Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes
Abstract
1. Introduction
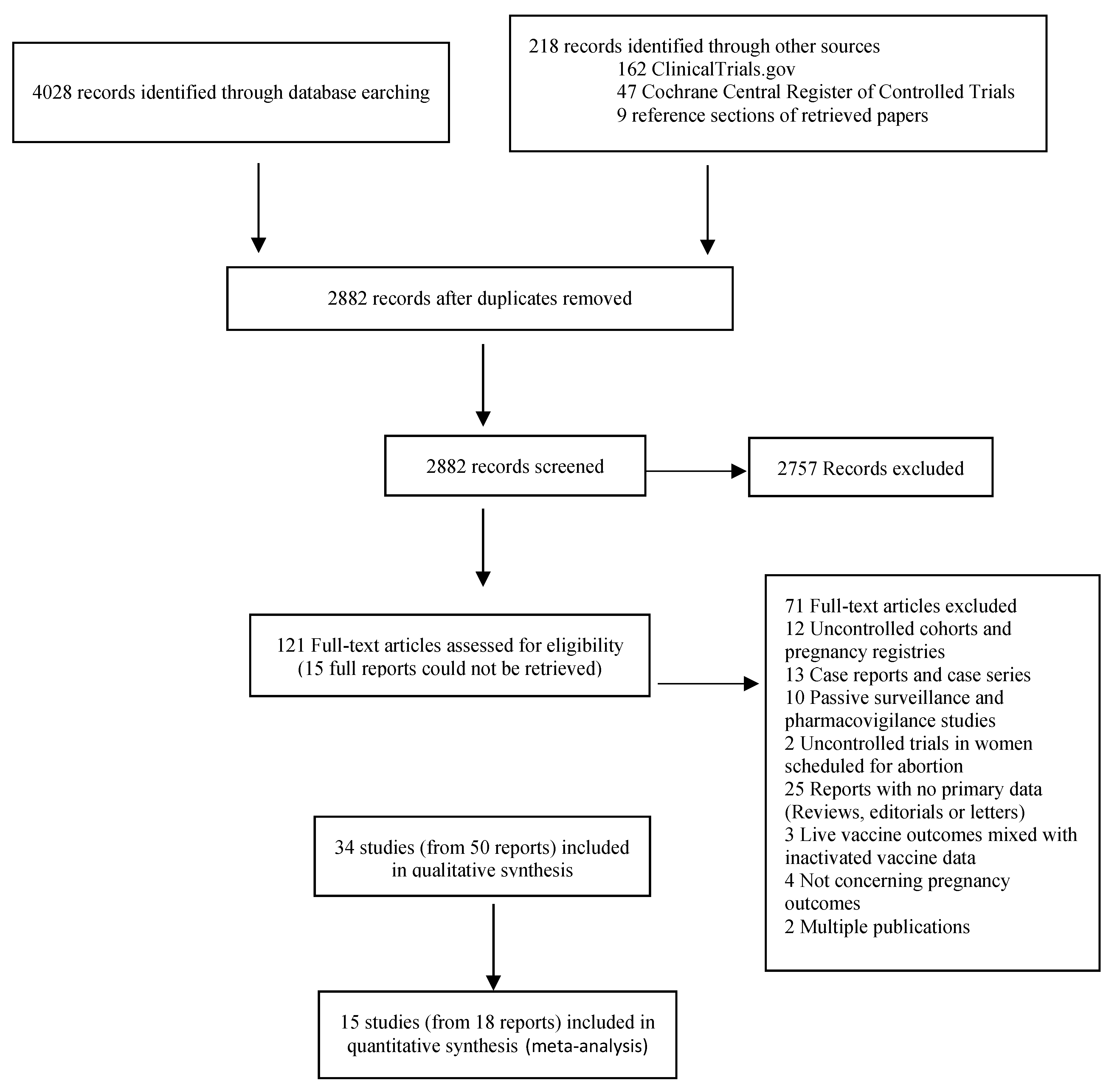
2. Materials and Methods
Data Analysis
3. Results
3.1. Meta-Analysis of Pregnancy Outcomes
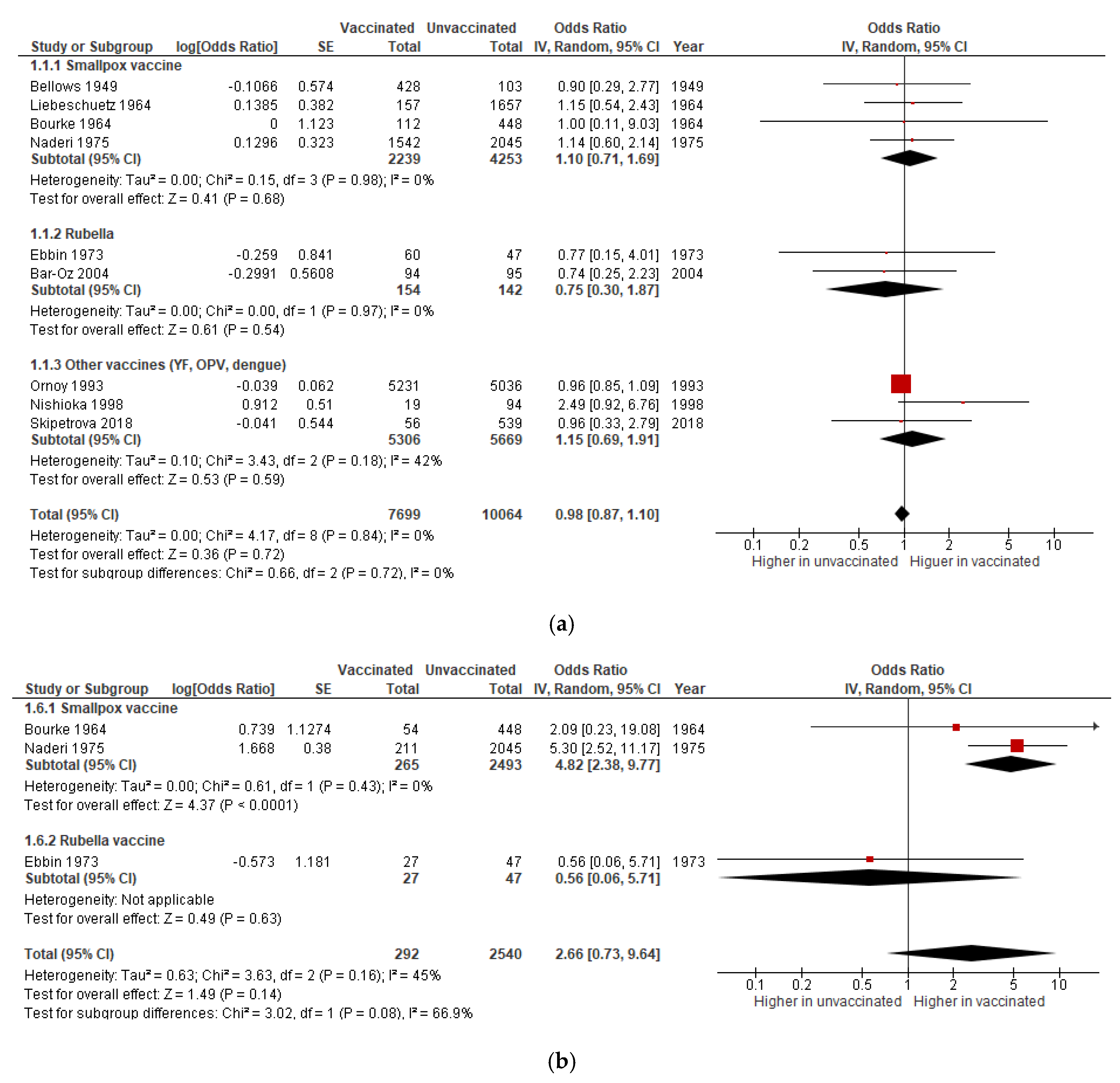
3.1.1. Miscarriage
3.1.2. Stillbirth
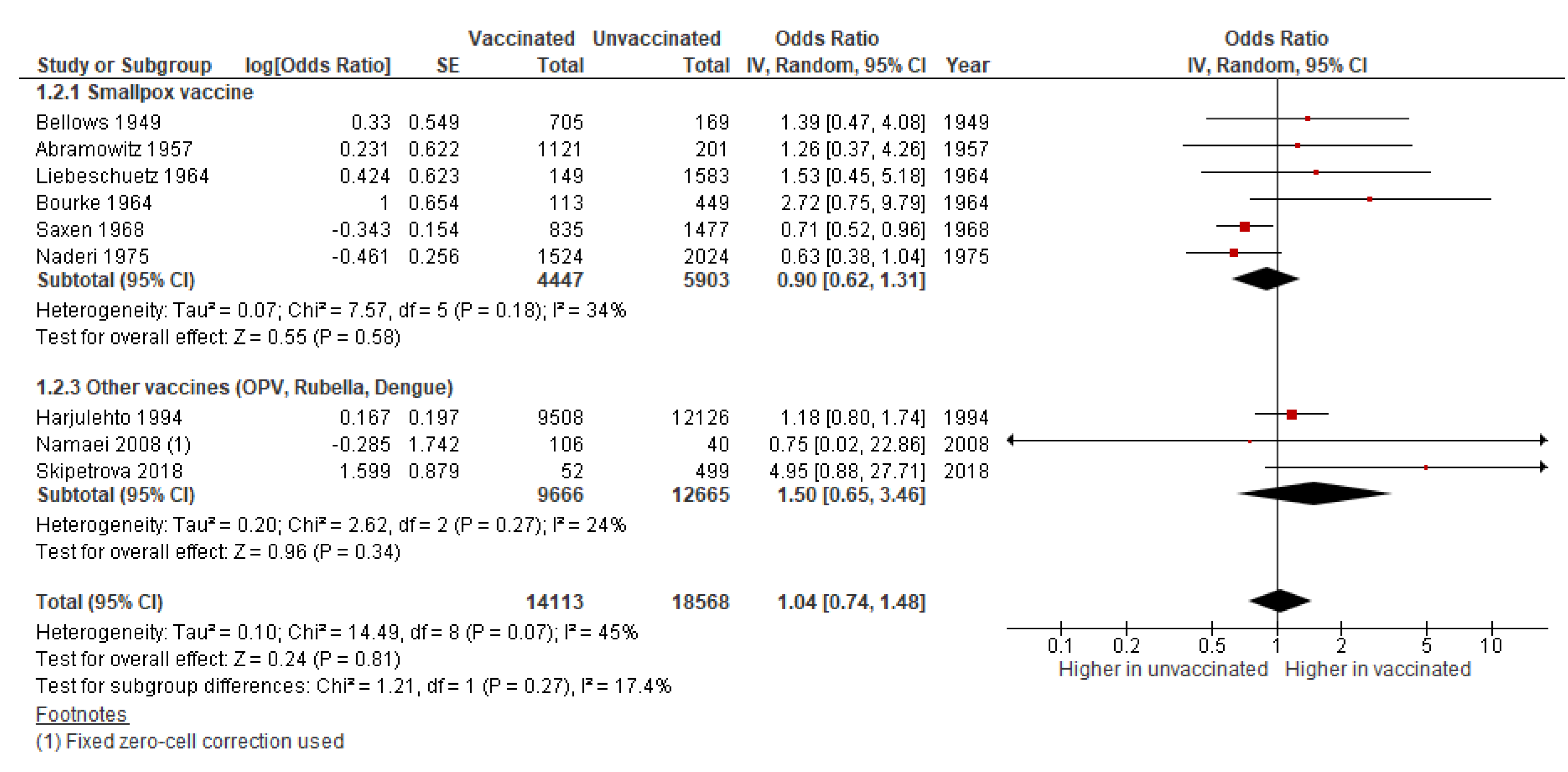
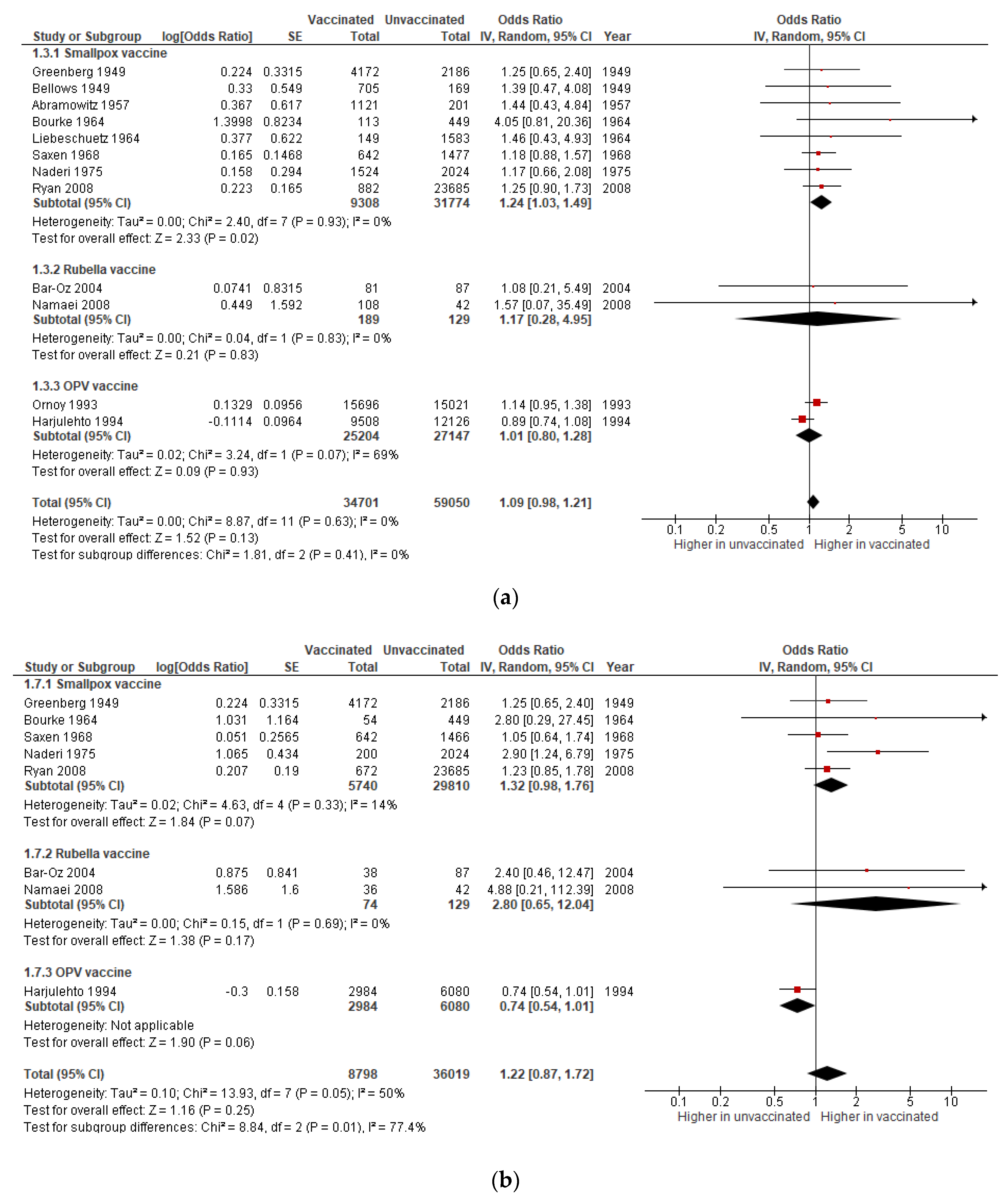
3.1.3. Congenital Anomalies
3.1.4. Preterm Birth
3.1.5. Neonatal Death
3.1.6. Sensitivity Analysis Excluding Studies at Critical Risk of Bias
3.2. Uncontrolled Cohorts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Qualitative Synthesis of Included Studies
Appendix A.1. Smallpox Vaccine
Appendix A.2. Rubella Vaccine
Appendix A.3. Oral Poliovirus Vaccine
Appendix A.4. Yellow Fever Vaccine
Appendix A.5. Dengue Vaccine
References
- Marshall, H.; Mcmillan, M.; Andrews, R.M.; Macartney, K.; Edwards, K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum. Vaccin. Immunother. 2016, 12, 848–856. [Google Scholar] [CrossRef]
- Nunes, M.C.; Aqil, A.R.; Omer, S.B.; Madhi, S.A. The Effects of Influenza Vaccination during Pregnancy on Birth Outcomes: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2016, 33, 1104–1114. [Google Scholar] [CrossRef]
- Amirthalingam, G.; Campbell, H.; Ribeiro, S.; Fry, N.K.; Ramsay, M.; Miller, E.; Andrews, N. Sustained Effectiveness of the Maternal Pertussis Immunization Program in England 3 Years Following Introduction. Clin. Infect. Dis. 2016, 63, S236–S243. [Google Scholar] [CrossRef]
- Torresi, J.; Richmond, P.C.; Heron, L.G.; Qiao, M.; Marjason, J.; Starr-Spires, L.; van der Vliet, D.; Jin, J.; Wartel, T.A.; Bouckenooghe, A. Replication and Excretion of the Live Attenuated Tetravalent Dengue Vaccine CYD-TDV in a Flavivirus-Naive Adult Population: Assessment of Vaccine Viremia and Virus Shedding. J. Infect. Dis. 2017, 216, 834–841. [Google Scholar] [CrossRef][Green Version]
- Wilkins, J.; Salvatore, M.A.; Leedom, J.M.; Portnoy, B. Viremia in a recipient of HPV-77 Rubella virus vaccine. Calif. Med. 1969, 110, 224–227. [Google Scholar] [PubMed]
- Silva, M.L.; Espírito-Santo, L.R.; Martins, M.A.; Silveira-Lemos, D.; Peruhype-Magalhaes, V.; Caminha, R.C.; de Andrade Maranhao-Filho, P.; Auxiliadora-Martins, M.; de Menezes Martins, R.; Galler, R.; et al. Clinical and immunological insights on severe, adverse neurotropic and viscerotropic disease following 17D yellow fever vaccination. Clin. Vaccine Immunol. 2010, 17, 118–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keller-Stanislawski, B.; Englund, J.A.; Kang, G.; Mangtani, P.; Neuzil, K.; Nohynek, H.; Pless, R.; Lambach, P.; Zuber, P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014, 32, 7057–7064. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.A.K.; Seward, J.F. Pregnancy, birth, and infant health outcomes from the National Smallpox Vaccine in Pregnancy Registry, 2003–2006. Clin. Infect. Dis. 2008, 46 (Suppl. S3), S221–S226. [Google Scholar] [CrossRef]
- Hood, C.K.; McKinnon, G.E. Prenatal vaccinia. Am. J. Obstet. Gynecol. 1963, 85, 238–240. [Google Scholar] [CrossRef]
- Rubella vaccination during pregnancy—United States, 1971–1988. JAMA 1989, 38, 289–293.
- Castillo-Solórzano, C.; Reef, S.E.; Morice, A.; Vascones, N.; Chevez, A.E.; Castalia-Soares, R.; Torres, C.; Vizzotti, C.; Ruiz Matus, C. Rubella vaccination of unknowingly pregnant women during mass campaigns for rubella and congenital rubella syndrome elimination, the Americas 2001–2008. J. Infect. Dis. 2011, 204 (Suppl. S2), S713–S717. [Google Scholar] [CrossRef] [PubMed]
- Hamkar, R.; Jalilvand, S.; Abdolbaghi, M.H.; Esteghamati, A.R.; Hagh-goo, A.; Jelyani, K.N.; Mohktari-Azad, T.; Zahraei, M.; Nategh, R. Inadvertent rubella vaccination of pregnant women: Evaluation of possible transplacental infection with rubella vaccine. Vaccine 2006, 24, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Kortung, M.; Pustowoit, B.; Faber, R.; Piskazeck, U.; Liebert, U.G. Persistent fetal rubella vaccine virus infection following inadvertent vaccination during early pregnancy. J. Med. Virol. 2000, 61, 155–158. [Google Scholar] [CrossRef]
- Jackson, A.L.; Louw, J.X. Poliomyelitis at birth due to transplacental infection: Report of a case. S. Afr. Med. J. 1959, 33, 357–358. [Google Scholar] [PubMed]
- Burton, A.E.; Robinson, E.T.; Harper, W.F.; Bell, E.J.; Boyd, J. Fetal damage after accidental polio vaccination of an immune mother. J. R. Coll. Gen. Pract. 1984, 34, 390–394. [Google Scholar]
- Suzano, C.E.S.; Amaral, E.; Sato, H.K.; Papaiordanou, P.M. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine 2006, 24, 1421–1426. [Google Scholar] [CrossRef]
- Nasidi, A.; Monath, T.P.; Vandenberg, J.; Tomori, O.; Calisher, C.H.; Hurtgen, X.; Munube, G.R.; Sorungbe, A.O.; Okafor, G.C.; Wali, S. Yellow fever vaccination and pregnancy: A four-year prospective study. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 337–339. [Google Scholar] [CrossRef]
- Krubiner, C.B.; Faden, R.R.; Karron, R.A.; Little, M.O.; Lyerly, A.D.; Abramson, J.S.; Beigi, R.H.; Cravioto, A.R.; Durbin, A.P.; Gellin, B.G.; et al. Pregnant women & vaccines against emerging epidemic threats: Ethics guidance for preparedness, research, and response. Vaccine 2019. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Skipetrova, A.; Wartel, T.A.; Gailhardou, S. Dengue vaccination during pregnancy—An overview of clinical trials data. Vaccine 2018, 36, 3345–3350. [Google Scholar] [CrossRef]
- Abramowitz, L.J. Vaccination and virus diseases during pregnancy. S. Afr. Med. J. 1957, 31, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bar-Oz, B.; Levichek, Z.; Moretti, M.E.; Mah, C.; Andreou, S.; Koren, G. Pregnancy outcome following rubella vaccination: A prospective controlled study. Am. J. Med. Genet. 2004, 130, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Bellows, M.T.; Hyman, M.E. Effect of smallpox vaccination on the outcome of pregnancy. Public Health Rep. 1949, 64, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Bourke, G.J.; Whitty, R.J. Smallpox vaccination in pregnancy: A prospective study. Br. Med. J. 1964, 1, 1544–1546. [Google Scholar] [CrossRef]
- Ebbin, A.J.; Wilson, M.G.; Chandor, S.B.; Wehrle, P.F. Inadvertent rubella immunization in pregnancy. Am. J. Obstet. Gynecol. 1973, 117, 505–512. [Google Scholar] [CrossRef]
- Greenberg, M.; Yankauer, A.J.; Krugman, S.; Osborn, J.J.; Ward, R.S.; Dancis, J. The effect of smallpox vaccination during pregnancy on the incidence of congenital malformations. Pediatrics 1949, 3, 456–467. [Google Scholar]
- Harjulehto-Mervaala, T.; Aro, T.; Hiilesmaa, V.K.; Hovi, T.; Saxen, H.; Saxen, L. Oral polio vaccination during pregnancy: Lack of impact on fetal development and perinatal outcome. Clin. Infect. Dis. 1994, 18, 414–420. [Google Scholar] [CrossRef]
- Harjulehto-Mervaala, T.; Aro, T.; Hiilesmaa, V.K.; Saxen, H.; Hovi, T.; Saxen, L. Oral polio vaccination during pregnancy: No increase in the occurrence of congenital malformations. Am. J. Epidemiol. 1993, 138, 407–414. [Google Scholar] [CrossRef]
- Harjulehto-Mervaala Hovi, T.; Aro, T.; Saxen, H. Oral poliovirus vaccination and pregnancy complications. Acta Obstet. Gynecol. Scand. 1995, 74, 262–265. [Google Scholar] [CrossRef]
- Liebeschuetz, H.J. The effects of vaccination in pregnancy on the foetus. J. Obstet. Gynaecol. 1964, 71, 132–134. [Google Scholar] [CrossRef]
- Naderi, S. Smallpox vaccination during pregnancy. Obstet. Gynecol. 1975, 46, 223–226. [Google Scholar] [PubMed]
- Namaei, M.H.; Ziaee, M.; Naseh, N. Congenital rubella syndrome in infants of women vaccinated during or just before pregnancy with measles-rubella vaccine. Indian J. Med. Res. 2008, 127, 551–554. [Google Scholar] [PubMed]
- De Nishioka, S.A.; Nunes-Araujo, F.R.; Pires, W.P.; Silva, F.A.; Costa, H.L. Yellow fever vaccination during pregnancy and spontaneous abortion: A case-control study. Trop. Med. Int. Health 1998, 3, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Arnon, J.; Feingold, M.; Ben Ishai, P. Spontaneous abortions following oral poliovirus vaccination in first trimester. Lancet 1990, 335, 800. [Google Scholar] [CrossRef]
- Ornoy, A.; Ben Ishai, P. Congenital anomalies after oral poliovirus vaccination during pregnancy. Lancet 1993, 341, 1162. [Google Scholar] [CrossRef]
- Ryan, M.A.; Gumbs, G.R.; Conlin, A.M.S.; Sevick, C.J.; Jacobson, I.G.; Snell, K.J.; Spooner, C.N.; Smith, T.C.; Department of Defense Birth Infant Health Registry Team. Evaluation of preterm births and birth defects in liveborn infants of US military women who received smallpox vaccine. Birth Defects Res. A Clin. Mol. Teratol. 2008, 82, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Saxen, L.; Cantell, K.; Hakama, M. Relation between smallpox vaccination and outcome of pregnancy. Am. J. Public Health Nations Health 1968, 58, 1910–1921. [Google Scholar] [CrossRef]
- Allan, B.C.; Hamilton, S.M.; Wiemers, M.A.; Winsor, H.; Gust, I.D. Pregnancy complicated by accidental rubella vaccination. Aust. N. Z. J. Obstet. Gynaecol. 1973, 13, 72–76. [Google Scholar] [CrossRef]
- Behnaz, F.; Mohammadzadeh, M.; Bafghi, M.S. Fetal risk associated with rubella mass vaccination. Indian J. Community Med. 2007, 32, 206–207. [Google Scholar] [CrossRef]
- Ergenoglu, A.M.; Yeniel, A.O.; Yildirim, N.; Kazandi, M.; Akercan, F.; Sagol, S. Rubella vaccination during the preconception period or in pregnancy and perinatal and fetal outcomes. Turk. J. Pediatr. 2012, 54, 230–233. [Google Scholar]
- Fleet, W.F.J.; Benz, E.W.J.; Karzon, D.T.; Lefkowitz, L.B.; Herrmann, K.L. Fetal consequences of maternal rubella immunization. J. Am. Med. Assoc. 1974, 227, 621–627. [Google Scholar] [CrossRef]
- Tookey, P.A.; Jones, G.; Miller, B.H.; Peckham, C.S. Rubella vaccination in pregnancy. CDR 1991, 1, R86–R88. [Google Scholar] [PubMed]
- Pardon, F.; Vilariño, M.; Barbero, P.; Garcia, G.; Outon, E.; Gil, C.; Vera, A.; Rossi, S.; Distefano, A. Rubella vaccination of unknowingly pregnant women during 2006 mass campaign in Argentina. J. Infect. Dis. 2011, 204 (Suppl. S2), S745–S747. [Google Scholar] [CrossRef] [PubMed]
- Bart, S.W.; Stetler, H.C.; Preblud, S.R.; Williams, N.M.; Orenstein, W.A.; Bart, K.J.; Hinman, A.R.; Herrmann, K.L. Fetal risk associated with rubella vaccine: An update. Rev. Infect. Dis. 1985, 7 (Suppl. S1), S95–S102. [Google Scholar] [CrossRef] [PubMed]
- Preblud, S.R.; Williams, N.M. Fetal risk associated with rubella vaccine: Implications for vaccination of susceptible women. Obstet. Gynecol. 1985, 66, 121–123. [Google Scholar] [PubMed]
- Preblud, S.R.; Stetler, H.C.; Frank, J.A.; Greaves, W.L.; Hinman, A.R.; Herrmann, K.L. Fetal risk associated with rubella vaccine. J. Am. Med. Assoc. 1981, 246, 1413–1417. [Google Scholar] [CrossRef]
- Modlin, J.F.; Herrmann, K.; Brandling-Bennett, A.D.; Eddins, D.L.; Hayden, G.F. Risk of congenital abnormality after inadvertent rubella vaccination of pregnant women. N. Engl. J. Med. 1976, 294, 972–974. [Google Scholar] [CrossRef]
- Wyll, S.A.; Herrmann, K.L. Inadvertent Rubella Vaccination of Pregnant Women: Fetal Risk in 215 Cases. JAMA 1973, 225, 1472–1476. [Google Scholar] [CrossRef]
- Wyll, S.A. Risk of rubella vaccination during pregnancy. Obstet. Gynecol. 1971, 38, 641–642. [Google Scholar]
- Larson, H.E.; Parkman, P.D.; Davis, W.J.; Hopps, H.E.; Meyer, H.M.J. Inadvertent rubella virus vaccination during pregnancy. N. Engl. J. Med. 1971, 284, 870–873. [Google Scholar] [CrossRef]
- Sheppard, S.; Smithells, R.W.; Dickson, A.; Holzel, H. Rubella vaccination and pregnancy: Preliminary report of a national survey. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 727. [Google Scholar] [CrossRef] [PubMed]
- Badilla, X.; Morice, A.; Avila-Aguero, M.L.; Saenz, E.; Cerda, I.; Reef, S.; Castillo-Solórzano, C. Fetal risk associated with rubella vaccination during pregnancy. Pediatr. Infect. Dis. J. 2007, 26, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.K.; Sanajotta, A.T.; Moraes, J.C.; Andrade, J.Q.; Duarte, G.; Cervi, M.C.; Curti, S.P.; Pannuti, C.S.; Milanez, H.; Pessoto, M.; et al. Rubella vaccination of unknowingly pregnant women: The Sao Paulo experience, 2001. J. Infect. Dis. 2011, 204 (Suppl. S2), S737–S744. [Google Scholar] [CrossRef] [PubMed]
- Minussi, L.; Mohrdieck, R.; Bercini, M.; Ranieri, T.; Sanseverino, M.T.V.; Momino, W.; Callegari-Jacques, S.M.; Schuler-Faccini, L. Prospective evaluation of pregnant women vaccinated against rubella in southern Brazil. Reprod. Toxicol. 2008, 25, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.C.; Siqueira, M.M.; Toscano, C.M.; Maia, M.D.L.S.; Flannery, B.; Sato, H.K.; Will, R.M.; Rodrigues, R.C.; Oliveira, I.C.; Barbosa, T.C.; et al. Follow-up study of unknowingly pregnant women vaccinated against rubella in Brazil, 2001–2002. J. Infect. Dis. 2011, 204 (Suppl. S1), S729–S736. [Google Scholar] [CrossRef] [PubMed]
- Mistchenko, A.S.; Keller, G.A.; Acevedo, M.E.; Jacquez, O.A.; Di Girolamo, G.; Diez, R.A. Inadvertent Rubella Vaccination of Pregnant Women during the Nationwide Rubella Vaccination Campaign in Buenos Aires, Argentina. Drug Saf. 2008, 31, 934–935. [Google Scholar]
- Da Silva e Sa, G.R.; Camacho, L.A.B.; Stavola, M.S.; Lemos, X.R.; Basilio de Oliveira, C.A.; Siqueira, M.M. Pregnancy outcomes following rubella vaccination: A prospective study in the state of Rio de Janeiro, Brazil, 2001–2002. J. Infect. Dis. 2011, 204 (Suppl. S2), S722–S728. [Google Scholar] [CrossRef]
- Wentworth, P. Studies on placentae and infants from women vaccinated for smallpox during pregnancy. J. Clin. Pathol. 1966, 19, 328–330. [Google Scholar] [CrossRef]
- MacArthur, P. Congenital vaccinia and vaccinia gravidarum. Lancet 1952, 2, 1104–1106. [Google Scholar] [CrossRef]
- Enders, G. Rubella antibody titers in vaccinated and nonvaccinated women and results of vaccination during pregnancy. Rev. Infect. Dis. 1985, 7 (Suppl. S1), S103–S107. [Google Scholar] [CrossRef]
- Nasiri, R.; Yoseffi, J.; Khajedaloe, M.; Sarafraz Yazdi, M.; Delgoshaei, F. Congenital rubella syndrome after rubella vaccination in 1–4 weeks periconceptional period. Indian J. Pediatr. 2009, 76, 279–282. [Google Scholar] [CrossRef]
- Cavalcanti, D.P.; Salomao, M.A.; Lopez-Camelo, J.; Pessoto, M.A. Early exposure to yellow fever vaccine during pregnancy. Trop. Med. Int. Health 2007, 12, 833–837. [Google Scholar] [CrossRef]
- Tsai, T.F.; Paul, R.; Lynberg, M.C.; Letson, G.W. Congenital yellow fever virus infection after immunization in pregnancy. J. Infect. Dis. 1993, 168, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Robert, E.; Vial, T.; Schaefer, C.; Arnon, J.; Reuvers, M. Exposure to yellow fever vaccine in early pregnancy. Vaccine 1999, 17, 283–285. [Google Scholar] [CrossRef]
- Marin, M.; Willis, E.D.; Marko, A.; Rasmussen, S.A.; Bialek, S.R.; Dana, A. Closure of varicella-zoster virus-containing vaccines pregnancy registry—United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 732–733. [Google Scholar] [PubMed]
- Willis, E.; Marko, A.; Marin, M.; Rasmussen, S.; Bialek, S.R.; Redfield, A.; Mcgee, M.; Dana, A. Pregnancy Registry for Varicella-Zoster Virus-Containing Vaccines: 18-Year Summary of Pregnancy Outcomes. Open Forum Infect. Dis. 2014, 1 (Suppl. S1), S307. [Google Scholar] [CrossRef]
- Update on Overall Prevalence of Major Birth Defects—Atlanta, Georgia, 1978–2005. JAMA 2008, 57, 1–5.
- World Health Organization. SAGE Interim Recommendations on Vaccination against Ebola Virus Disease (EVD). Available online: https://www.who.int/immunization/interim_ebola_recommendations_feb_2019.pdf?ua=1 (accessed on 12 December 2019).
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier: NCT03611946. Evaluation of the Safety and Immunogenicity of the Live Attenuated Zika Vaccine RZIKV/D4Δ30-713 in Flavivirus-Naïve Adults. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03611946 (accessed on 3 December 2019).
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier: NCT02996890. Zika-Vaccine Dose Finding Study Regarding Safety, Immunogenicity and Tolerability. Available online: https://clinicaltrials.gov/ct2/show/NCT02996890 (accessed on 3 December 2019).
- Kochhar, S.; Bonhoeffer, J.; Jones, C.E.; Muñoz, F.M.; Honrado, A.; Bauwens, J.; Sobanjo-ter Meulen, A.; Hirschfeld, S. Immunization in pregnancy clinical research in low- and middle-income countries—Study design, regulatory and safety considerations. Vaccine 2017, 35, 6575–6581. [Google Scholar] [CrossRef]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG Transplacental Transfer on Early Life Immunity. Immunohorizons 2018, 2, 14–25. [Google Scholar] [CrossRef]
- Edwards, K.M. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015, 33, 6469–6472. [Google Scholar] [CrossRef]
- Schwartz, D.A. Maternal and Infant Death and the rVSV-ZEBOV Vaccine Through Three Recent Ebola Virus Epidemics-West Africa, DRC Équateur and DRC Kivu: 4 Years of Excluding Pregnant and Lactating Women and Their Infants from Immunization. Curr. Trop. Med. Rep. 2019, 6, 213–222. [Google Scholar] [CrossRef]
- Okogbenin, S.; Okoeguale, J.; Akpede, G.; Colubri, A.; Barnes, K.G.; Mehta, S.; Eifediyi, R.; Okogbo, F.; Eigbefoh, J.; Momoh, M.; et al. Retrospective cohort study of lassa fever in pregnancy, southern Nigeria. Emerg. Infect. Dis. 2019, 25, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, B.; Jain, P.; Holder, B.S.; Lindsey, B.; Regan, L.; Kampmann, B. What determines uptake of pertussis vaccine in pregnancy? A cross sectional survey in an ethnically diverse population of pregnant women in London. Vaccine 2015, 33, 5822–5828. [Google Scholar] [CrossRef] [PubMed]
| Study | Location | Study Design | Exposure | Participants | Exposure in 1st Trimester | Previous Immunity | Control Group | Measured Outcomes |
|---|---|---|---|---|---|---|---|---|
| Abramowitz et al. (1957) [21] | Cape Town, South Africa | Retrospective cohort | Smallpox vaccine before 20 weeks gestation | 1121 vaccinated women (510 with successful vaccination *) | NR | NR | 201 women not vaccinated during pregnancy | Stillbirth, birth defects, neonatal death. Outcome definitions not reported |
| Bar-Oz et al. (2004) [22] | Toronto, Canada | Prospective cohort | Rubella (RA 27/3) vaccination ≤3 months before/after conception | 94 women counselled about safety of rubella vaccination during pregnancy through a telephone service | 38 women | NR | 95 women counselled at similar gestational ages for non-teratogenic exposures, not vaccinated during pregnancy | Miscarriage, birth defects, congenital rubella syndrome, neonatal death. Outcomes reported by mother and physician ≥6 months after expected DOB |
| Bellows et al. (1949) [23] | New York, U.S. | Prospective cohort | Smallpox vaccination in pregnancy during a mass vaccination campaign | 720 vaccinated women (571 successful vaccination *) ≤4 months pregnant at admission to antenatal clinic | 247 women | 210/720 with accelerated reaction, suggestive of partial immunity | 173 women admitted to the same antenatal clinics before 4 months gestation, not vaccinated during pregnancy | Miscarriage (before 5 months), stillbirth (after 5 months), birth defects (physical exam, fundoscopy, X-ray, follow-up for 12 months), neonatal death |
| Bourke et al. (1964) [24] | Dublin, Ireland | Prospective cohort | Successful smallpox vaccination * at any stage of pregnancy | 112 vaccinated women attending antenatal clinics in 4 hospitals that account for >80% of births in Dublin | 54 women | NR | 448 women attending the antenatal clinics on the same day (4 adjacent charts), not vaccinated during pregnancy | Miscarriage, stillbirth, birth defects (including stillbirths), neonatal death. Outcome ascertained from medical records. Definitions not reported |
| Ebbin et al. (1973) [25] | Los Angeles County, U.S. | Retrospective cohort | Rubella vaccination during pregnancy or within 3 months before conception | 60 vaccinated women admitted to 7 participating hospitals or referred from private physicians | 27 women | 9/60 previously susceptible, rest unknown | 47 controls from hospital or private practice, matched for age, race, parity, sex of the infant, and private/non-private hospital status | Miscarriage, congenital infection (viral isolation in products of conception from abortion cases, or in pharyngeal and rectal swabs from live born infants) |
| Greenberg et al. (1949) [26] | New York City, U.S. | Retrospective cohort | Smallpox vaccination in 1st trimester of pregnancy during a mass campaign | 4172 † infants born to vaccinated women in participating hospitals and health stations | 4172 infants | NR | 2186 infants born to non-vaccinated women in the same period, identified in participating hospitals and health stations | Birth defects (excluding club foot, hydrocele, inguinal hernia and haemagiomas), LBW |
| Harjulehto et al. (1993, 1994 and 1995) [27,28,29] | Greater Helsinki Region, Finland | Retrospective cohort (population-based) | OPV vaccination during mass campaign; exposure not determined at the individual level | 9508 ‡ births in the 3 hospitals that serve the region, born of women pregnant during the mass vaccination campaign | 2984 § births | Most women likely immune (IPV included in national schedule) | 12,126 live and stillbirths reported in the same hospitals from July to December 1984 and 1986 | Stillbirth (after 22 weeks EGA), prematurity, SGA, birth defects (BPA criteria, including autopsies), neonatal death (in the first 7 days of life) |
| Liebeschuetz et al. (1964) [30] | London, UK | Retrospective cohort | Successful smallpox vaccination * in pregnancy during a mass campaign | 157 vaccinated women attending a maternity hospital during a 6-month period after mass campaign | 131 women | 105/157 had received smallpox vaccine in the past | 1657 women attending the hospital during the same period who were not vaccinated or “unsuccessfully” vaccinated* | Miscarriage, stillbirth, birth defects, fetal vaccinia. Outcome definitions not reported |
| Naderi et al. (1975) [31] | Shiraz, Iran | Prospective cohort | Successful smallpox vaccination * in pregnancy during mass campaign | 1542 infants of 1522 women attending university hospital clinics within 10 months after campaign | 211 infants | All exposed women received smallpox vaccine in the past | 2045 infants of 2014 women not vaccinated during pregnancy and attending the same clinics during the following year | Miscarriage, stillbirth, prematurity, birth defects, clubfoot. Outcome definitions not reported |
| Namaei et al. (2008) [32] | Birjand, Iran | Prospective cohort | Rubella vaccine ≤3 months before/after conception, during a mass campaign | 106 vaccinated women receiving antenatal care and delivering at a university hospital | 71 women | Women with previous infection or vaccination were excluded | 40 women not vaccinated during pregnancy. No details of recruitment provided | Stillbirth, prematurity, congenital infection (serology in cord or infant blood), congenital rubella syndrome (CDC’s clinical criteria) |
| Nishioka et al. (1998) [33] | Uberlandia, Brazil | Case control | Yellow fever (17D) vaccination in pregnancy during a mass campaign § | CASES: 39 women attended for miscarriage at university hospital, with LMP ≥15 days before mass campaign | NR | NR | 74 women living in the same city who attended the antenatal clinic at the university hospital | Miscarriage (pregnancy loss before 28 weeks EGA) |
| Ornoy et al. (1990 and 1993) [34,35] | Israel | Historically-controlled cohort (population-based) | OPV vaccination during a mass campaign with 90% coverage; exposure not determined at the individual level | Women attending hospitals in West Jerusalem within 4 months of campaign (miscarriage) or ≤12 months after (birth defects, LBW) ‡ N = 20,926 annual births | NR | Most women likely immune (poliovirus vaccine in national schedule since 1950s) | Women attending the included hospitals during the same period in the previous year, who were not vaccinated during pregnancy N = 20,143 annual births | Miscarriage (in relation to the number of annual births), birth defects (as proportion of annual live births), LBW (<2500 grs birthweight). Outcomes obtained from hospital records |
| Ryan et al. (2008) [36] | U.S. | Retrospective cohort. (Dept. of Defense databases) | Smallpox vaccination at any stage of pregnancy | 882 infants born during 2003-2004 to active-duty military women vaccinated inadvertently during pregnancy | 672 women | NR, but probably not previous immunity (routine vaccination stopped in 1972) | 23,685 infants born to military women not vaccinated against smallpox; 6853 infants born to active-duty women vaccinated before or after pregnancy | Prematurity (birth before 37 weeks EGA), birth defects (NBDPN definitions used, records up to 12 months of age reviewed). Outcomes defined using ICD-9-CM codes |
| Saxen et al. (1968) [37] | Finland | Case control | Smallpox vaccination before or during pregnancy in the context of a country-wide campaign | CASES 835 stillbirths and 642 infants with birth defects notified to the National Board of Health | NR | 73% of mothers in study group and 77% in control group previously vaccinated | 1477 infants born next after stillbirth/malformed infants in the same district | Stillbirth, birth defects Outcome definitions not reported |
| Skipetrova et al. (2018) [20] | Several countries (mostly Latin America) | Secondary analysis of data from clinical trials | Dengue (CYD-TDV) vaccination during pregnancy or <30 days before LMP (“risk period”) | 58 women inadvertently vaccinated during the “risk period” in CYD-TDV clinical trials | Most women vaccinated before or shortly after conception | NR | 341 pregnant women vaccinated outside the “risk period”, 30 received placebo during “risk period”, 179 received placebo outside the “risk period” | Miscarriage (pregnancy loss before 20 weeks EGA), stillbirth (fetal death after 20 weeks EGA) |
| Vaccination during Pregnancy Compared with No Vaccination. | ||||
| Patient or population: Pregnant women and their fetuses/infants. Intervention: Administration of live vaccines during pregnancy or shortly before conception. Comparison: No exposure to live vaccines during pregnancy or shortly before conception. | ||||
| Outcomes | Relative Effect (95% CI) | Number of Participants (Studies) | Quality of the Evidence (GRADE) | Comments |
| Miscarriage | OR 0·98 (0.87 to 1.10) | 17,763 (9 studies) | Very low | Includes data on smallpox (4 studies), rubella (2), OPV (1), dengue (1), and YF (1) vaccines. |
| Stillbirth | OR 1·04 (0.74 to 1.48) | 32,701 (9 studies) | Very low | Includes data on smallpox (6 studies), rubella (1), OPV (1), and dengue (1) vaccines. |
| Congenital anomalies | OR 1.09 (0.98 to 1.21) | 93,751 (12 studies) | Very low | Includes data on smallpox (8 studies), rubella (2), and OPV (2) vaccines. |
| Preterm birth | OR 0.99 (0.90 to 1.08) | 49,995 (5 studies) | Very low | Includes data on smallpox (2 studies), rubella (2,) and OPV (1) vaccines. |
| Neonatal death | OR 1.06 (0.68 to 1.65) | 24,499 (5 studies) | Very low | Includes data on smallpox (3 studies), rubella (1), and OPV (1) vaccines. |
| Miscarriage after 1st trimester vaccination | OR 2.66|(0.73 to 9.64) | 2832 (3 studies) | Very low | Includes data on smallpox (1 study) and rubella (1) vaccines. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laris-González, A.; Bernal-Serrano, D.; Jarde, A.; Kampmann, B. Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines 2020, 8, 124. https://doi.org/10.3390/vaccines8010124
Laris-González A, Bernal-Serrano D, Jarde A, Kampmann B. Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines. 2020; 8(1):124. https://doi.org/10.3390/vaccines8010124
Chicago/Turabian StyleLaris-González, Almudena, Daniel Bernal-Serrano, Alexander Jarde, and Beate Kampmann. 2020. "Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes" Vaccines 8, no. 1: 124. https://doi.org/10.3390/vaccines8010124
APA StyleLaris-González, A., Bernal-Serrano, D., Jarde, A., & Kampmann, B. (2020). Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines, 8(1), 124. https://doi.org/10.3390/vaccines8010124





