Antigen-Based Nano-Immunotherapy Controls Parasite Persistence, Inflammatory and Oxidative Stress, and Cardiac Fibrosis, the Hallmarks of Chronic Chagas Cardiomyopathy, in A Mouse Model of Trypanosoma cruzi Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Composition of Immunotherapy
2.3. Mice, Challenge Infection, and Treatment with Immunotherapy
2.4. Flow Cytometry
2.5. Real Time RT-qPCR
2.6. Parasite Burden
2.7. Histology
2.8. Serum Markers of Oxidative Stress and Inflammation
2.9. Western Blotting
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
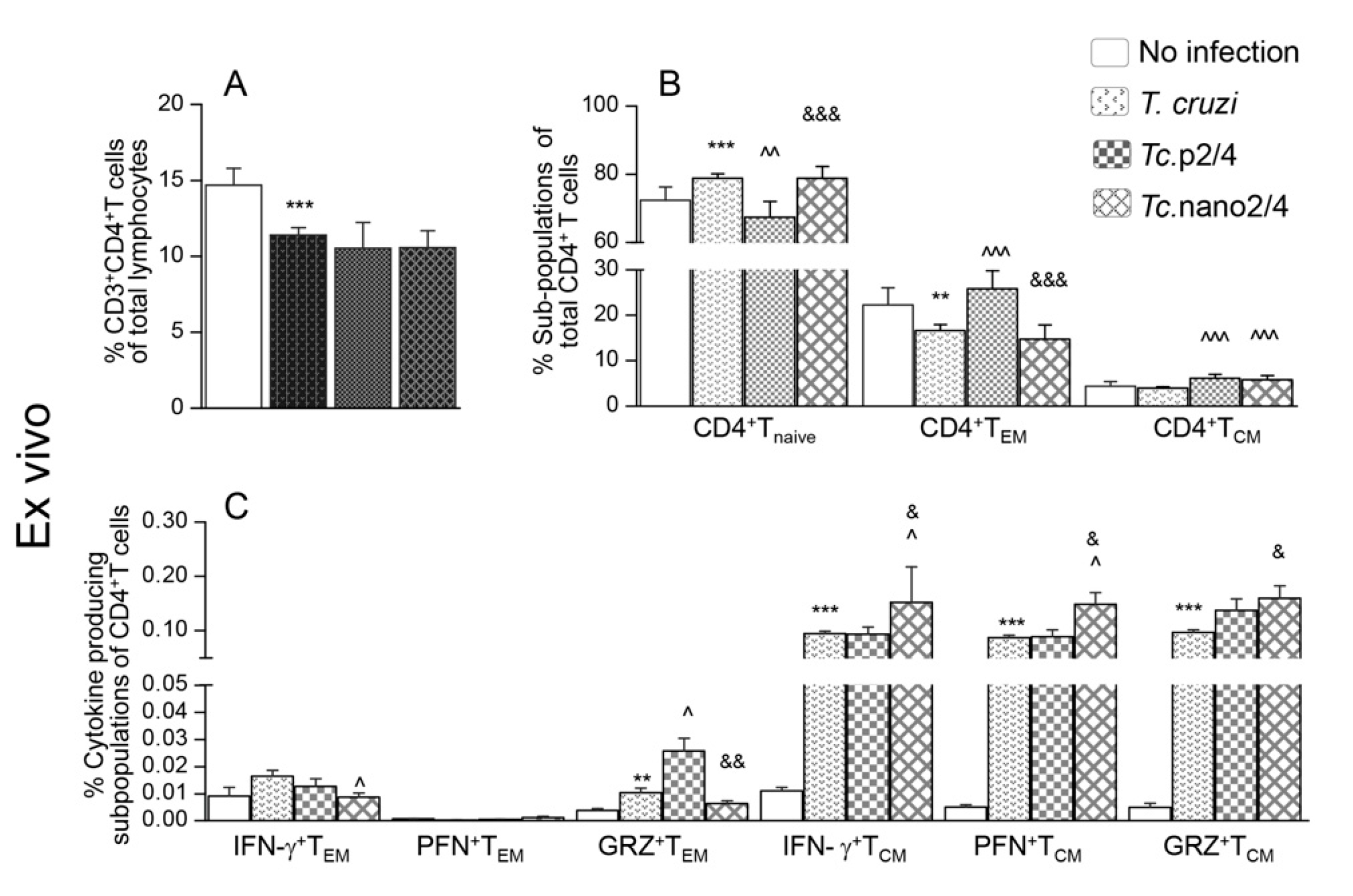
3.1. Effects of Nano-Immunotherapy on Functional Activation and Recall Response of CD4+T Cells in Chagas Mice
3.2. Effects of Nano-Immunotherapy on Functional Activation and Recall Response of CD8+ T Cells in Chagas Mice
3.3. Splenic Expression of Cytokines in Chagas Mice (± Immunotherapy)
3.4. Parasite Persistence and Inflammatory Pathology (± Nano-Immunotherapy)
3.5. Efficacy of Nano2/4 in Arresting Peripheral and Myocardial Oxidative Stress in Chagas Mice
3.6. Tissue Fibrosis and Profibrotic Macrophages in Chagas Disease (± Nano Therapy)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lopez, M.; Tanowitz, H.B.; Garg, N.J. Pathogenesis of chronic Chagas disease: Macrophages, mitochondria, and oxidative Stress. Curr. Clin. Microbiol. Rep. 2018, 5, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and pathogenesis of Chagas heart disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L. Council on Chagas disease of the interamerican society of cardiology. Chagas disease: An overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013, 62, 767–776. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Marin, J.A.N.; Rassi, A. Chronic Chagas cardiomyopathy: A review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem. Inst. Oswaldo Cruz 2017, 112, 224–235. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.M.; Myler, P.J.; Bartholomeu, D.C.; Nilsson, D.; Aggarwal, G.; Tran, A.N.; Ghedin, E.; Worthey, E.A.; Delcher, A.L.; Blandin, G.; et al. The genome sequence of Trypanosoma cruzi etiologic agent of Chagas disease. Science 2005, 309, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Sinha, M.; Luxon, B.; Garg, N.J. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: In silico and in vitro screening. Infect. Immun. 2004, 72, 6245–6254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhatia, V.; Garg, N.J. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin. Vaccine Immunol. 2008, 15, 1158–1164. [Google Scholar] [CrossRef]
- Aparicio-Burgos, J.E.; Ochoa-Garcia, L.; Zepeda-Escobar, J.A.; Gupta, S.; Dhiman, M.; Martínez, J.S.; de Oca-Jiménez, R.M.; Val Arreola, M.; Barbabosa-Pliego, A.; Vázquez-Chagoyán, J.C.; et al. Testing the efficacy of a multi- component DNA-prime/DNA-boost vaccine against Trypanosoma cruzi infection in dogs. PLoS Negl. Trop. Dis. 2011, 5, e1050. [Google Scholar] [CrossRef]
- Gupta, S.; Wan, X.-X.; Zago, M.P.; Sellers, V.C.; Silva, T.S.; Assiah, D.; Dhiman, M.; Nuñez, S.; Petersen, J.R.; Vázquez-Chagoyán, J.C.; et al. Antigenicity and diagnostic potential of vaccine candidates in human Chagas disease. PLoS Negl. Trop. Dis. 2013, 7, e2018. [Google Scholar] [CrossRef]
- Gupta, S.; Garg, N.J. Prophylactic efficacy of TcVac2R against Trypanosoma cruzi in mice. PLoS Negl. Trop. Dis. 2010, 4, e797. [Google Scholar] [CrossRef]
- Gupta, S.; Garg, N.J. Delivery of antigenic candidates by a DNA/MVA heterologous approach elicits effector CD8+T cell mediated immunity against Trypanosoma cruzi. Vaccine 2012, 12, 1464–1478. [Google Scholar] [CrossRef]
- Gupta, S.; Garg, N.J. TcVac3 induced control of Trypanosoma cruzi infection and chronic myocarditis in mice. PLoS ONE 2013, 8, e59434. [Google Scholar] [CrossRef]
- Gupta, S.; Garg, N.J. A two-component DNA-prime/protein-boost vaccination strategy for eliciting long- term, protective T cell immunity against Trypanosoma cruzi. PLoS Pathog. 2015, 11, e1004828. [Google Scholar] [CrossRef][Green Version]
- Aparicio-Burgos, J.E.; Zepeda-Escobar, J.A.; de Oca-Jimenez, R.M.; Estrada-Franco, J.G.; Barbabosa-Pliego, A.; Ochoa-García, L.; Alejandre-Aguilar, R.; Rivas, N.; Peñuelas-Rivas, G.; Val-Arreola, M.; et al. Immune protection against Trypanosoma cruzi induced by TcVac4 in a canine model. PLoS Negl. Trop. Dis. 2015, 9, e0003625. [Google Scholar] [CrossRef]
- Dumonteil, E. DNA Vaccines against Protozoan Parasites: Advances and Challenges. J. Biomed. Biotechnol. 2007, 2007, 90520. [Google Scholar] [CrossRef]
- Vázquez-Chagoyán, J.C.; Gupta, S.; Garg, N.J. Vaccine development against Trypanosoma cruzi and Chagas disease. Adv. Parasitol. 2011, 75, 121–146. [Google Scholar]
- Dos Santos Virgilio, F.; Pontes, C.; Dominguez, M.R.; Ersching, J.; Rodrigues, M.M.; Vasconcelos, J.R. CD8(+) T cell-mediated immunity during Trypanosoma cruzi infection: A path for vaccine development? Mediators Inflamm. 2014, 2014, 243786. [Google Scholar] [CrossRef]
- Rios, L.E.; Vazquez-Chagoyan, J.C.; Pacheco, A.O.; Zago, M.P.; Garg, N.J. Immunity and vaccine development efforts against Trypanosoma cruzi. Acta Trop. 2019, 200, 105168. [Google Scholar] [CrossRef] [PubMed]
- Borggren, M.; Nielsen, J.; Bragstad, K.; Karlsson, I.; Krog, J.S.; Williams, J.A.; Fomsgaard, A. Vector optimization and needle-free intradermal application of a broadly protective polyvalent influenza A DNA vaccine for pigs and humans. Hum. Vaccin. Immunother. 2015, 11, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Carnes, A.E.; Hodgson, C.P. Plasmid DNA vaccine vector design: Impact on efficacy, safety and upstream production. Biotechnol. Adv. 2009, 27, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Chowdhury, I.H.; Jie, Z.; Choudhuri, S.; Garg, N.J. Origin of monocytes/macrophages contributing to chronic inflammation in Chagas disease: Role of SIRT1 inhibition of FAK-NFkB- dependent proliferation and proinflammatory activation of macrophages. Cells 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.J.; Yin, Y.W.; Garg, N.J. PARP1 depletion improves mitochondrial and heart function in Chagas disease: Effects on POLG dependent mtDNA maintenance. PLoS Pathog. 2018, 14, e1007065. [Google Scholar] [CrossRef]
- Dhiman, M.; Garg, N.J. NADPH oxidase inhibition ameliorates Trypanosoma cruzi-induced myocarditis during Chagas disease. J. Pathol. 2011, 225, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Cabibi, D.; Bronte, F.; Porcasi, R.; Ingrao, S.; Giannone, A.G.; Maida, M.; Grazia Bavetta, M.; Petta, S.; Di Marco, V.; Calvaruso, V. Comparison of histochemical stainings in evaluation of liver fibrosis and correlation with transient elastography in chronic hepatitis. Anal. Cell. Pathol. 2015, 2015, 431750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhiman, M.; Estrada-Franco, J.G.; Pando, J.; Ramirez-Aguilar, F.J.; Spratt, H.; Vazquez-Corzo, S.; Perez-Molina, G.; Gallegos-Sandoval, R.; Moreno, R. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in chagasic patients. Clin. Vaccine Immunol. 2009, 16, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-X.; Gupta, S.; Zago, M.P.; Davidson, M.M.; Dousset, P.; Amoroso, A.; Garg, N.J. Defects of mtDNA replication impaired the mitochondrial biogenesis during Trypanosoma cruzi infection in human cardiomyocytes and chagasic patients: The role of Nrf1/2 and antioxidant response. J. Am. Heart Assoc. 2012, 1, e003855. [Google Scholar] [CrossRef]
- Garg, N.J.; Bhatia, V.; Gerstner, A.; de Ford, J.; Papaconstantinou, J. Gene expression analysis in mitochondria from chagasic mice: Alterations in specific metabolic pathways. Biochem. J. 2004, 381, 743–752. [Google Scholar] [CrossRef]
- Wen, J.J.; Nagajyothi, F.; Machado, F.S.; Weiss, L.M.; Scherer, P.E.; Tanowitz, H.B.; Garg, N.J.A. Markers of oxidative stress in adipose tissue during Trypanosoma cruzi infection. Parasitol. Res. 2014, 113, 3159–3165. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Sanchez-Burgos, G.; Mezquita-Vega, R.G.; Escobedo-Ortegon, J.; Ramirez-Sierra, M.J.; Arjona-Torres, A.; Ouaissi, A.; Rodrigues, M.M.; Dumonteil, E. Comparative evaluation of therapeutic DNA vaccines against Trypanosoma cruzi in mice. FEMS Immunol. Med. Microbiol. 2007, 50, 333–341. [Google Scholar] [CrossRef]
- Zapata-Estrella, H.; Hummel-Newell, C.; Sanchez-Burgos, G.; Escobedo-Ortegon, J.; Ramirez-Sierra, M.J.; Arjona-Torres, A.; Dumonteil, E. Control of Trypanosoma cruzi infection and changes in T-cell populations induced by a therapeutic DNA vaccine in mice. Immunol. Lett. 2006, 103, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Limon-Flores, A.Y.; Cervera-Cetina, R.; Tzec-Arjona, J.L.; Ek-Macias, L.; Sánchez-Burgos, G.; Ramirez-Sierra, M.J.; Cruz-Chan, J.V.; VanWynsberghe, N.R.; Dumonteil, E. Effect of a combination DNA vaccine for the prevention and therapy of Trypanosoma cruzi infection in mice: Role of CD4+ and CD8+ T cells. Vaccine 2010, 28, 7414–7419. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.; Campos, E.E.; Menon, R.; Zago, M.P.; Garg, N.J. Epidemiology and pathogenesis of fetal- transplacental transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. BBA Mol. Basis Dis. 2019, 1866, 165591. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-J.; Bhatia, V.; Popov, V.L.; Garg, N.J. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas disease. Am. J. Pathol. 2006, 169, 1953–1964. [Google Scholar] [CrossRef][Green Version]
- Caldas, I.S.; Talvani, A.; Caldas, S.; Carneiro, C.M.; de Lana, M.; da Matta Guedes, P.M.; Bahia, M.T. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol. Res. 2008, 103, 413–421. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Wen, J.-J.; Gupta, S.; Guan, Z.; Dhiman, M.; Condon, D.; Lui, C.; Garg, N.J. Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J. Am. Coll. Cardiol. 2010, 55, 2499–2508. [Google Scholar] [CrossRef]
- Dhiman, M.; Wan, X.-X.; Popov, L.V.; Vargas, G.; Garg, N.J. MnSODtg mice control myocardial inflammatory and oxidative stress and remodeling responses elicited in chronic Chagas disease. J. Am. Heart Assoc. 2013, 2, e000302. [Google Scholar] [CrossRef]
- Gupta, S.; Smith, C.; Auclair, S.; Delgadillo Ade, J.; Garg, N.J. Therapeutic efficacy of a subunit vaccine in controlling chronic Trypanosoma cruzi infection and Chagas disease is enhanced by glutathione peroxidase over-expression. PLoS ONE 2015, 10, e0130562. [Google Scholar] [CrossRef]
- Wen, J.J.; Wan, X.; Thacker, J.; Garg, N.J. Chemotherapeutic efficacy of phosphodiesterase inhibitors in Chagas cardiomyopathy. JACC Basic Transl. Sci. 2016, 1, 235–250. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wen, J.J.; Koo, S.J.; Liang, L.Y.; Garg, N.J. SIRT1-PGC1alpha-NFkappaB pathway of oxidative and inflammatory stress during Trypanosoma cruzi infection: Benefits of SIRT1-targeted therapy in improving heart function in Chagas disease. PLoS Pathog. 2016, 12, e1005954. [Google Scholar] [CrossRef] [PubMed]
- Senra, T.; Ianni, B.M.; Costa, A.C.P.; Mady, C.; Martinelli-Filho, M.; Kalil-Filho, R.; Rochitte, C.E. Long-term prognostic value of myocardial fibrosis in patients with Chagas cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Kania, G.; Blyszczuk, P.; Eriksson, U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc. Med. 2009, 19, 247–252. [Google Scholar] [CrossRef]
- da Costa, A.W.F.; do Carmo Neto, J.R.; Braga, Y.L.L.; Silva, B.A.; Lamounier, A.B.; Silva, B.O.; Dos Reis, M.A.; de Oliveira, F.A.; Celes, M.R.N.; Machado, J.R. Cardiac Chagas disease: MMPs, TIMPs, Galectins, and TGF-beta as tissue remodeling players. Dis. Markers 2019, 2019, 3632906. [Google Scholar] [CrossRef]
- Deng, X.S.; Meng, X.; Li, F.; Venardos, N.; Fullerton, D.; Jaggers, J. MMP-12-induced pro-osteogenic responses in human aortic valve interstitial cells. J. Surg. Res. 2019, 235, 44–51. [Google Scholar] [CrossRef]
- Kraen, M.; Frantz, S.; Nihlen, U.; Engström, G.; Löfdahl, C.G.; Wollmer, P.; Dencker, M. Matrix metalloproteinases in COPD and atherosclerosis with emphasis on the effects of smoking. PLoS ONE 2019, 14, e0211987. [Google Scholar] [CrossRef]
- Stawski, L.; Haines, P.; Fine, A.; Rudnicka, L.; Trojanowska, M. MMP-12 deficiency attenuates angiotensin II-induced vascular injury, M2 macrophage accumulation, and skin and heart fibrosis. PLoS ONE 2014, 9, e109763. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef]







| Immunotherapy | Heart Mean ± SD (Score Range) | Skeletal Muscle Mean ± SD (Score Range) |
|---|---|---|
| A. Inflammation score | ||
| No infection | 0 | 0 |
| T. cruzi only | 2.17 ± 0.17*** (0–3) | 2.91 ± 0.08*** (1–3) |
| Tc.p2/4 | 0.83 ± 0.11^^^ (0–1) | 1 ± 0^^^ (1) |
| Tc.nano2/4 | 0.91 ± 0.08^^^ (0–1) | 0.91 ± 0.08^^^ (0–1) |
| B. Fibrosis score | ||
| No infection | 0 | 0 |
| T. cruzi only | 5.1 ± 0.48*** | 6.4 ± 0.8*** |
| Tc.p2/4 | 0.73 ± 0.02^^^ | 0.74 ± 0.6^^^ |
| Tc.nano2/4 | 0.4 ± 0.04^^^, & | 0.51 ± 0.05^^^, & |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokugamage, N.; Choudhuri, S.; Davies, C.; Chowdhury, I.H.; Garg, N.J. Antigen-Based Nano-Immunotherapy Controls Parasite Persistence, Inflammatory and Oxidative Stress, and Cardiac Fibrosis, the Hallmarks of Chronic Chagas Cardiomyopathy, in A Mouse Model of Trypanosoma cruzi Infection. Vaccines 2020, 8, 96. https://doi.org/10.3390/vaccines8010096
Lokugamage N, Choudhuri S, Davies C, Chowdhury IH, Garg NJ. Antigen-Based Nano-Immunotherapy Controls Parasite Persistence, Inflammatory and Oxidative Stress, and Cardiac Fibrosis, the Hallmarks of Chronic Chagas Cardiomyopathy, in A Mouse Model of Trypanosoma cruzi Infection. Vaccines. 2020; 8(1):96. https://doi.org/10.3390/vaccines8010096
Chicago/Turabian StyleLokugamage, Nandadeva, Subhadip Choudhuri, Carolina Davies, Imran Hussain Chowdhury, and Nisha Jain Garg. 2020. "Antigen-Based Nano-Immunotherapy Controls Parasite Persistence, Inflammatory and Oxidative Stress, and Cardiac Fibrosis, the Hallmarks of Chronic Chagas Cardiomyopathy, in A Mouse Model of Trypanosoma cruzi Infection" Vaccines 8, no. 1: 96. https://doi.org/10.3390/vaccines8010096
APA StyleLokugamage, N., Choudhuri, S., Davies, C., Chowdhury, I. H., & Garg, N. J. (2020). Antigen-Based Nano-Immunotherapy Controls Parasite Persistence, Inflammatory and Oxidative Stress, and Cardiac Fibrosis, the Hallmarks of Chronic Chagas Cardiomyopathy, in A Mouse Model of Trypanosoma cruzi Infection. Vaccines, 8(1), 96. https://doi.org/10.3390/vaccines8010096






