Addition of Partial Envelope Domain II into Envelope Domain III of Dengue Virus Antigen Potentiates the Induction of Virus-Neutralizing Antibodies and Induces Protective Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Animals
2.2. Cell Culture
2.3. Production of Recombinant Ags
2.4. Immunization and Ab Purification
2.5. Virus Propagation, Titration, and Neutralization
2.6. Ab Binding Assay
2.7. Neutralization Assay Using U937-DC-SIGN Cells
2.8. DENV Challenge Infection in AG129 Mice
2.9. Blood Cell Isolation and Serum Cytokine Analysis
2.10. Reverse-Transcription Quantitative PCR (RT-qPCR)
2.11. ADE Assay Using U937 Cells
2.12. Statistical Analysis
3. Results
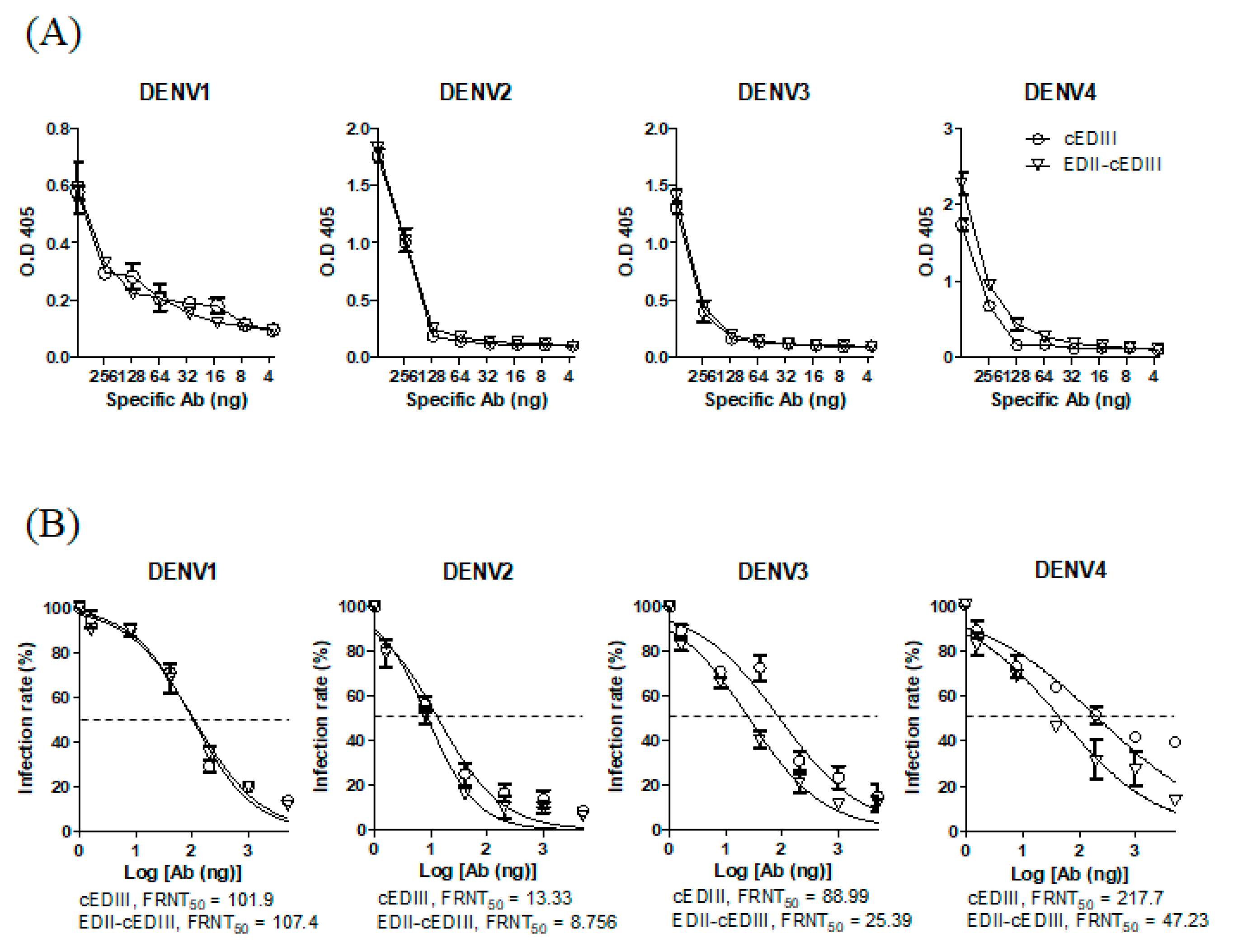
3.1. EDII of DENV4 Enhanced the Induction of Abs Capable of Binding to DENV4 and Neutralizing DENV3 and DENV4
3.2. Anti-EDII-cEDIII Abs Neutralized the Four DENV Serotypes in U937-DC-SIGN Cells
3.3. ADE Was Triggered by Anti-EDII-cEDIII and Anti-cEDIII Abs
3.4. Anti-EDII-cEDIII Ab Increased the Survival of DENV-Infected Mice
3.5. Anti-EDII-cEDIII Ab Reduced Initial DENV Levels in Axillary Lymph Nodes and Spleen
3.6. Anti-EDII-cEDIII Ab Inhibited DENV Infection of Lymphocytes
3.7. Anti-EDII-cEDIII Ab Inhibited Secondary Infection of the Liver and Small Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.; Ismail, H.I.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Hadinegoro, S.R.; Arredondo-Garcia, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J. Biol. Med. 1970, 42, 350–362. [Google Scholar] [PubMed]
- Halstead, S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003, 60, 421–467. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Rodriguez-Barraquer, I.; Dorigatti, I.; Mier, Y.T.-R.L.; Laydon, D.J.; Cummings, D.A. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science 2016, 353, 1033–1036. [Google Scholar] [CrossRef]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-Garcia, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramirez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Whitehead, S.S.; Pierce, K.K.; Tibery, C.M.; Grier, P.L.; Hynes, N.A.; Larsson, C.J.; Sabundayo, B.P.; Talaat, K.R.; Janiak, A.; et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016, 8, 330–336. [Google Scholar] [CrossRef]
- Osorio, J.E.; Wallace, D.; Stinchcomb, D.T. A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone. Expert Rev. Vaccines 2016, 15, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Jones, J.O.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.K.; Shrivastava, A. Recent developments in recombinant protein-based dengue vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef] [PubMed]
- Vartak, A.; Sucheck, S.J. Recent advances in subunit vaccine carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef]
- Govindarajan, D.; Meschino, S.; Guan, L.; Clements, D.E.; ter Meulen, J.H.; Casimiro, D.R.; Coller, B.A.; Bett, A.J. Preclinical development of a dengue tetravalent recombinant subunit vaccine: Immunogenicity and protective efficacy in nonhuman primates. Vaccine 2015, 33, 4105–4116. [Google Scholar] [CrossRef]
- Shukla, R.; Rajpoot, R.K.; Arora, U.; Poddar, A.; Swaminathan, S.; Khanna, N. Pichia pastoris-expressed bivalent virus-like particulate vaccine induces domain III-focused bivalent neutralizing antibodies without antibody-dependent enhancement in vivo. Front. Microbiol. 2017, 8, 2644. [Google Scholar] [CrossRef]
- Oscherwitz, J. The promise and challenge of epitope-focused vaccines. Hum. Vaccines Immunother. 2016, 12, 2113–2116. [Google Scholar] [CrossRef]
- Yap, Y.K.; Smith, D.R. Strategies for the plant-based expression of dengue subunit vaccines. Biotechnol. Appl. Biochem. 2010, 57, 47–53. [Google Scholar] [CrossRef]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef]
- Costin, J.M.; Zaitseva, E.; Kahle, K.M.; Nicholson, C.O.; Rowe, D.K.; Graham, A.S.; Bazzone, L.E.; Hogancamp, G.; Figueroa Sierra, M.; Fong, R.H.; et al. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J. Virol. 2013, 87, 52–66. [Google Scholar] [CrossRef]
- Lai, C.Y.; Williams, K.L.; Wu, Y.C.; Knight, S.; Balmaseda, A.; Harris, E.; Wang, W.K. Analysis of cross-reactive antibodies recognizing the fusion loop of envelope protein and correlation with neutralizing antibody titers in Nicaraguan dengue cases. PLoS Negl. Trop. Dis. 2013, 7, e2451. [Google Scholar] [CrossRef]
- Smith, S.A.; de Alwis, A.R.; Kose, N.; Harris, E.; Ibarra, K.D.; Kahle, K.M.; Pfaff, J.M.; Xiang, X.; Doranz, B.J.; de Silva, A.M.; et al. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 2013, 4, e00873-13. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.H.; Liu, S.J.; Tsai, J.P.; Li, Y.S.; Chen, M.Y.; Liu, H.H.; Lien, S.P.; Yueh, A.; Hsiao, K.N.; Lai, L.W.; et al. A novel dengue vaccine candidate that induces cross-neutralizing antibodies and memory immunity. Microbes Infect. 2009, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bal, J.; Jung, H.Y.; Nguyen, L.N.; Park, J.; Jang, Y.S.; Kim, D.H. Evaluation of cell-surface displayed synthetic consensus dengue EDIII cells as a potent oral vaccine candidate. Microb. Cell Factories 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Kim, S.H.; Park, J.; Son, Y.; Yoo, H.S.; Lee, K.Y.; Jang, Y.S. Nasal immunization with major epitope-containing ApxIIA toxin fragment induces protective immunity against challenge infection with Actinobacillus pleuropneumoniae in a murine model. Vet. Immunol. Immunopathol. 2013, 151, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, R.; Vasudevan, S.G. Dengue: Methods and Protocols, 1st ed.; Springer’s Humana Press: Totowa, NJ, USA, 2014; pp. 4–14. [Google Scholar]
- Ayala-Nunez, N.V.; Wilschut, J.; Smit, J.M. Monitoring virus entry into living cells using DiD-labeled dengue virus particles. Methods 2011, 55, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cha, J.D.; Choi, K.M.; Lee, K.Y.; Han, K.M.; Jang, Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef]
- Cruz-Oliveira, C.; Freire, J.M.; Conceicao, T.M.; Higa, L.M.; Castanho, M.A.; Da Poian, A.T. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol. Rev. 2015, 39, 155–170. [Google Scholar] [CrossRef]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef]
- Pokidysheva, E.; Zhang, Y.; Battisti, A.J.; Bator-Kelly, C.M.; Chipman, P.R.; Xiao, C.; Gregorio, G.G.; Hendrickson, W.A.; Kuhn, R.J.; Rossmann, M.G. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 2006, 124, 485–493. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. A game of numbers: The stoichiometry of antibody-mediated neutralization of flavivirus infection. Prog. Mol. Biol. Transl. Sci. 2015, 129, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Dorner, M.; Feulner, M.; Imanaka, N.; Murphy, M.Y.; Ploss, A.; Rice, C.M. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA 2012, 109, 14610–14615. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Rouvinski, A.; Dejnirattisai, W.; Guardado-Calvo, P.; Vaney, M.C.; Sharma, A.; Duquerroy, S.; Supasa, P.; Wongwiwat, W.; Haouz, A.; Barba-Spaeth, G.; et al. Covalently linked dengue virus envelope glycoprotein dimers reduce exposure of the immunodominant fusion loop epitope. Nat. Commun. 2017, 8, 15411. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- St John, A.L.; Abraham, S.N.; Gubler, D.J. Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat. Rev. Microbiol. 2013, 11, 420–426. [Google Scholar] [CrossRef]
- Lin, Y.W.; Wang, K.J.; Lei, H.Y.; Lin, Y.S.; Yeh, T.M.; Liu, H.S.; Liu, C.C.; Chen, S.H. Virus replication and cytokine production in dengue virus-infected human B lymphocytes. J. Virol. 2002, 76, 12242–12249. [Google Scholar] [CrossRef]
- Silveira, G.F.; Wowk, P.F.; Cataneo, A.H.D.; Dos Santos, P.F.; Delgobo, M.; Stimamiglio, M.A.; Lo Sarzi, M.; Thomazelli, A.; Conchon-Costa, I.; Pavanelli, W.R.; et al. Human T lymphocytes are permissive for dengue virus replication. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.M.; Lee, C.K.; Wu-Hsieh, B.A. Intrahepatic infiltrating NK and CD8 T cells cause liver cell death in different phases of dengue virus infection. PLoS ONE 2012, 7, e46292. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, H.-Y.; Khai, L.T.; Thuy, N.T.T.; Mai, L.Q.; Jang, Y.-S. Addition of Partial Envelope Domain II into Envelope Domain III of Dengue Virus Antigen Potentiates the Induction of Virus-Neutralizing Antibodies and Induces Protective Immunity. Vaccines 2020, 8, 88. https://doi.org/10.3390/vaccines8010088
Park J, Lee H-Y, Khai LT, Thuy NTT, Mai LQ, Jang Y-S. Addition of Partial Envelope Domain II into Envelope Domain III of Dengue Virus Antigen Potentiates the Induction of Virus-Neutralizing Antibodies and Induces Protective Immunity. Vaccines. 2020; 8(1):88. https://doi.org/10.3390/vaccines8010088
Chicago/Turabian StylePark, Jisang, Hyun-Young Lee, Ly Tuan Khai, Nguyen Thi Thu Thuy, Le Quynh Mai, and Yong-Suk Jang. 2020. "Addition of Partial Envelope Domain II into Envelope Domain III of Dengue Virus Antigen Potentiates the Induction of Virus-Neutralizing Antibodies and Induces Protective Immunity" Vaccines 8, no. 1: 88. https://doi.org/10.3390/vaccines8010088
APA StylePark, J., Lee, H.-Y., Khai, L. T., Thuy, N. T. T., Mai, L. Q., & Jang, Y.-S. (2020). Addition of Partial Envelope Domain II into Envelope Domain III of Dengue Virus Antigen Potentiates the Induction of Virus-Neutralizing Antibodies and Induces Protective Immunity. Vaccines, 8(1), 88. https://doi.org/10.3390/vaccines8010088




