Vaccination with Consensus H7 Elicits Broadly Reactive and Protective Antibodies against Eurasian and North American Lineage H7 Viruses
Abstract
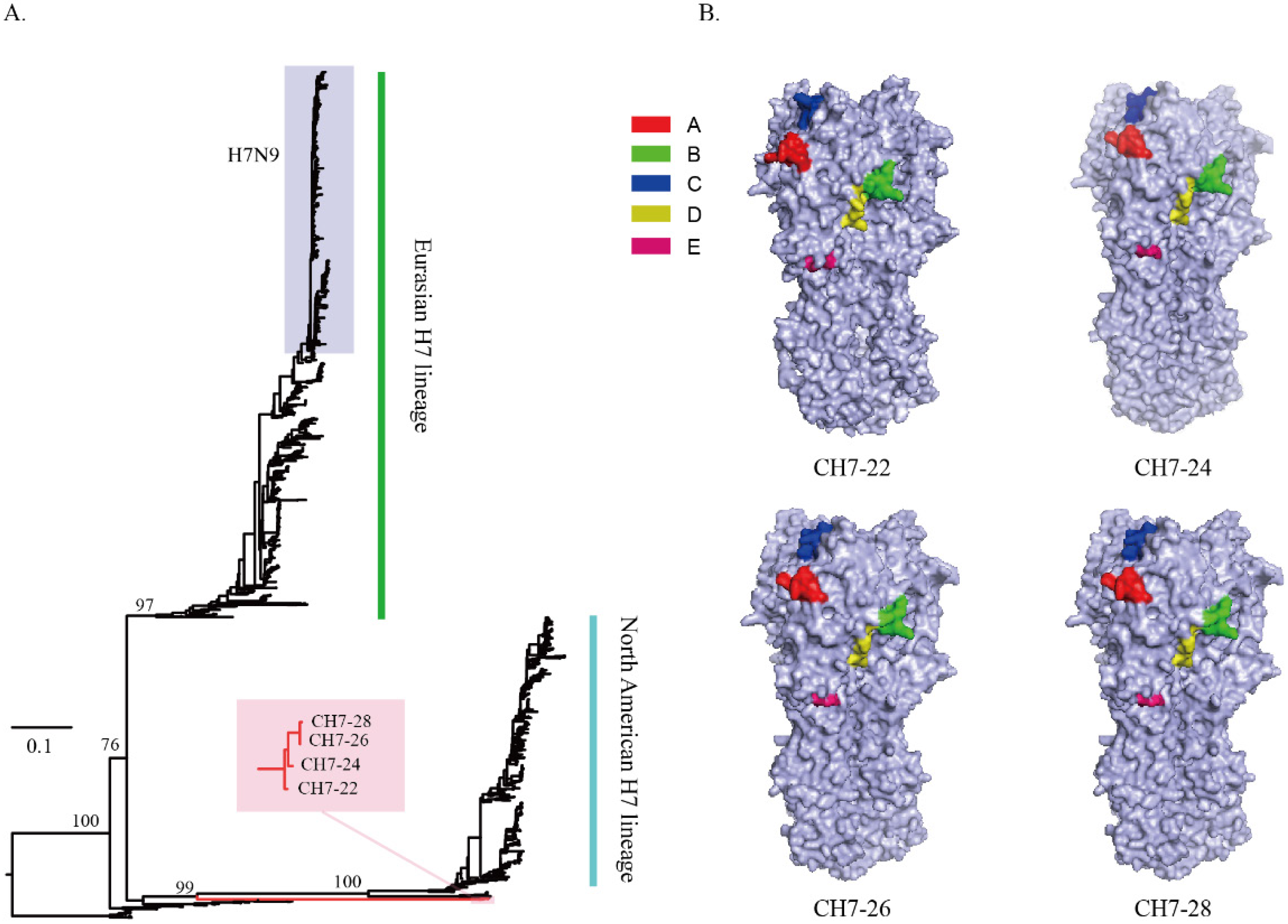
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
2.3. Antigenic Construction and Characterization
2.4. Gene Synthesis and Plasmid Construction
2.5. In Vitro Expression of Consensus H7 HAs
2.6. Vaccination and Antibody Response Analysis
2.6.1. Hemagglutinin Inhibition (HAI) Assay
2.6.2. Microneutralization (MN) Assay
2.6.3. ELISA
2.7. Adaptaion of H7N3 Influenza Virus in Mice
2.8. Viral Infection and Titration
2.9. Statistical Analysis
3. Results
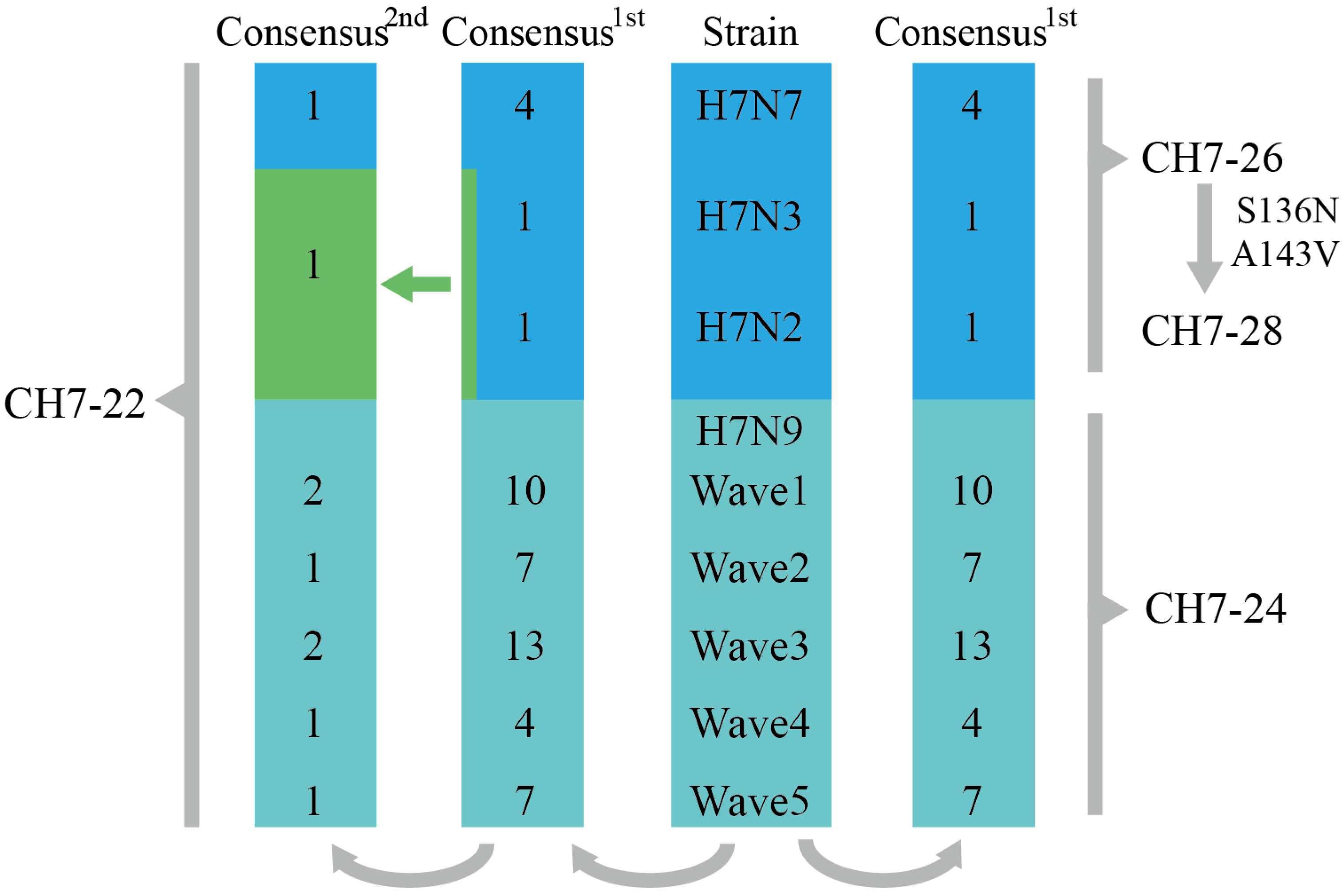
3.1. Design of the Four Consensus H7 Proteins
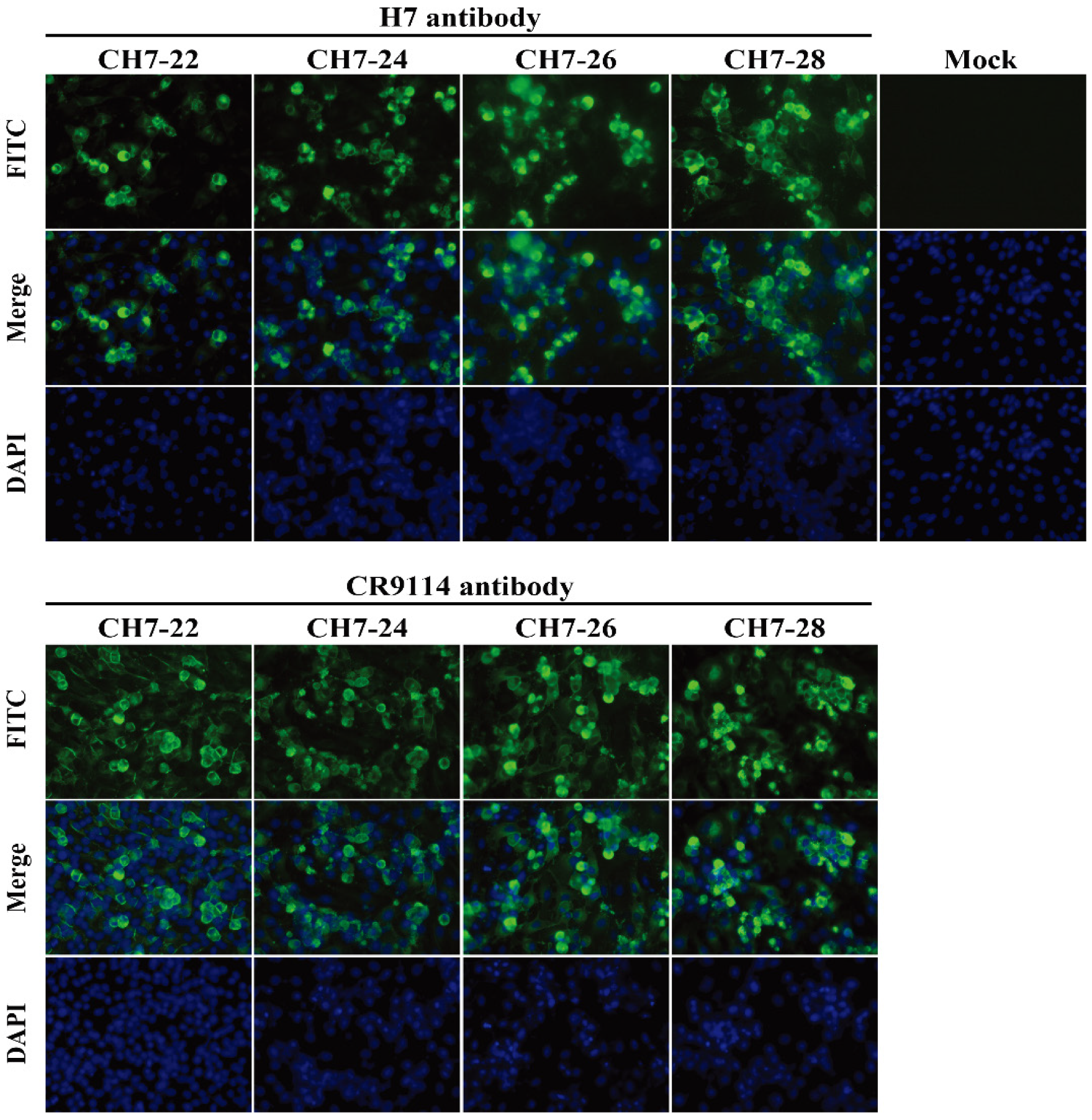
3.2. Characterization of the Four Consensus H7 Proteins
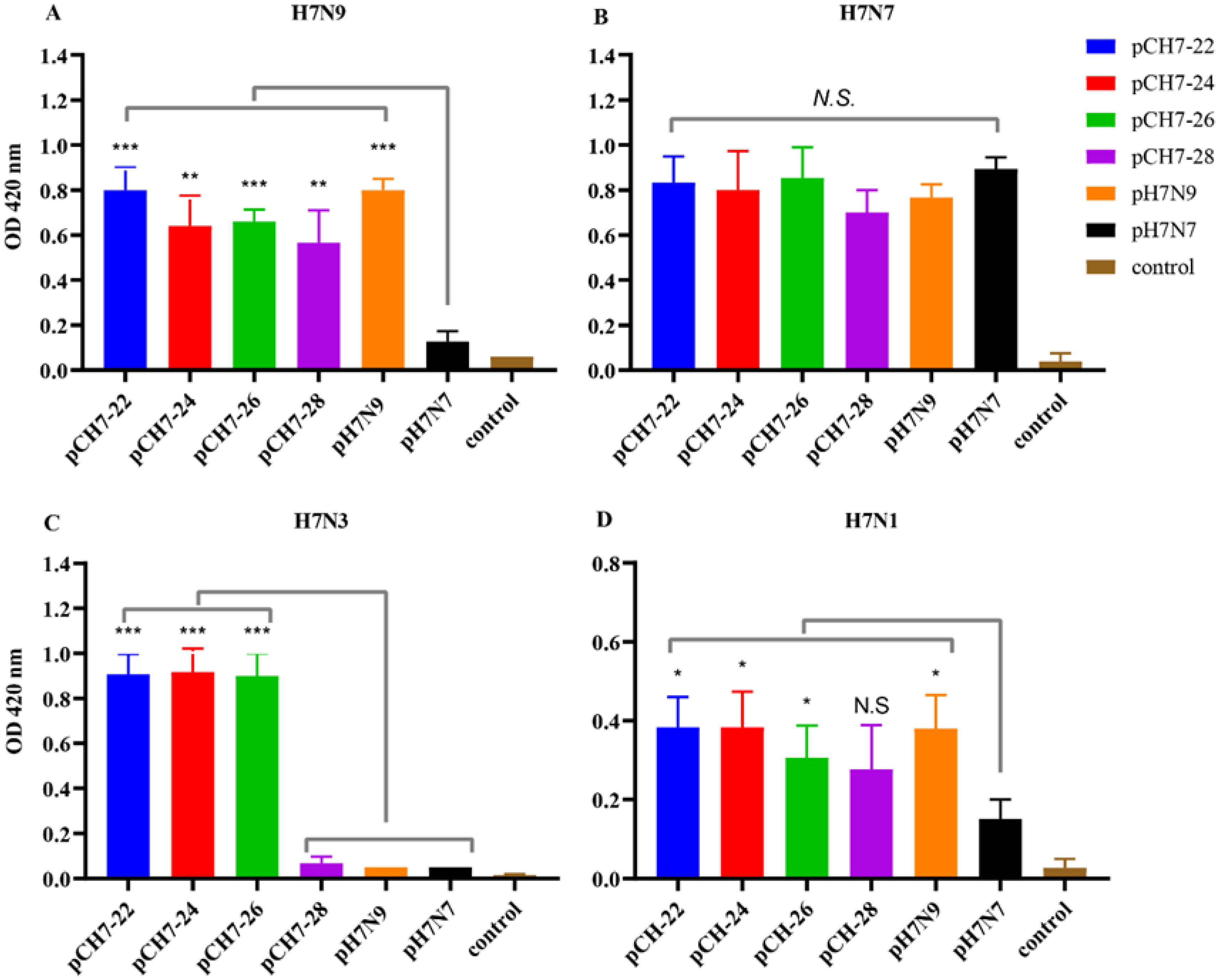
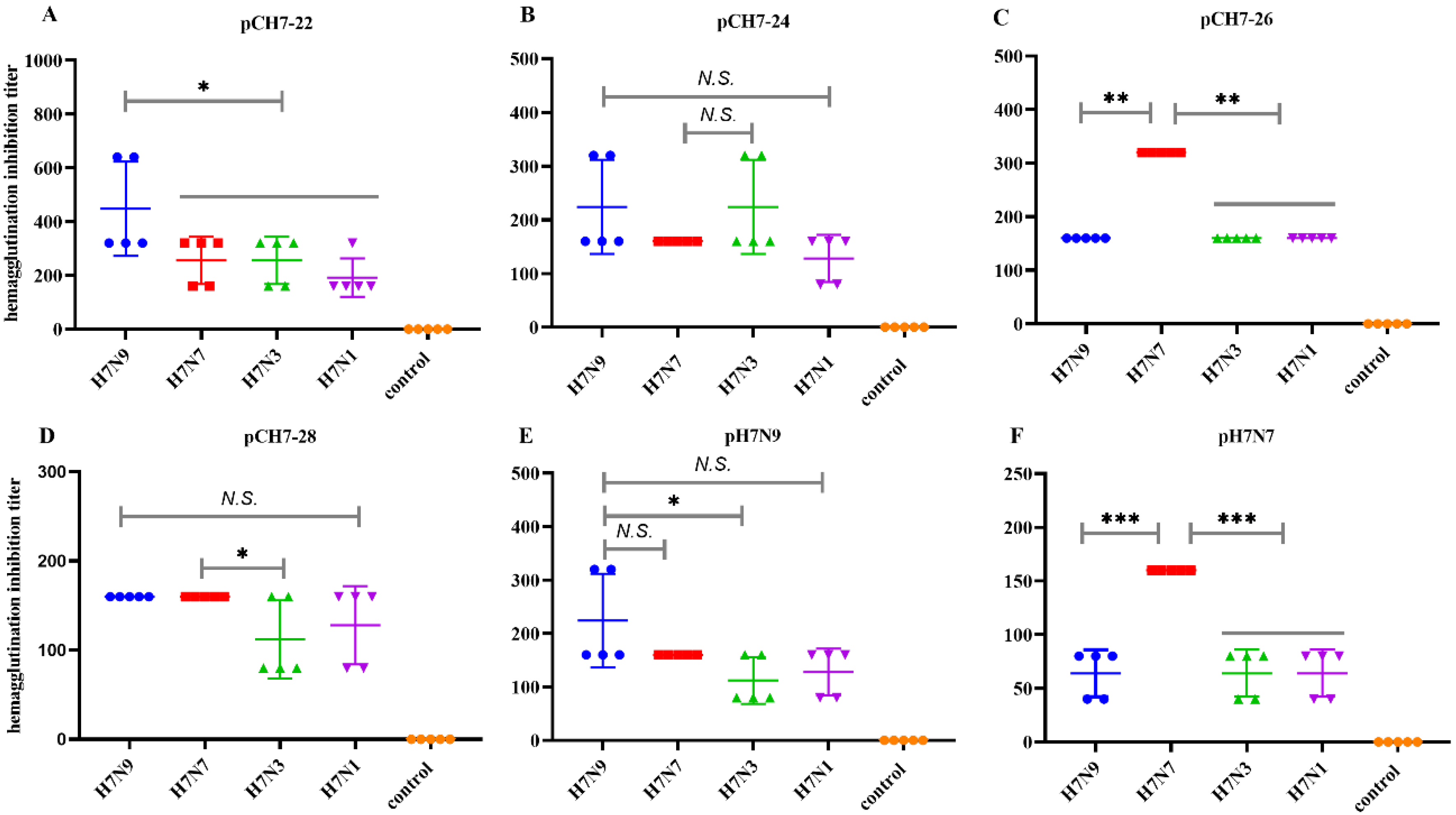
3.3. DNA Vaccines Encoding Consensus H7 Proteins Elicit Broadly Reactive Antibody Responses in Mice
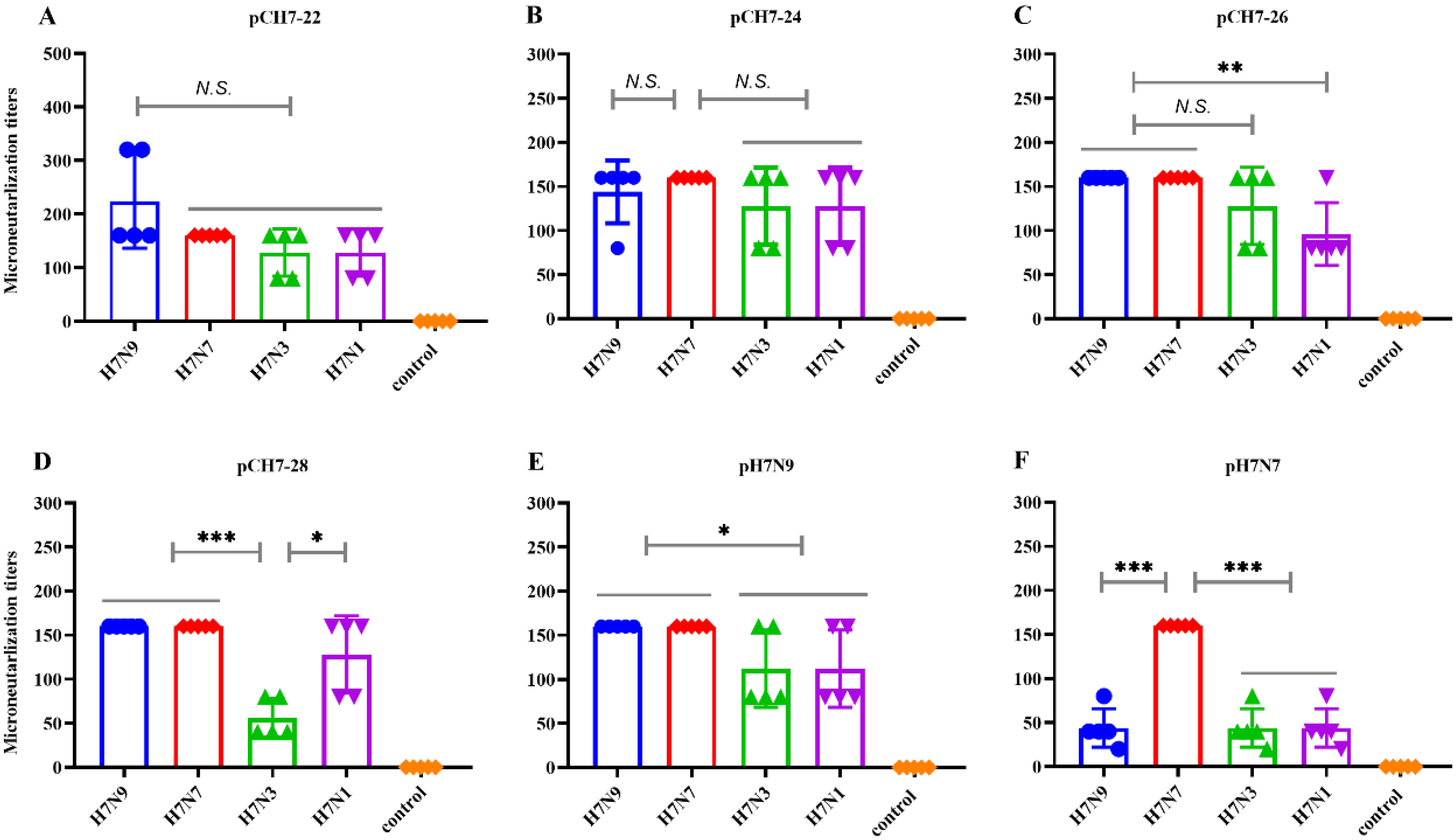
3.4. DNA Vaccines Encoding COBRA H7 Proteins Elicit Broadly Neutralizing Antibody Responses in Mice
3.5. DNA Vaccination with Consensuses H7 Confers Protection against Lethal H7N9 Influenza Virus Challenge in Mice
3.6. DNA Vaccines Elicit Protection against H7N3 Influenza Virus Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belser, J.A.; Bridges, C.B.; Katz, J.M.; Tumpey, T.M. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg. Infect. Dis. 2009, 15, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Abdelwhab, E.M.; Veits, J.; Mettenleiter, T.C. Prevalence and control of H7 avian influenza viruses in birds and humans–ERRATUM. Epidemiol. Infect. 2014, 142, 921. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Cui, L.B.; Chen, C.; Wang, D.Y.; Qi, X.; Zhou, M.H.; Guo, X.L.; Wang, F.M.; Liu, W.J.; Kong, W.R.; et al. Severe human infection with a novel avian-origin influenza A(H7N4) virus. Sci. Bull. 2018, 63, 1043–1050. [Google Scholar] [CrossRef]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.M.; Schneeberger, P.M.; Rozendaal, F.W.; Broekman, J.M.; Kemink, S.A.G.; Munster, V.; Kuiken, T.; Rimmelzwaan, G.F.; Schutten, M.; Van Doornum, G.J.J.; et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 1356–1361. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, L.; Zhou, M.; Chen, Z.; Li, F.; Wu, H.; Xiang, N.; Chen, E.; Tang, F.; Wang, D.; et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N. Engl. J. Med. 2014, 370, 520–532. [Google Scholar] [CrossRef]
- Zhu, H.; Lam, T.T.-Y.; Smith, D.K.; Guan, Y. Emergence and development of H7N9 influenza viruses in China. Curr. Opin. Virol. 2016, 16, 106–113. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Zhou, B.; Wang, J.; Chai, Y.; Shen, Y.; Chen, X.; Ma, C.; Hong, W.; Chen, Y.; Zhang, Y.; et al. Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature 2015, 522, 102–105. [Google Scholar] [CrossRef]
- Qi, W.; Jia, W.; Liu, D.; Li, J.; Bi, Y.; Xie, S.; Li, B.; Hu, T.; Du, Y.; Xing, L.; et al. Emergence and Adaptation of a Novel Highly Pathogenic H7N9 Influenza Virus in Birds and Humans from a 2013 Human-Infecting Low-Pathogenic Ancestor. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, H.; Xiong, C.; Di, L.; Shi, W.; Li, M.; Liu, S.; Chen, J.; Chen, G.; Li, Y.; et al. Novel avian influenza A (H5N6) viruses isolated in migratory waterfowl before the first human case reported in China, 2014. Sci. Rep. 2016, 6, 29888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ren, R.; Yang, L.; Bao, C.; Wu, J.; Wang, D.; Li, C.; Xiang, N.; Wang, Y.; Li, D.; et al. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September-December 2016. West. Pac. Surveill. Response J. 2017, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, W.; Shi, Y.; Wang, D.; Xiao, H.; Li, W.; Bi, Y.; Wu, Y.; Li, X.; Yan, J.; et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: Phylogenetic, structural, and coalescent analyses. Lancet 2013, 381, 1926–1932. [Google Scholar] [CrossRef]
- World Health Organization. Influenza at the Human-Animal Interface. Available online: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_07_25_2017.pdf (accessed on 25 July 2017).
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef]
- Sautto, G.A.; Kirchenbaum, G.A.; Ross, T.M. Towards a universal influenza vaccine: Different approaches for one goal. Virol. J. 2018, 15, 17. [Google Scholar] [CrossRef]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortes-Garcia, G.; Vogel, T.U.; et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef]
- Wong, T.M.; Allen, J.D.; Bebin-Blackwell, A.G.; Carter, D.M.; Alefantis, T.; DiNapoli, J.; Kleanthous, H.; Ross, T.M. Computationally Optimized Broadly Reactive Hemagglutinin Elicits Hemagglutination Inhibition Antibodies against a Panel of H3N2 Influenza Virus Cocirculating Variants. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Comparison of several adjuvants for inactivated H7N9 influenza virus vaccine. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-ZGYC201609009.htm (accessed on 18 March 2020).
- Liu, H.; Xiong, C.; Chen, J.; Chen, G.; Zhang, J.; Li, Y.; Xiong, Y.; Wang, R.; Cao, Y.; Chen, Q.; et al. Two genetically diverse H7N7 avian influenza viruses isolated from migratory birds in central China. Emerg. Microbes. Infect. 2018, 7, 62. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Wang, Q.; Hao, M.; Zhang, X.; Liu, L.; Yu, X.; Yang, C.; Xu, J.; Chen, J.; et al. A cross-reactive human monoclonal antibody targets the conserved H7 antigenic site A from fifth wave H7N9-infected humans. Antivir. Res. 2019, 170, 104556. [Google Scholar] [CrossRef]
- WHO. Serological Detection of Avian Influenza A(H7N9) Infections by Microneutralization Assay; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Kwon, H.I.; Kim, Y.I.; Park, S.J.; Song, M.S.; Kim, E.H.; Kim, S.M.; Si, Y.J.; Lee, I.W.; Song, B.M.; Lee, Y.J.; et al. Evaluation of the Immune Responses to and Cross-Protective Efficacy of Eurasian H7 Avian Influenza Viruses. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Chen, Q.; Xiong, C.; Yao, Y.; Wang, H.; Wang, H.; Chen, Z. Comparative analysis of antibody induction and protection against influenza virus infection by DNA immunization with HA, HAe, and HA1 in mice. Arch. Virol. 2014, 159, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yoon, H.; Kumar, S.; Ramanathan, M.P.; Corbitt, N.; Kutzler, M.; Dai, A.; Boyer, J.D.; Weiner, D.B. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol. Ther. 2007, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Highly conserved protective epitopes on influenza B viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, H.; Chen, J.; Shao, Z.; He, B.; Chen, J.; Lan, J.; Chen, Q.; Chen, Z. Protection against homo and hetero-subtypic in fl uenza A virus by optimized M2e DNA vaccine. Emerg. Microbes Infect. 2019, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Aihara, H.; Miyazaki, J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998, 16, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, J.B.; Dushoff, J. Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc. Natl. Acad. Sci. USA 2003, 100, 7152–7157. [Google Scholar] [CrossRef] [PubMed]
- Joseph, U.; Su, Y.C.; Vijaykrishna, D.; Smith, G.J. The ecology and adaptive evolution of influenza A interspecies transmission. Influenza Other Respir. Viruses 2017, 11, 74–84. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Tsvetnitsky, V.; Donnelly, J.J. Universal influenza vaccines: Shifting to better vaccines. Vaccine 2016, 34, 2926–2933. [Google Scholar] [CrossRef]
- Sautto, G.A.; Kirchenbaum, G.A.; Abreu, R.B.; Ecker, J.W.; Pierce, S.R.; Kleanthous, H.; Ross, T.M. A Computationally Optimized Broadly Reactive Antigen Subtype-Specific Influenza Vaccine Strategy Elicits Unique Potent Broadly Neutralizing Antibodies against Hemagglutinin. J. Immunol. 2020, 204, 375–385. [Google Scholar] [CrossRef]
- World Health Organization. Human Infection with Avian Influenza A(H7N9) Virus-The Total Number of Fatal Cases is Published on a Monthly Basis by the China National Health and Family Planning Commission; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. 201709 Antigenic and Genetic Characteristics of Zoonotic Influenza Viruses and Development of Candidate Vaccine Viruses for Pandemic Preparedness; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Su, S.; Gu, M.; Liu, D.; Cui, J.; Gao, G.F.; Zhou, J.; Liu, X. Epidemiology, Evolution, and Pathogenesis of H7N9 Influenza Viruses in Five Epidemic Waves since 2013 in China. Trends Microbiol. 2017, 25, 713–728. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Zhu, W.; Zhang, Y.; Zou, S.; Bo, H.; Gao, R.; Dong, J.; Huang, W.; Guo, J.; et al. Two Outbreak Sources of Influenza A (H7N9) Viruses Have Been Established in China. J. Virol. 2016, 90, 5561–5573. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Mok, C.K.P.; Zhu, W.; Zhou, H.; He, J.; Guan, W.; Wu, J.; Song, W.; Wang, D.; Liu, J.; et al. Human Infection with Highly Pathogenic Avian Influenza A(H7N9) Virus, China. Emerg. Infect. Dis. 2017, 23, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Obenauer, J.C.; Denson, J.; Mehta, P.K.; Su, X.; Mukatira, S.; Finkelstein, D.B.; Xu, X.; Wang, J.; Ma, J.; Fan, Y.; et al. Large-scale sequence analysis of avian influenza isolates. Science 2006, 311, 1576–1580. [Google Scholar] [CrossRef]
- Carter, D.M.; Bloom, C.E.; Kirchenbaum, G.A.; Tsvetnitsky, V.; Isakova-Sivak, I.; Rudenko, L.; Ross, T.M. Cross-protection against H7N9 influenza strains using a live-attenuated H7N3 virus vaccine. Vaccine 2015, 33, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Goff, P.H.; Krammer, F.; Hai, R.; Seibert, C.W.; Margine, I.; Garcia-Sastre, A.; Palese, P. Induction of Cross-Reactive Antibodies to Novel H7N9 Influenza Virus by Recombinant Newcastle Disease Virus Expressing a North American Lineage H7 Subtype Hemagglutinin. J. Virol. 2013, 87, 8235–8240. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Flyer, D.C.; Raghunandan, R.; Liu, Y.; Wei, Z.P.; Wu, Y.Y.; Kpamegan, E.; Courbron, D.; Fries, L.F.; Glenn, G.M. Development of influenza H7N9 virus like particle (VLP) vaccine: Homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine 2013, 31, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, Z.Y.; Cheng, X.; Xu, L.; Jin, H. Evaluation of Live Attenuated H7N3 and H7N7 Vaccine Viruses for Their Receptor Binding Preferences, Immunogenicity in Ferrets and Cross Reactivity to the Novel H7N9 Virus. PLoS ONE 2013, 8, e76884. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadlallah, G.M.; Ma, F.; Zhang, Z.; Hao, M.; Hu, J.; Li, M.; Liu, H.; Liang, B.; Yao, Y.; Gong, R.; et al. Vaccination with Consensus H7 Elicits Broadly Reactive and Protective Antibodies against Eurasian and North American Lineage H7 Viruses. Vaccines 2020, 8, 143. https://doi.org/10.3390/vaccines8010143
Fadlallah GM, Ma F, Zhang Z, Hao M, Hu J, Li M, Liu H, Liang B, Yao Y, Gong R, et al. Vaccination with Consensus H7 Elicits Broadly Reactive and Protective Antibodies against Eurasian and North American Lineage H7 Viruses. Vaccines. 2020; 8(1):143. https://doi.org/10.3390/vaccines8010143
Chicago/Turabian StyleFadlallah, Gendeal M., Fuying Ma, Zherui Zhang, Mengchan Hao, Juefu Hu, Mingxin Li, Haizhou Liu, Biling Liang, Yanfeng Yao, Rui Gong, and et al. 2020. "Vaccination with Consensus H7 Elicits Broadly Reactive and Protective Antibodies against Eurasian and North American Lineage H7 Viruses" Vaccines 8, no. 1: 143. https://doi.org/10.3390/vaccines8010143
APA StyleFadlallah, G. M., Ma, F., Zhang, Z., Hao, M., Hu, J., Li, M., Liu, H., Liang, B., Yao, Y., Gong, R., Zhang, B., Liu, D., & Chen, J. (2020). Vaccination with Consensus H7 Elicits Broadly Reactive and Protective Antibodies against Eurasian and North American Lineage H7 Viruses. Vaccines, 8(1), 143. https://doi.org/10.3390/vaccines8010143







