Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Feeding Regime
2.2. Cells
2.3. Virus
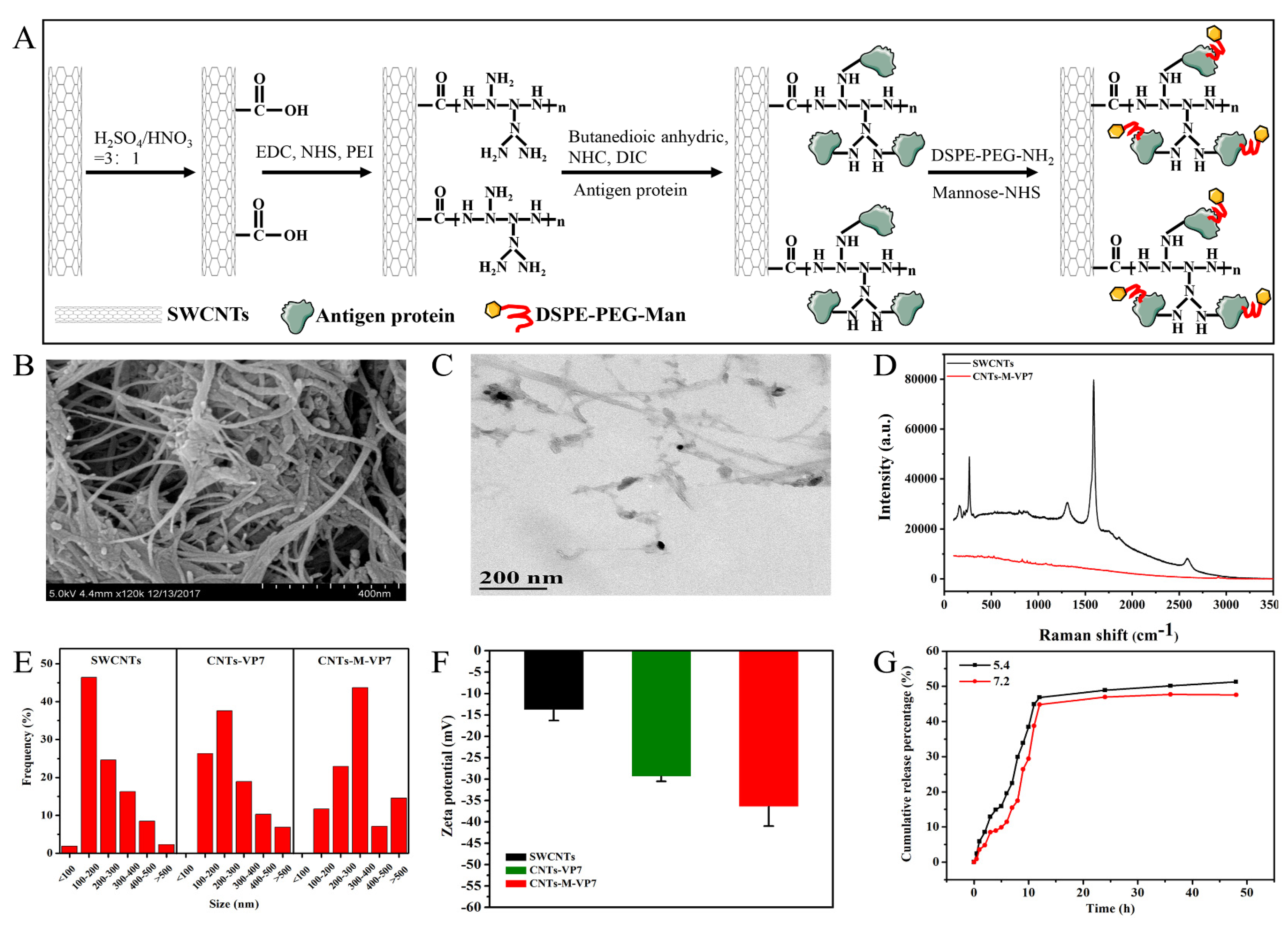
2.4. Functionalized SWCNTs
2.5. Synthesis and Characterization of Targeted Nanovaccine
2.6. Safety Evaluation of the Constructed Vaccines
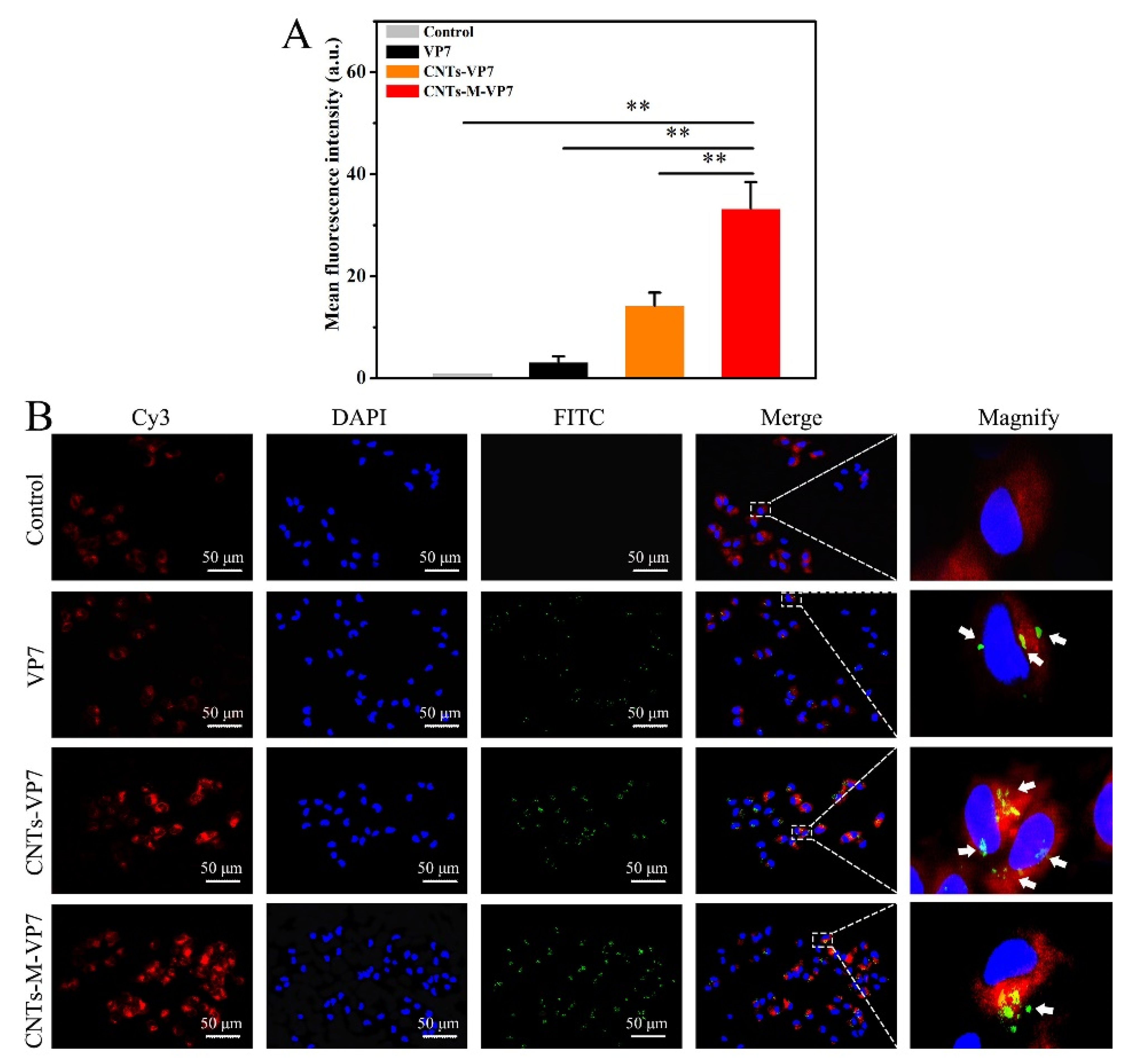
2.7. Cellular and Tissular Uptake of Nanovaccine
2.8. APCs Activation Analysis
2.9. In Vivo Fluorescence Imaging
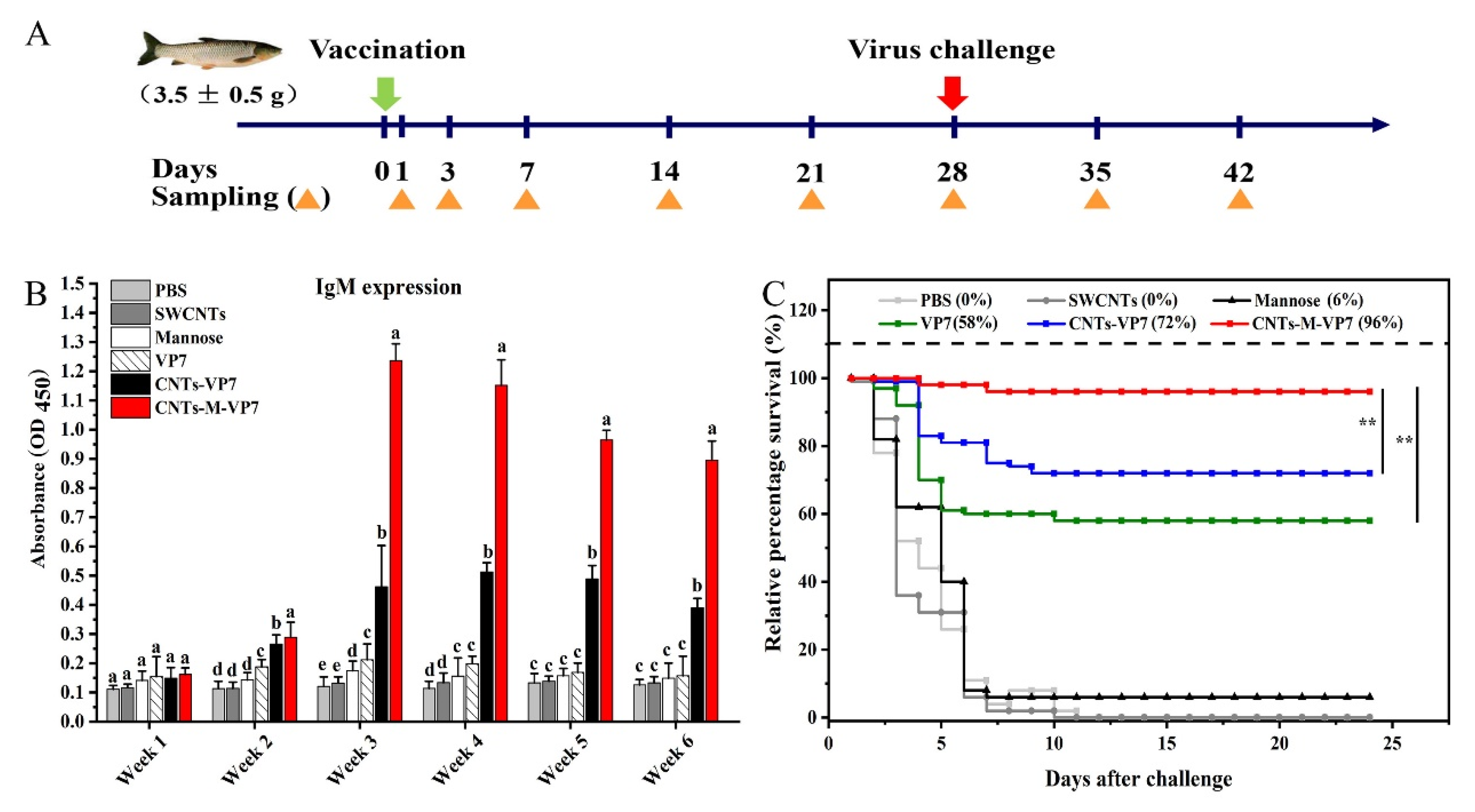
2.10. Vaccination
2.11. Serum Antibody Production, Enzyme Activities, and Virus Challenge
3. Results and Discussion
3.1. Construction and Characterization of Targeted Delivery System
3.2. APCs Targeting Ability of the Constructed Nanovaccines In Vivo and In Vitro
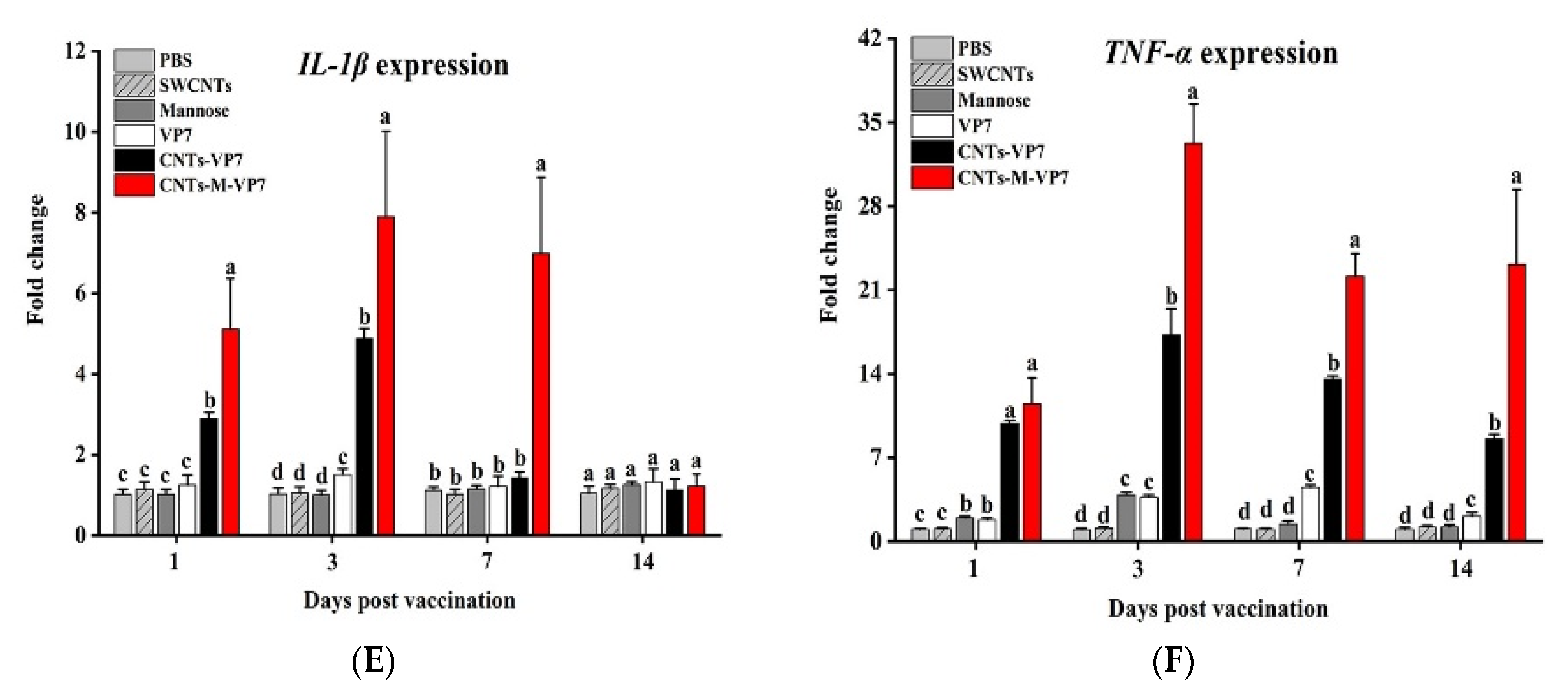
3.3. In Vivo APCs Activation by Nanovaccines
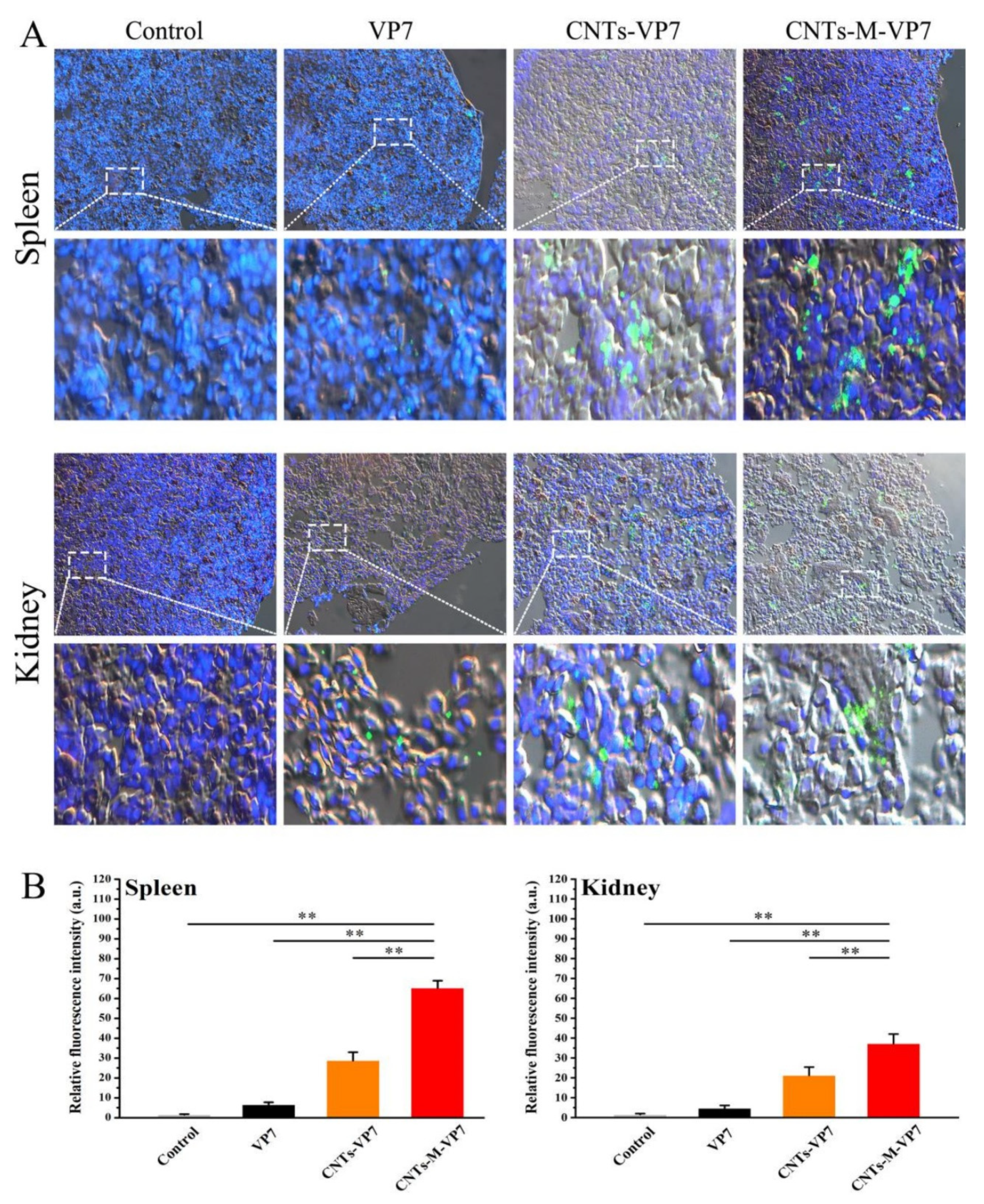
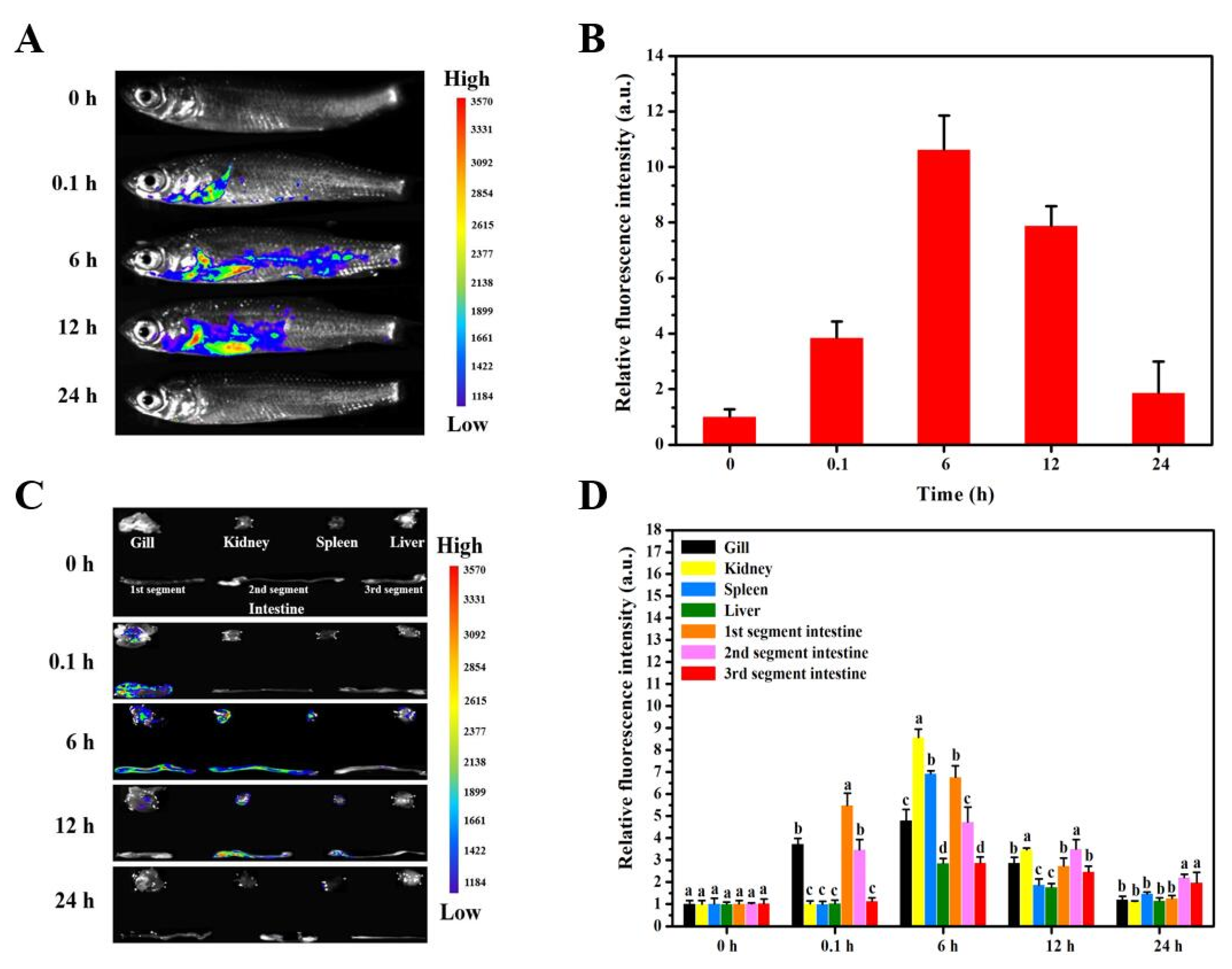
3.4. Delivery Kinetics of Targeted Nanovaccine
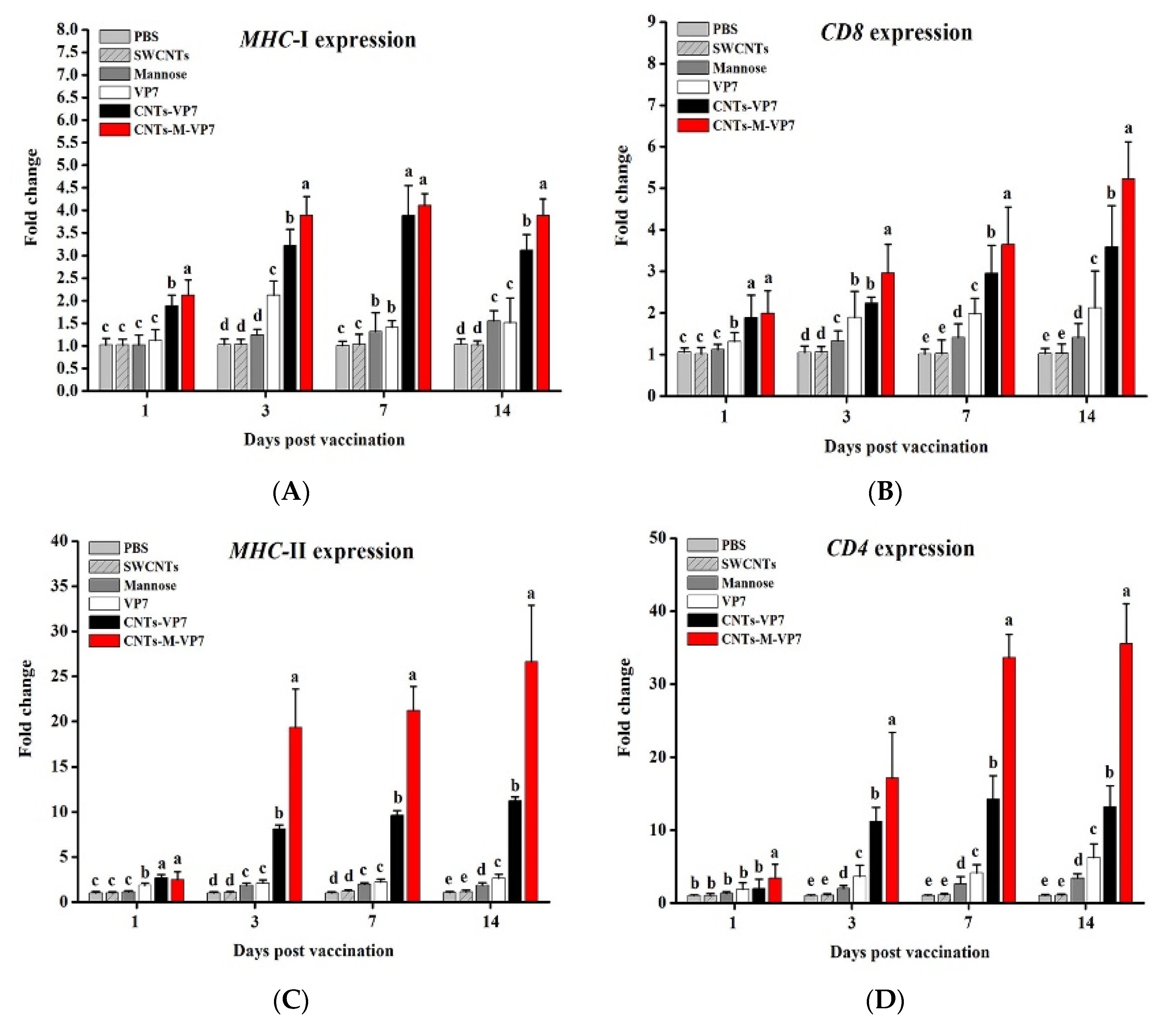
3.5. Immune Responses Induced by CNTs-M-VP7 Nanovaccine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pikarsky, E.; Ronen, A.; Abramowitz, J.; Levavi-Sivan, B.; Hutoran, M.; Shapira, Y.; Steinitz, M.; Perelberg, A.; Soffer, D.; Kotler, M. Pathogenesis of acute viral disease induced in fish by carp interstitial nephritis and gill necrosis virus. J. Virol. 2004, 78, 9544–9551. [Google Scholar] [CrossRef] [PubMed]
- Bedendo, G.; Panzarin, V.; Fortin, A.; Zamperin, G.; Pretto, T.; Buratin, A.; Quartesan, R.; Sabbion, M.; Salogni, C.; Pascoli, F.; et al. Detection and characterization of a rhabdovirus causing mortality in black bullhead catfish. Ameiurus Melas J. Fish Dis. 2018, 41, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chu, Q.; Cui, J.; Bi, D. Inducible microRNA-3570 feedback inhibits the RIG-I-dependent innate immune response to rhabdovirus in teleost fish by targeting MAVS/IPS-1. J. Virol. 2017, 2, e01594. [Google Scholar] [CrossRef] [PubMed]
- Skinner, L.A.; Lapatra, S.E.; Adams, A.; Thompsonc, K.D.; Balfryd, S.K.; McKinleyad, R.S.; Schultee, P.M. Concurrent injection of a rhabdovirus-specific DNA vaccine with a polyvalent, oil-adjuvanted vaccine delays the specific anti-viral immune response in Atlantic salmon, Salmo Salar L. Fish Shellfish Immunol. 2018, 28, 579–586. [Google Scholar] [CrossRef]
- Black, J.G.; Lawson, K.F. The safety and efficacy of immunizing foxes (Vulpes vulpes) using bait containing attenuated rabies virus vaccine. Can. J. Comp. Med. 1980, 44, 169176. [Google Scholar]
- Jechlinger, W. Optimization and delivery of plasmid DNA for vaccination. Expert Rev. Vaccines 2014, 5, 803–825. [Google Scholar] [CrossRef]
- Plant, K.P.; Lapatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Cain, K.D. Optimization of efficacy of a live attenuated Flavobacterium psychrophilum immersion vaccine. Fish Shellfish Immunol. 2016, 56, 169–180. [Google Scholar] [CrossRef]
- Caruffo, M.; Maturana, C.; Kambalapally, S.; Larenas, J.; Tobar, J.A. Protective oral vaccination against infectious Salmon Anaemia virus in Salmo Salar. Fish Shellfish Immunol. 2016, 54, 54–59. [Google Scholar] [CrossRef]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a polyvalent immersion vaccine against Flavobacterium psychrophilum and evaluation of immune response to vaccination in rainbow trout fry (Onchorynchus mykiss L.). Vet. Res. 2017, 48, 43. [Google Scholar] [CrossRef]
- Xu, Z.; Parra, D.; Gomez, D.; Salinas, I.; Zhang, Y.A.; Jorgensen, L.G.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Sunyer, J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Takizawa, F.; Parra, D.; Gomez, D.; Jorgensen, L.v.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef] [PubMed]
- Sallinas, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From vaccines to memory and back. Immunity 2010, 33, 451–463. [Google Scholar]
- Rombout, J.H.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish. Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Rigaudeau, D.; Tacchi, L.; Pijlman, G.P.; Kampers, L.; Vesely, T.; Pokorova, D.; Boudinot, P.; Wiegertjes, G.F.; Forlenza, M. Vaccination of carp against SVCV with an oral DNA vaccine or an insect cells-based subunit vaccine. Fish Shellfish Immunol. 2018, 85, 66–77. [Google Scholar] [CrossRef]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.; Phillips, B.; Carter, M.B.; et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 2011, 10, 389–397. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Guo, R.; Shen, M.; Zhang, M.; Zhu, M.; Shi, X. Targeted delivery of doxorubicin into cancer cells using a folic acid–dendrimer conjugate. Polym. Chem. 2011, 2, 1754–1760. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gulbake, A.; Jain, A.; Vyas, S.P.; Jain, S.K. Targeted delivery of an anti-cancer agent via steroid coupled liposomes. Drug Deliv. 2009, 16, 437–447. [Google Scholar] [CrossRef]
- Zarghami, N.; Grass, L.; Sauter, E.R.; Diamandis, E.P. Prostate-specific antigen in serum during the menstrual cycle. Clin. Chem. 1997, 43, 1862–1867. [Google Scholar] [CrossRef]
- Kreutz, F.T.; Suresh, M.R. Novel bispecific immunoprobe for rapid and sensitive detection of prostate-specific antigen. Clin. Chem. 1997, 43, 649–656. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Yu, F.; Liu, L. Grass carp reovirus outer capsid proteins VP5 and VP7 interact in vitro. Arch. Virol. 2017, 162, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liao, Z.W.; Yuan, G.; Su, J. A plasmid containing CpG ODN as vaccine adjuvant against grass carp reovirus in grass carp Ctenopharyngodon Idella. Oncotarget 2017, 8, 86576–86591. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, G.L.; Gong, Y.X.; Ling, F.; Wang, G.X. Protective immunity of grass carp immunized with DNA vaccine encoding the vp7 gene of grass carp reovirus using carbon nanotubes as a carrier molecule. Fish Shellfish Immunol. 2015, 42, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.B.D.; Santos, L.I.D.; Coelho, J.P.; Ribeiro, H.B.; Pimenta, M.A.; Ladeira, L.O.; Gomes, D.A.; Furtado, C.A.; Gazzinelli, R.T. Oxidized multiwalled carbon nanotubes as antigen delivery system to promote superior CD8+ T cell response and protection against cancer. Nano. Lett. 2014, 14, 5458–5470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; You, H.; Yang, X.; Lin, B.; Zhu, Z.; Lu, Z.; Li, X.; Zhao, Y.; Mao, L.; Shen, S.; et al. Functional single-walled carbon nanotubes ‘CAR’ for targeting dopamine delivery into the brain of parkinsonian mice. Nanoscale 2017, 9, 10832–10845. [Google Scholar] [CrossRef] [PubMed]
- Kafa, H.; Wang, T.W.; Rubio, N.; Venner, K.; Anderson, G.; Pach, E.; Ballesteros, B.; Preston, L.E.; Abbott, N.J.; Al-Jamal, K.T. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials 2015, 53, 437–452. [Google Scholar] [CrossRef]
- Gottardi, R.; Douradinha, B. Carbon nanotubes as a novel tool for vaccination against infectious diseases and cancer. J. Nanobiotechnol. 2013, 11, 30. [Google Scholar] [CrossRef]
- Colic, M.; Dzopalic, T.; Tomic, S.; Rajkovic, J.; Rudolf, R.; Vukovic, G.; Marinkovic, A.; Uskokovic, P. Immunomodulatory effects of carbon nanotubes functionalized with a Toll-like receptor 7 agonist on human dendritic cells. Carbon 2014, 67, 273–287. [Google Scholar] [CrossRef]
- Mulvey, J.J.; Villa, C.H.; Mcdevitt, M.R.; Escorcia, F.E.; Casey, E.; Scheinberg, D.A. Self-assembly of carbon nanaotubes and antibodies on tumors for targeted amplified delivery. Nat. Nanotechnol. 2013, 8, 763. [Google Scholar] [CrossRef]
- Rajput, M.K.; Kesharwani, S.S.; Muley, S.K.p.; Narisetty, S.; Tummala, S. Dendritic cell-targeted nanovaccine delivery system prepared with an immune-active polymer. ACS Appl. Mater. Interfaces 2018, 10, 27589–27602. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.Q.; Chen, Q.; Zhao, Y.H.; Peng, R.; Liu, Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Seng, E.K.; Ding, Q.Q.; Zhang, L.L. Characterization of infectious particles of grass carp reovirus by treatment with proteases. Arch. Virol. 2008, 153, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, G.L.; Gong, Y.X.; Lin, F.; Song, L.S.; Wang, G.X. Single-walled carbon nanotubes as candidate recombinant subunit vaccine carrier for immunization of grass carp against grass carp reovirus. Fish Shellfish Immunol. 2014, 41, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Shi, H.L.; Zhao, H.; Wang, X.; Yang, Y.; Yu, C.; Hao, C.Q.; Du, J.; Hu, H.; Yang, S.P. Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials 2014, 35, 5369–5380. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. International symposium on fish biologics: Serodiagnostics and vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon naonotubes: An emerging drug carrier for targeting cancer cells. J. Drug Deliv. 2014, 2014, 670815. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Krishnakumar, K.; Bhatt, A.R.; Paul, D.; Cherian, J.; John, A.; Suresh, S. Single-walled and Multi-walled carbon nanotubes based drug delivery system: Cancer therapy: A review. Indian J. Cancer 2015, 52, 262. [Google Scholar] [CrossRef]
- Hassan, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and changes. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.C.; Zhao, Y.; Zhao, X.H.; Xi, M.J.; Wei, S.; Fang, R.; Ji, W.; Chen, N.; Gu, Z.M.; et al. Molecular cloning and expression analysis of mannose receptor C type 1 in grass carp (Ctenopharyngodon idella). Dev. Comp. Immunol. 2014, 43, 54–58. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, 5692. [Google Scholar] [CrossRef] [PubMed]
- Reczek, D.; Schwake, M.; Schröder, J.; Hughes, H.; Blanz, J.; Jin, X.; Brondyk, W.; van Patten, S.; Edmunds, T.; Saftig, P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 2007, 131, 770–783. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.H.; Wang, J.; Zhao, Z.; Li, J.; Tu, X.; Huang, A.G.; Zhu, G.X.W.B. Enhanced protective immunity against spring viremia of carp virus infection can be induced by recombinant subunit vaccine conjugated to single-walled carbon nanotubes. Vaccine 2018, 36, 6334–6344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Z.; Zha, J.W.; Wang, G.X.; Zhu, B. Single-walled carbon nanotubes as delivery vehicles enhance the immunoprotective effect of a DNA vaccine against spring viremia of carp virus in common carp. Fish Shellfish Immunol. 2017, 71, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.; Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Norihiro, K.; Masato, N.; Kohei, M.; Kazuhiro, Y.; Makoto, E.; Junko, N. Pulmonary and systemic responses of highly pure and well-dispersed single-wall carbon nanotubes after intratracheal instillation in rats. Inhal. Toxicol. 2011, 23, 814–828. [Google Scholar]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano. Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Montesfonseca, S.L.; Orrantiaborunda, E.; Aguilarelguezabal, A.; González, H.C.; Talamásrohana, P.; Sánchezramírez, B. Cytotoxicity of functionalized carbon nanotubes in J774A macrophages. New Biotechnol. 2012, 29, S203. [Google Scholar] [CrossRef]
- Shi, X.; Balaji, S.; Pham, Q.P.; Spicer, P.P.; Hudson, J.L.; Wilson, L.J.; Tour, J.M.; Raphael, R.M.; Mikos, A.G. In vitro cytotoxicity of single-walled carbon nanotube/biodegradable polymer nanocomposites. J. Biomed. Mater. Res. A 2010, 86, 813–823. [Google Scholar]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef]
- Carrillo-Conde, B.; Song, E.H.; Chavez-Santoscopy, A.; Phanse, Y.; Ramer-Tait, A.E.; Pohl, N.L.; Wannemuehler, M.J.; Bellaire, B.H.; Narasimhan, B. Mannose-functionalized ‘’Pathogen-like” Poly-anhydride nanoparticles target C-type lectin receptors on dendritic cells. Mol. Pharm. 2011, 8, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Hardonk, M.J.; Dijkhuis, F.W.J.; Hulstaert, C.E.; Koudstaal, J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride elimination and repopulation. J. Leukoc. Biol. 1992, 52, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S. Macrophages and kidney disease: Macrophages and immunological inflammation of the kidney. Semin. Nephrol. 2010, 30, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Lore, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Christensen, D.; Henriksen-Lacey, M.; Kamath, A.T.; Lindenstrom, T.; Korsholm, K.S.; Christensen, J.P.; Rochat, A.F.; Lambert, P.H.; Andersen, P.; Siegrist, C.A.; et al. Cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J. Control. Release 2012, 160, 468–476. [Google Scholar] [CrossRef]
- Hateren, A.V.; Bailey, A.; Elliott, T. Recent advances in Major Histocompatibility Complex (MHC) class I antigen presentation: Plastic MHC molecules and TAPBPR-mediated quality control. F1000Res 2017, 6, 158. [Google Scholar] [CrossRef]
- Sansonetti, P.J.; Phalipon, A.; Arondel, J.; Thirumalai, K.; Banerjee, S.; Akira, S.; Takeda, K.; Zychlinsky, A. Caspase-1 Activation of IL-1β and IL-18 Are Essential for Shigella flexneri–Induced Inflammation. Immunity 2000, 12, 581–590. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Rook, G.A. The role of TNF-α in T-cell mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology 1994, 82, 591–595. [Google Scholar]
- Valladão, G.M.R.; Gallani, S.U.G.; Pala, G.; Jesus, R.B.; Kotzent, S.; Costa, J.C.; Silva, T.F.A.; Pilarski, F. Practical diets with essential oils of plants activate the complement system and alter the intestinal morphology of Nile tilapia. Aqua. Res. 2017, 48, 5640–5649. [Google Scholar] [CrossRef]
- Yin, F.; Gong, H.; Ke, Q.; Li, A. Stress, antioxidant defense and mucosal immune responses of the large yellow croaker Pseudosciaena crocea challenged with Cryptocaryon irritans. Fish Shellfish Immunol. 2015, 47, 344–351. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhang, W.B.; Xu, W.; Mai, K.S.; Zhang, Y.J.; Liufu, Z.G. Effects of potential probiotic bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicas. Fish Shellfish Immunol. 2012, 32, 750–755. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Zhang, C.; Zhao, Z.; Wang, G.-X. Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease. Vaccines 2020, 8, 87. https://doi.org/10.3390/vaccines8010087
Zhu B, Zhang C, Zhao Z, Wang G-X. Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease. Vaccines. 2020; 8(1):87. https://doi.org/10.3390/vaccines8010087
Chicago/Turabian StyleZhu, Bin, Chen Zhang, Zhao Zhao, and Gao-Xue Wang. 2020. "Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease" Vaccines 8, no. 1: 87. https://doi.org/10.3390/vaccines8010087
APA StyleZhu, B., Zhang, C., Zhao, Z., & Wang, G.-X. (2020). Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease. Vaccines, 8(1), 87. https://doi.org/10.3390/vaccines8010087





