J. Med. Virol. 1991, 33, 224–227. [Google Scholar] [CrossRef]
- Alvarez, G.; Aldudo, J.; Alonso, M.; Santana, S.; Valdivieso, F. Hepres simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells. J. Neurosci. Res. 2012, 90, 1020–1029. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Itzhaki, R.F.; Shipley, S.J.; Dobson, C.B. Herpes simplex virus infection causes cellular β-amyloid accumulation and secretase upregulation. Neurosci. Lett. 2007, 429, 95–100. [Google Scholar] [CrossRef]
- De Chiara, G.; Piacentini, R.; Fabiani, M.; Mastrodonato, A.; Marcocci, M.E.; Limongi, D.; Napoletani, G.; Protto, V.; Coluccio, P.; Celestino, I.; et al. Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019, 15, e1007617. [Google Scholar]
- Egan, K.P.; Wu, S.; Wigdahl, B.; Jennings, S.R. Immunological control of herpes simplex virus infections. J. Neurovirol. 2013, 19, 328–345. [Google Scholar]
- Dorshkind, K.; Montecino-Rodriguez, E.; Signer, R.A. The ageing immune system: Is it ever too old to become young again? Nat. Rev. Immunol. 2009, 9, 57–62. [Google Scholar] [PubMed]
- Amin, I.; Younas, S.; Afzal, S.; Shahid, M.; Idrees, M. Herpes Simplex Virus Type 1 and Host Antiviral Immune Responses: An Update. Viral Immunol. 2019, 32, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Baglio, F.; Cabinio, M.; Calabrese, E.; Hernis, A.; Nemni, R.; Clerici, M. Titers of herpes simplex virus type 1 antibodies positively correlate with grey matter volumes in Alzheimer’s disease. J. Alzheimers Dis. 2014, 33, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Baglio, F.; Agostini, S.; Cabinio, M.; Laganà, M.M.; Hernis, A.; Margaritella, N.; Guerini, F.R.; Zanzottera, M.; Nemni, R.; et al. Relationship between herpes simplex virus-1-specific antibody titers and cortical brain damage in Alzheimer’s disease and amnestic mild cognitive impairment. Front. Aging Neurosci. 2014, 6, 285. [Google Scholar] [CrossRef]
- Agostini, S.; Mancuso, R.; Hernis, A.; Costa, A.S.; Nemni, R.; Clerici, M. HSV-1-specific IgG subclasses distribution and serum neutralizing activity in Alzheimer’s disease and in Mild Cognitive Impairment. J. Alzheimers Dis. 2018, 63, 131–138. [Google Scholar] [CrossRef]
- Wiger, D.; Michaelsen, T.E. Binding site and subclass specificity of the herpes simplex virus type 1-induced Fc receptor. Immunology 1985, 54, 565–572. [Google Scholar]
- Dubin, G.; Scololof, E.; Frank, I.; Friedman, H.M. Herpes simplex virus type 1 Fc receptor protects infected cells from antiody-dependent cellular cytotoxicity. J. Virol. 1991, 65, 7046–7050. [Google Scholar]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Costa, A.S.; Agostini, S.; Guerini, F.R.; Mancuso, R.; Zanzottera, M.; Ripamonti, E.; Racca, V.; Nemni, R.; Clerici, M. Modulation of immune response to herpes simplex virus type 1 by IFNL3 and IRF7 polymorphisms: A study in Alzheimer’s disease. J. Alzheimers Dis. 2017, 60, 1055–1063. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Klapwijk, E.T.; van de Kamp, F.; van der Meulen, M.; Peters, S.; Wierenga, L.M. Qoala-T: A supervised-learning tool for quality control of FreeSurfer segmented MRI data. Neuroimage 2019, 189, 116–129. [Google Scholar] [PubMed]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. Alzheimer’s Disease Neuroimaging Initiative. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015, 115, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [PubMed]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R., Jr.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Rathore, S.; Hames, M.; Iftikhar, M.A.; Shacklett, A.; Davatzikos, C. A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer’s disease and its prodromal stages. Neuroimage 2017, 155, 530–548. [Google Scholar] [PubMed]
- Esiri, M.M. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J. Neurol. Sci. 1982, 54, 209–226. [Google Scholar] [CrossRef]
- Damasio, A.R.; Van Hoesen, G.W. The limbic system and the localisation of herpes simplex encephalitis. J. Neurol. Neurosurg. Psychiatry 1985, 48, 297–301. [Google Scholar]
- Baringer, J.R.; Pisani, P. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann. Neurol. 1994, 36, 823–829. [Google Scholar]
- Agostini, S.; Mancuso, R.; Baglio, F.; Cabinio, M.; Hernis, A.; Costa, A.S.; Calabrese, E.; Nemni, R.; Clerici, M. High avidity HSV-1 antibodies correlate with absence of amnestic Mild Cognitive Impairment conversion to Alzheimer’s disease. Brain Behav. Immun. 2016, 58, 254–260. [Google Scholar] [CrossRef]
- Ball, M.J. Limbic predilection in Alzheimer dementia: Is reactivated herpesvirus involved? Can. J. Neurol. Sci. 1982, 9, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.R.; Shang, D.; Wilcock, G.K.; Itzhaki, R.F. Alzheimer’s disease, herpes simplex virus type 1, cold sores and apolipoprotein E4. Biochem. Soc. Trans. 1995, 23, 594S. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, R.F.; Lin, W.R.; Shang, D.; Wilcock, G.K.; Faragher, B.; Jamieson, G.A. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet 1997, 349, 241–244. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Shipley, S.J.; Combrinck, M.; Wilcock, G.K.; Itzhaki, R.F. Productive Herpes Simplex Virus in Brain of Elderly Normal Subjects and Alzheimer’s Disease Patients. J. Med. Virol. 2005, 75, 300–306. [Google Scholar] [CrossRef]
- Marques, A.R.; Straus, S.E.; Fahle., G.; Weir, S.; Csako, G.; Fischer, S.H. Lack of association between HSV-1 DNA in the brain, Alzheimer’s disease and apolipoprotein E4. J. Neurovirol. 2001, 7, 82–83. [Google Scholar]
- Hemling, N.; Röyttä, M.; Rinne, J.; Pollanen, P. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann. Neurol. 2003, 54, 267–271. [Google Scholar] [CrossRef]
- Mori, I.; Kimura, Y.; Naiki, H.; Matsubara., R.; Takeuchi, T.; Yokochi, T.; Nishiyama, Y. Reactivation of HSV-1 in the brain of patients with familial Alzheimer’s disease. J. Med. Virol. 2004, 73, 605–611. [Google Scholar] [CrossRef]
- Olsson, J.; Lövheim, H.; Honkala, E.; Karhunen, P.J.; Elgh, F.; Kok, E.H. HSV presence in brains of individuals without dementia: The TASTY brain series. Dis. Model. Mech. 2016, 9, 1349–1355. [Google Scholar] [CrossRef]
- Damelang, T.; Rogerson, S.J.; Kent, S.J.; Chung, A.W. Role of IgG3 in infectious diseases. Trends Immunol. 2019, 40, 197–211. [Google Scholar] [CrossRef]
- Cabinio, M.; Saresella, M.; Piancone, F.; La Rosa, F.; Marventano, I.; Guerini, F.R.; Nemni, R.; Baglio, F.; Clerici, M. Association between hippocampal shape, neuroinflammation, and cognitive decline in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 1131–1144. [Google Scholar] [CrossRef]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [PubMed]
| Parameters | AD | MCI | HC | p-Value |
|---|---|---|---|---|
| N. | 70 | 61 | 67 | |
| Gender (M:F) | 28:42 | 30:31 | 28:39 | n.s. |
| Age, years | 75.8 ± 6.4 | 74.0 ± 5.6 | 72.3 ± 7.8 | n.s. |
| MMSE | 20.7 ± 3.2 | 25.1 ± 2.1 | 29.3 ± 0.5 | <0.0001 |
| Level of education, years | 8.7 ± 3.6 | 9.7 ± 4.3 | 8.6 ± 3.9 | n.s. |
| APOE ε-4 carriers (%) | 44 | 36 | 18 | <0.05 |
| HSV-1-specific IgG, % and AI | 90% 9.1; 7.2–10.1 | 95% 8.4; 6.2–9.8 | 88% 7.7; 6.2–8.6 | n.s. <0.05 |
| HSV-1-specific IgG3, % and OD | 77% 0.6; 0.5–0.8 | 88% 0.6; 0.5–0.9 | 66% 0.6; 0.5–0.9 | <0.05 n.s |
| Parameters | AD | MCI | HC-MRI | p-Value |
|---|---|---|---|---|
| N. | 27 | 30 | 33 | |
| Gender (M:F) | 10:17 | 15:15 | 9:24 | n.s. |
| Age, years | 75.8 ± 5.6 | 72.9 ± 6.2 | 72.7 ± 5.0 | n.s. |
| MMSE | 22.6 ± 0.3 | 26.7 ± 0.3 | 29.2 ± 0.3 | < 0.001 |
| Right hippocampal volume | 2592.8 ± 77.3 | 2922.5 ± 73.3 | 3129.1 ± 70.1 | < 0.001 |
| Left hippocampal volume | 2435.3 ± 83.9 | 2828.3 ± 79.6 | 3087.5 ± 76.1 | < 0.001 |
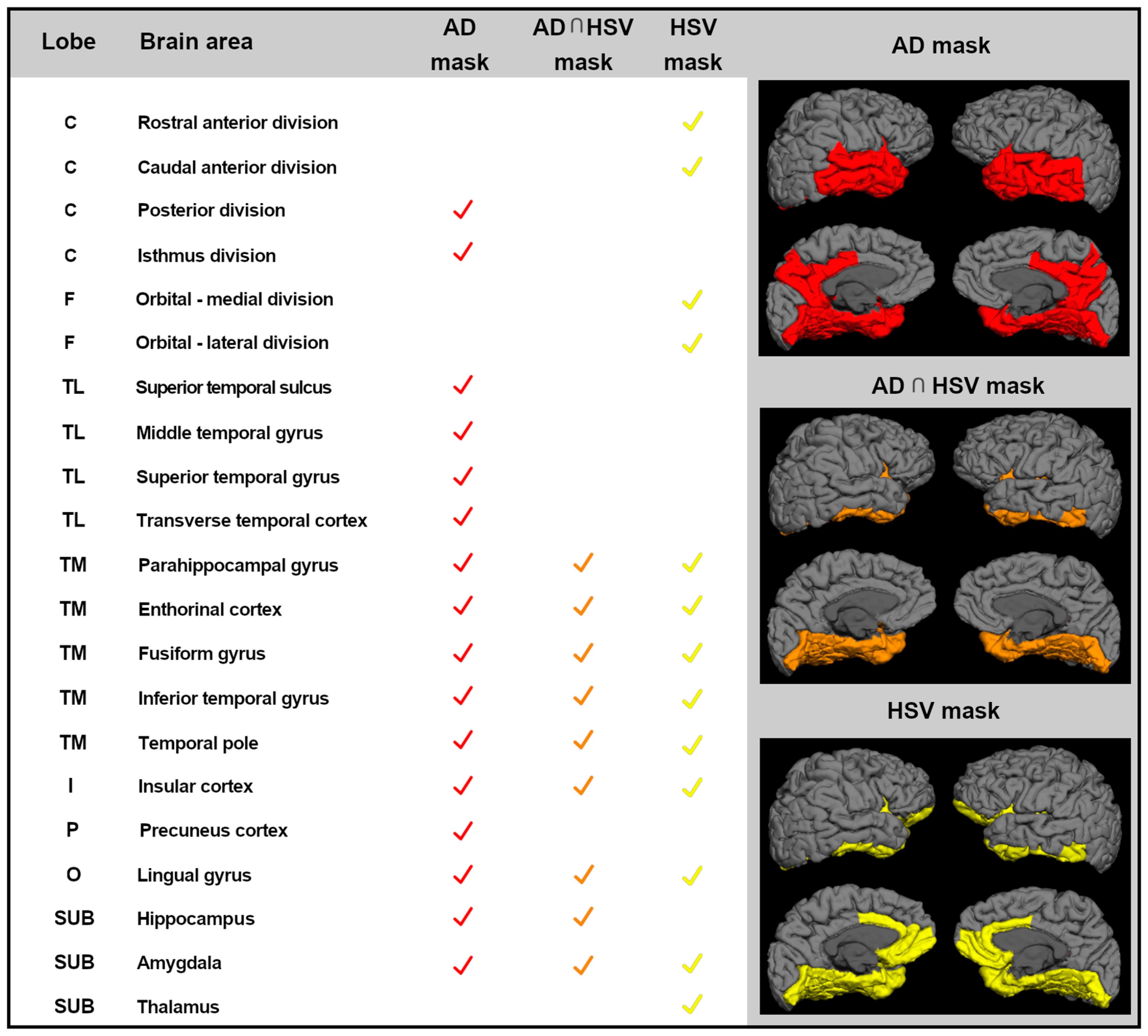
| Lobe | Brain Area | MCI | AD | AD Mask | AD ∩ HSV | HSV Mask | |||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | ||||||
| C | Isthmus division | r | −0.467 | 0.07561 | −0.2187 | −0.3781 | x | ||
| p-value | 0.0162 * | 0.7136 | 0.3162 | 0.0752 | |||||
| TL | Middle temporal gyrus | r | −0.2268 | −0.1363 | −0.5654 | −0.1744 | x | ||
| p-value | 0.2653 | 0.5067 | 0.0049 * | 0.4262 | |||||
| TL | Superior temporal gyrus | r | −0.2703 | −0.496 | −0.3303 | −0.4252 | x | ||
| p-value | 0.1816 | 0.01 * | 0.1237 | 0.0431 * | |||||
| TL | Transverse temporal cortex | r | −0.2483 | −0.4874 | −0.1729 | −0.4337 | x | ||
| p-value | 0.2213 | 0.0115 * | 0.4302 | 0.0387 * | |||||
| TM | Parahippocampal gyrus | r | 0.0799 | 0.01633 | −0.5272 | −0.3543 | x | x | X |
| p-value | 0.6981 | 0.9369 | 0.0097 * | 0.0972 | |||||
| TM | Fusiform gyrus | r | −0.3602 | −0.3018 | −0.4156 | −0.1747 | x | x | X |
| p-value | 0.0706 | 0.1341 | 0.0486 * | 0.4254 | |||||
| TM | Inferior temporal gyrus | r | −0.2018 | −0.3054 | −0.4832 | −0.2864 | x | x | X |
| p-value | 0.3228 | 0.1293 | 0.0195 * | 0.1853 | |||||
| TM | Temporal pole | r | −0.0795 | −0.1542 | −0.5661 | −0.4616 | x | x | X |
| p-value | 0.6996 | 0.452 | 0.0049 * | 0.0266 * | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, R.; Cabinio, M.; Agostini, S.; Baglio, F.; Clerici, M. HSV-1-Specific IgG3 Titers Correlate with Brain Cortical Thinning in Individuals with Mild Cognitive Impairment and Alzheimer’s Disease. Vaccines 2020, 8, 255. https://doi.org/10.3390/vaccines8020255
Mancuso R, Cabinio M, Agostini S, Baglio F, Clerici M. HSV-1-Specific IgG3 Titers Correlate with Brain Cortical Thinning in Individuals with Mild Cognitive Impairment and Alzheimer’s Disease. Vaccines. 2020; 8(2):255. https://doi.org/10.3390/vaccines8020255
Chicago/Turabian StyleMancuso, Roberta, Monia Cabinio, Simone Agostini, Francesca Baglio, and Mario Clerici. 2020. "HSV-1-Specific IgG3 Titers Correlate with Brain Cortical Thinning in Individuals with Mild Cognitive Impairment and Alzheimer’s Disease" Vaccines 8, no. 2: 255. https://doi.org/10.3390/vaccines8020255
APA StyleMancuso, R., Cabinio, M., Agostini, S., Baglio, F., & Clerici, M. (2020). HSV-1-Specific IgG3 Titers Correlate with Brain Cortical Thinning in Individuals with Mild Cognitive Impairment and Alzheimer’s Disease. Vaccines, 8(2), 255. https://doi.org/10.3390/vaccines8020255






