Low-Energy Electron Irradiation Efficiently Inactivates the Gram-Negative Pathogen Rodentibacter pneumotropicus—A New Method for the Generation of Bacterial Vaccines with Increased Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cultivation of Rodentibacter Pneumotropicus
2.3. Pathogen Inactivation
2.3.1. Low-energy Electron Irradiation (LEEI)
2.3.2. Formaldehyde Inactivation
2.4. ELISA
2.5. LPS Based Reporter Assay
2.6. Immunization and Challenge
2.7. Determination of Bacterial Load
2.8. Statistical Analysis
3. Results
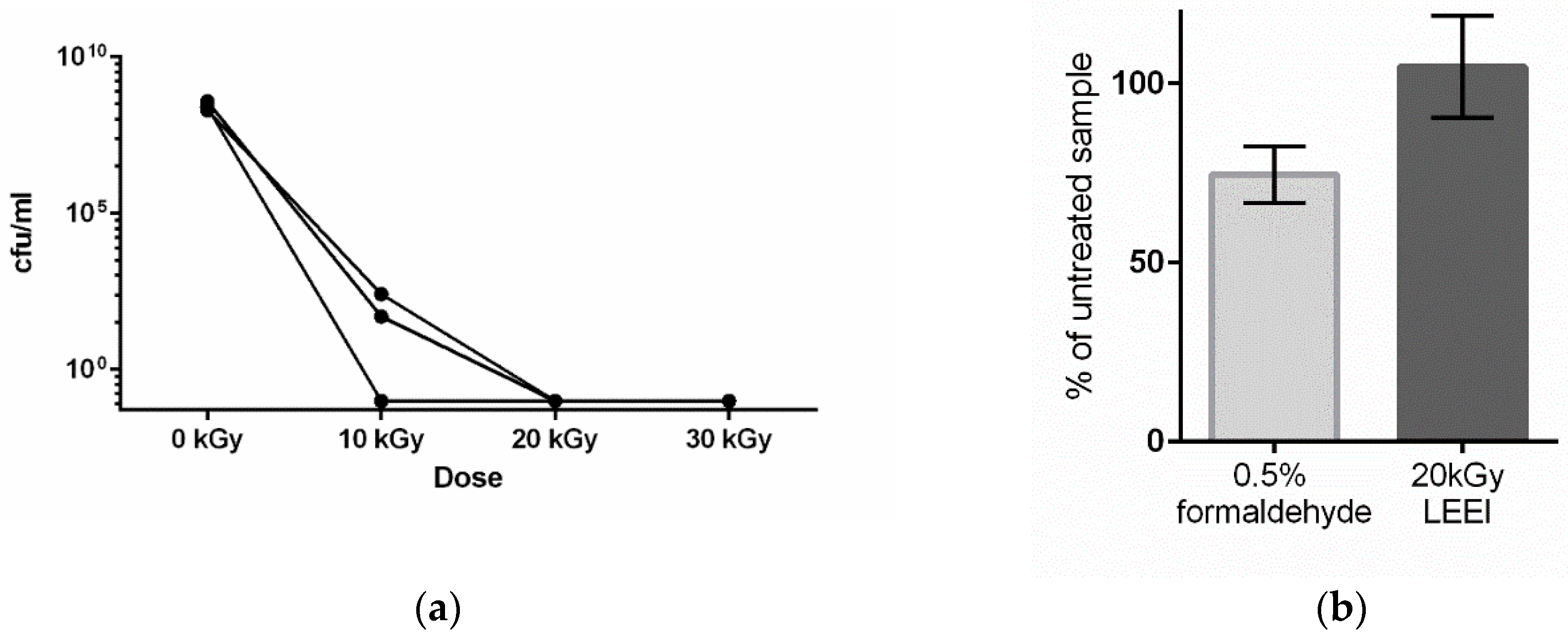
3.1. Low-Energy Electron Irradiation Inactivates Bacteria with High Antigen Conservation
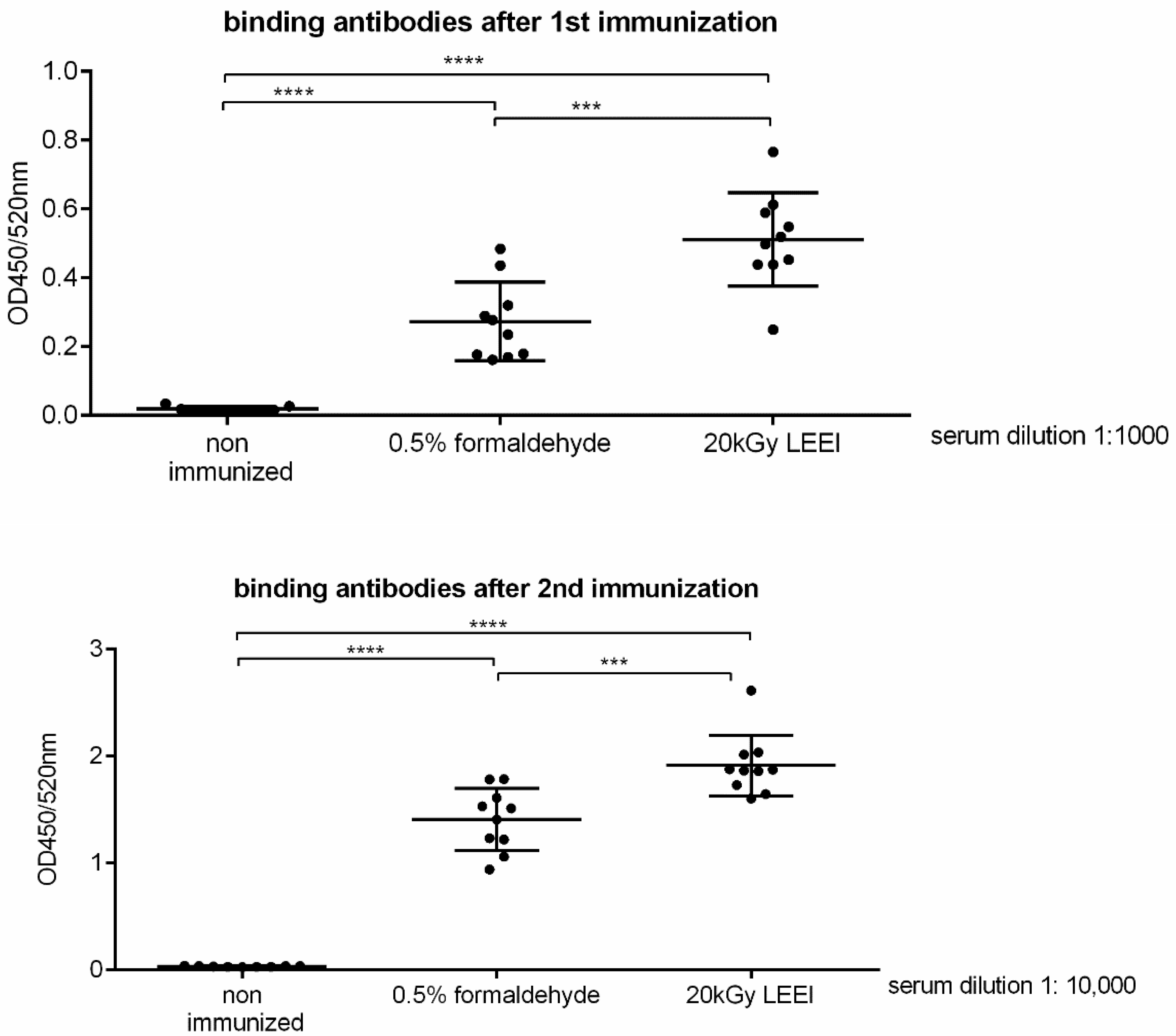
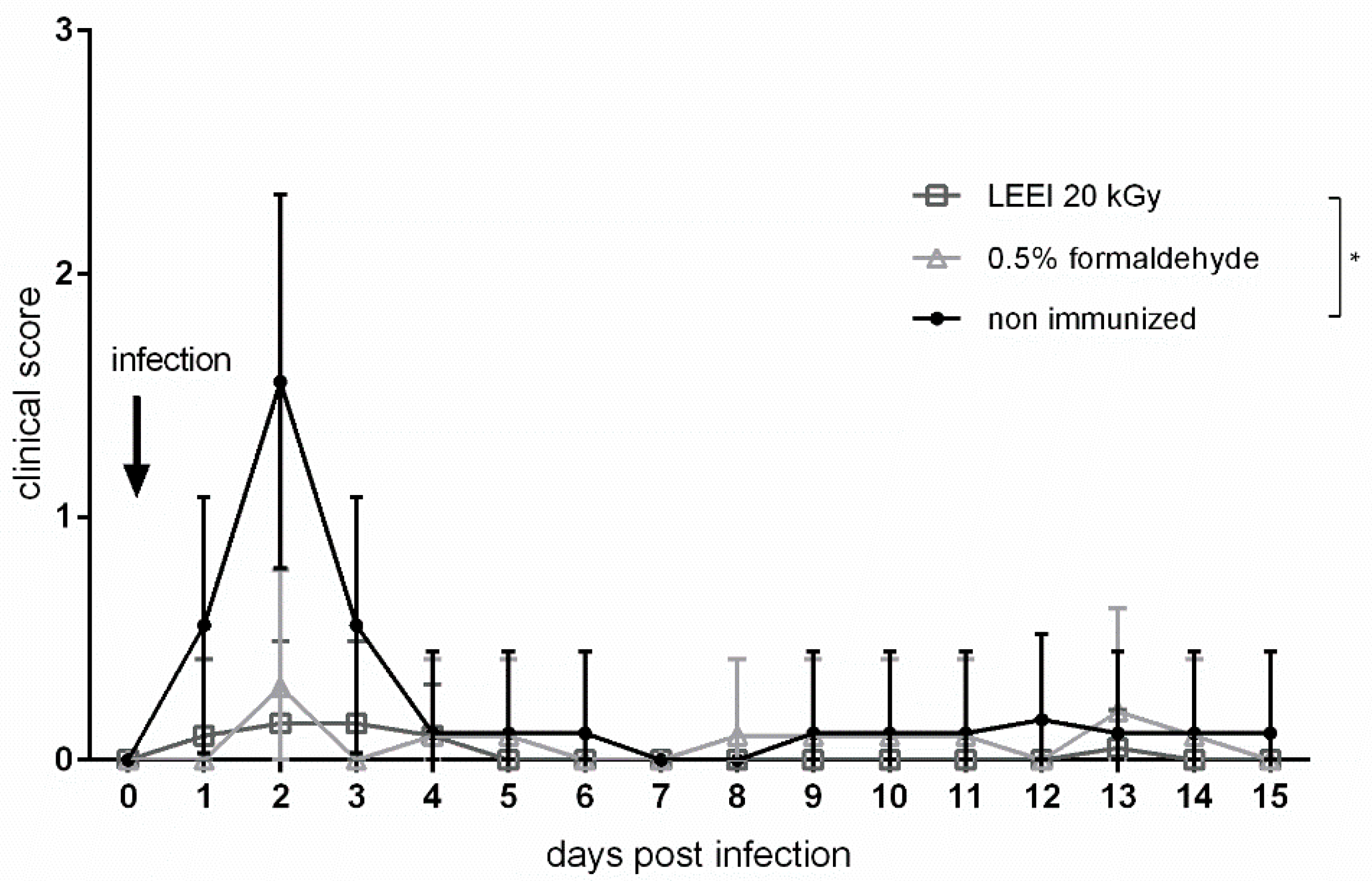
3.2. Low-Energy Electron Irradiation Elicits Strong Immune Responses after Vaccination of Mice
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldstein, M.A.; Tauraso, N.M. Effect of formalin, beta-propiolactone, merthiolate, and ultraviolet light upon influenza virus infectivity chicken cell agglutination, hemagglutination, and antigenicity. Appl. Microbiol. 1970, 19, 290–294. [Google Scholar] [CrossRef]
- Amanna, I.J.; Raué, H.-P.; Slifka, M.K. Development of a new hydrogen peroxide–based vaccine platform. Nat. Med. 2012, 18, 974–979. [Google Scholar] [CrossRef]
- Seo, H.S. Application of radiation technology in vaccines development. Clin. Exp. Vaccine Res. 2015, 4, 145. [Google Scholar] [CrossRef]
- Fan, Y.-C.; Chiu, H.-C.; Chen, L.-K.; Chang, G.-J.J.; Chiou, S.-S. Formalin Inactivation of Japanese Encephalitis Virus Vaccine Alters the Antigenicity and Immunogenicity of a Neutralization Epitope in Envelope Protein Domain III. PLoS Negl. Trop. Dis. 2015, 9, e0004167. [Google Scholar] [CrossRef]
- Brown, F. Formaldehyde as an inactivant. Vaccine 1995, 13, 231. [Google Scholar] [CrossRef]
- Ferguson, M.; Wood, D.J.; Minor, P.D. Antigenic structure of poliovirus in inactivated vaccines. J. Gen. Virol. 1993, 74, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Babb, R.; Chen, A.; Hirst, T.R.; Kara, E.E.; McColl, S.R.; Ogunniyi, A.D.; Paton, J.C.; Alsharifi, M. Intranasal vaccination with γ-irradiated Streptococcus pneumoniae whole-cell vaccine provides serotype-independent protection mediated by B-cells and innate IL-17 responses. Clin. Sci. 2016, 130, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Dabral, N.; Martha-Moreno-Lafont, N.S.; Vemulapalli, R. Oral immunization of mice with gamma-irradiated Brucella neotomae induces protection against intraperitoneal and intranasal challenge with virulent B. abortus 2308. PLoS ONE 2014, 9, e107180. [Google Scholar] [CrossRef] [PubMed]
- Bordin, A.I.; Pillai, S.D.; Brake, C.; Bagley, K.B.; Bourquin, J.R.; Coleman, M.; Oliveira, F.N.; Mwangi, W.; McMurray, D.N.; Love, C.C.; et al. Immunogenicity of an Electron Beam Inactivated Rhodococcus equi Vaccine in Neonatal Foals. PLoS ONE 2014, 9, e105367. [Google Scholar] [CrossRef] [PubMed]
- Jwa, M.Y.; Jeong, S.; Ko, E.B.; Kim, A.R.; Kim, H.Y.; Kim, S.K.; Seo, H.S.; Yun, C.-H.; Han, S.H. Gamma-irradiation of Streptococcus pneumoniae for the use as an immunogenic whole cell vaccine. J. Microbiol. 2018, 56, 579–585. [Google Scholar] [CrossRef]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, F. Chemical Changes Induced in DNA by Ionizing Radiation. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 32, pp. 115–154. ISBN 9780125400329. [Google Scholar]
- Bayer, L.; Fertey, J.; Ulbert, S.; Grunwald, T. Immunization with an adjuvanted low-energy electron irradiation inactivated respiratory syncytial virus vaccine shows immunoprotective activity in mice. Vaccine 2018, 36, 1561–1569. [Google Scholar] [CrossRef]
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated virus vaccines from chemistry to prophylaxis: Merits, risks and challenges. Expert Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef]
- Silindir, M.; Özer, A.Y. Sterilization Methods and the Comparison of E-Beam Sterilization with Gamma Radiation Sterilization. FABAD J. Pharm. Sci. 2009, 34, 43–53. [Google Scholar]
- IAEA. IAEA Safety standards. In Radiation Safety of Gamma, Electron and X-ray Irradiation Facilities; IAEA: Vienna, Austria, 2010; ISBN 978-92-0-103710-7. [Google Scholar]
- Fertey, J.; Bayer, L.; Grunwald, T.; Pohl, A.; Beckmann, J.; Gotzmann, G.; Casado, J.; Schönfelder, J.; Rögner, F.-H.; Wetzel, C.; et al. Pathogens Inactivated by Low-Energy-Electron Irradiation Maintain Antigenic Properties and Induce Protective Immune Responses. Viruses 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.; Schmäschke, R.; Fertey, J.; Bangoura, B.; Schönfelder, J.; Lendner, M.; Ulbert, S.; Daugschies, A. Eimeria tenella oocysts attenuated by low energy electron irradiation (LEEI) induce protection against challenge infection in chickens. Vet. Parasitol. 2019, 266, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Fornefett, J.; Krause, J.; Klose, K.; Fingas, F.; Hassert, R.; Benga, L.; Grunwald, T.; Müller, U.; Schrödl, W.; Baums, C.G. Comparative analysis of humoral immune responses and pathologies of BALB/c and C57BL/6 wildtype mice experimentally infected with a highly virulent Rodentibacter pneumotropicus (Pasteurella pneumotropica) strain. BMC Microbiol. 2018, 18, 314. [Google Scholar] [CrossRef] [PubMed]
- Benga, L.; Benten, W.P.M.; Engelhardt, E.; Bleich, A.; Gougoula, C.; Sager, M. Development of a multiplex PCR assay based on the 16S–23S rRNA internal transcribed spacer for the detection and identification of rodent Pasteurellaceae. J. Microbiol. Methods 2013, 95, 256–261. [Google Scholar] [CrossRef]
- Burger-Kentischer, A.; Abele, I.S.; Finkelmeier, D.; Wiesmüller, K.-H.; Rupp, S. A new cell-based innate immune receptor assay for the examination of receptor activity, ligand specificity, signalling pathways and the detection of pyrogens. J. Immunol. Methods 2010, 358, 93–103. [Google Scholar] [CrossRef]
- See, S.B.; Thomas, W.R. Protective anti-outer membrane protein immunity against Pasteurella pneumotropica infection of mice. Microbes Infect. 2013, 15, 470–479. [Google Scholar] [CrossRef]
- Scott, G.H.; McCaul, T.F.; Williams, J.C. Inactivation of Coxiella burnetii by gamma irradiation. J. Gen. Microbiol. 1989, 135, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Stanislavsky, E.S.; Makarenko, T.A.; Kholodkova, E.V.; Lugowski, C. R-form lipopolysaccharides (LPS) of Gram-negative bacteria as possible vaccine antigens. FEMS Immunol. Med. Microbiol. 1997, 18, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zariri, A.; van der Ley, P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev. Vaccines 2015, 14, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Hieke, A.-S.C.; Pillai, S.D. Escherichia coli Cells Exposed to Lethal Doses of Electron Beam Irradiation Retain Their Ability to Propagate Bacteriophages and Are Metabolically Active. Front. Microbiol. 2018, 9, 2138. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fertey, J.; Bayer, L.; Kähl, S.; Haji, R.M.; Burger-Kentischer, A.; Thoma, M.; Standfest, B.; Schönfelder, J.; Portillo Casado, J.; Rögner, F.-H.; et al. Low-Energy Electron Irradiation Efficiently Inactivates the Gram-Negative Pathogen Rodentibacter pneumotropicus—A New Method for the Generation of Bacterial Vaccines with Increased Efficacy. Vaccines 2020, 8, 113. https://doi.org/10.3390/vaccines8010113
Fertey J, Bayer L, Kähl S, Haji RM, Burger-Kentischer A, Thoma M, Standfest B, Schönfelder J, Portillo Casado J, Rögner F-H, et al. Low-Energy Electron Irradiation Efficiently Inactivates the Gram-Negative Pathogen Rodentibacter pneumotropicus—A New Method for the Generation of Bacterial Vaccines with Increased Efficacy. Vaccines. 2020; 8(1):113. https://doi.org/10.3390/vaccines8010113
Chicago/Turabian StyleFertey, Jasmin, Lea Bayer, Sophie Kähl, Rukiya M. Haji, Anke Burger-Kentischer, Martin Thoma, Bastian Standfest, Jessy Schönfelder, Javier Portillo Casado, Frank-Holm Rögner, and et al. 2020. "Low-Energy Electron Irradiation Efficiently Inactivates the Gram-Negative Pathogen Rodentibacter pneumotropicus—A New Method for the Generation of Bacterial Vaccines with Increased Efficacy" Vaccines 8, no. 1: 113. https://doi.org/10.3390/vaccines8010113
APA StyleFertey, J., Bayer, L., Kähl, S., Haji, R. M., Burger-Kentischer, A., Thoma, M., Standfest, B., Schönfelder, J., Portillo Casado, J., Rögner, F.-H., Baums, C. G., Grunwald, T., & Ulbert, S. (2020). Low-Energy Electron Irradiation Efficiently Inactivates the Gram-Negative Pathogen Rodentibacter pneumotropicus—A New Method for the Generation of Bacterial Vaccines with Increased Efficacy. Vaccines, 8(1), 113. https://doi.org/10.3390/vaccines8010113




