Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain–Barré Syndrome
Abstract
:1. Introduction
2. Materials and Methods
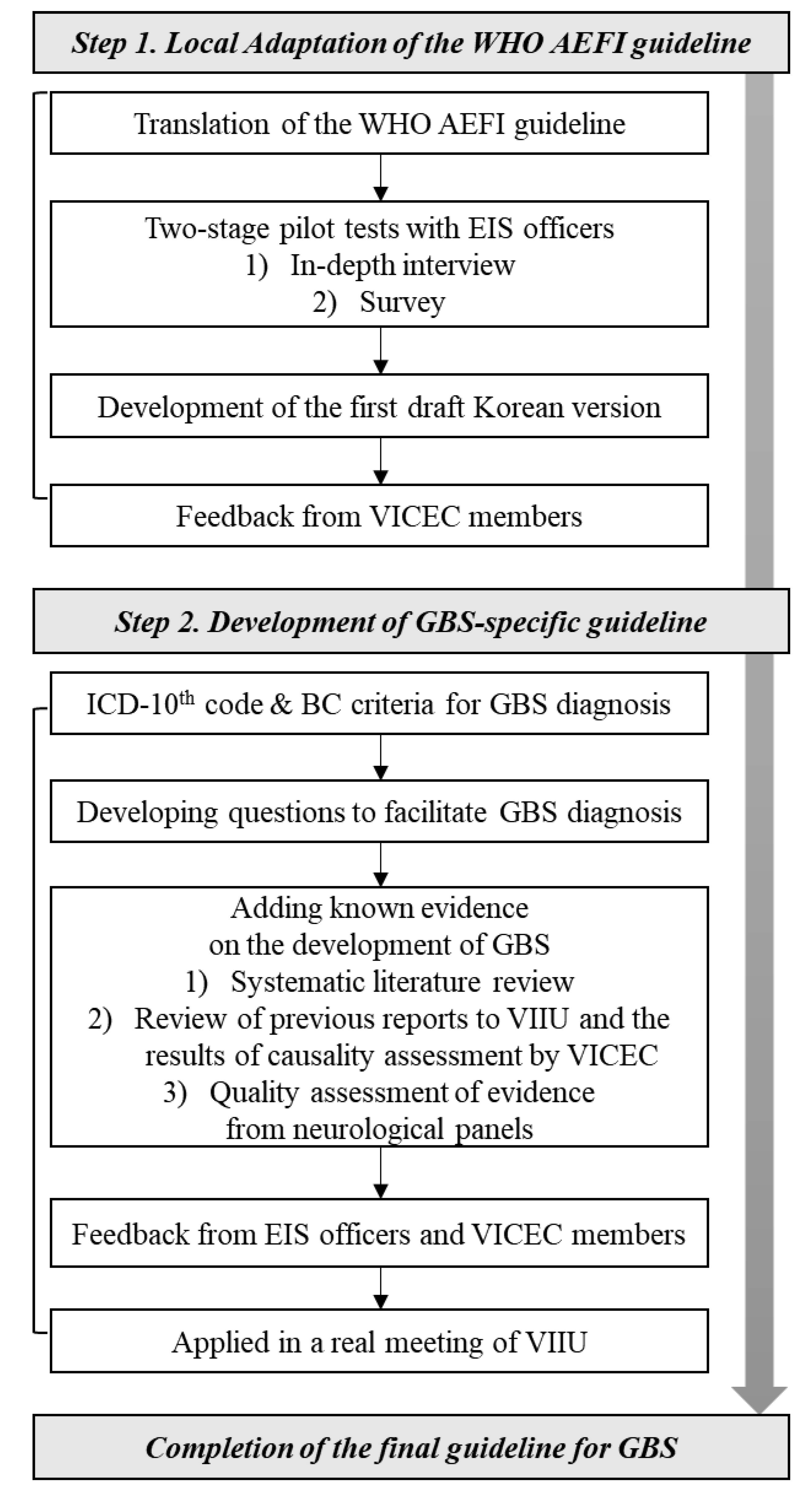
2.1. Local Adaptation of the Global WHO Guidelines
2.2. Development of GBS-Specific Guidelines
3. Results
3.1. Structure of the Guidelines
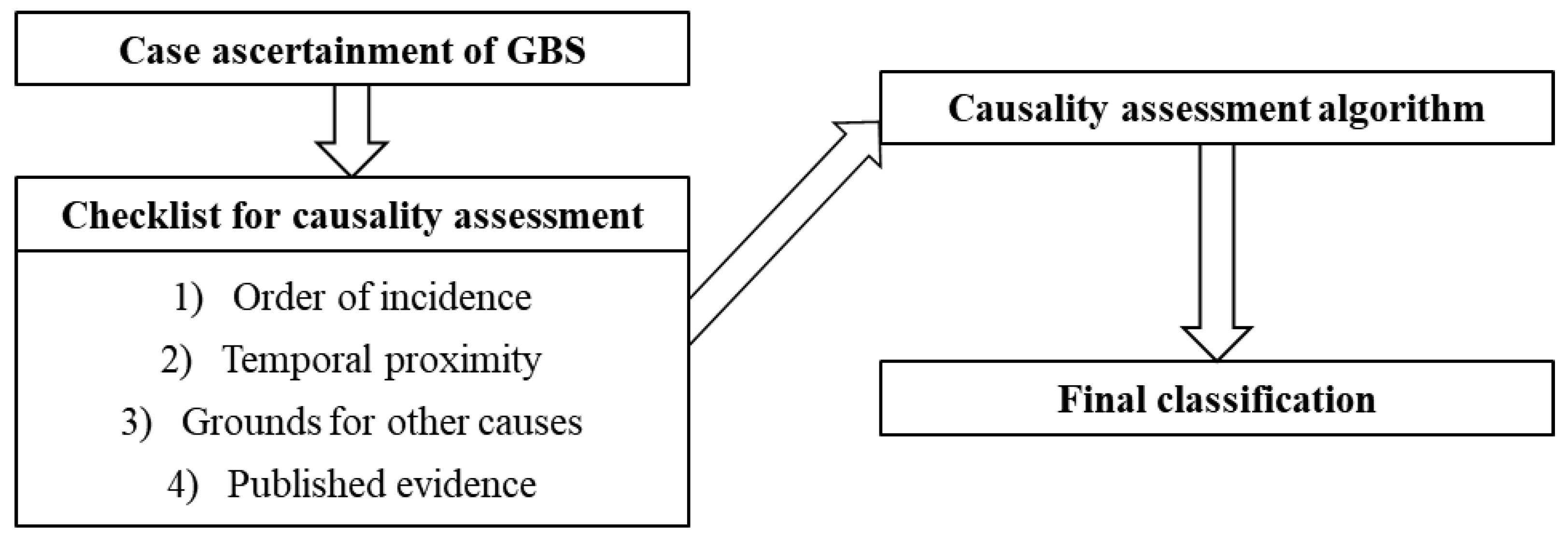
3.2. Case Ascertainment of GBS (Eligibility)
3.3. Checklist
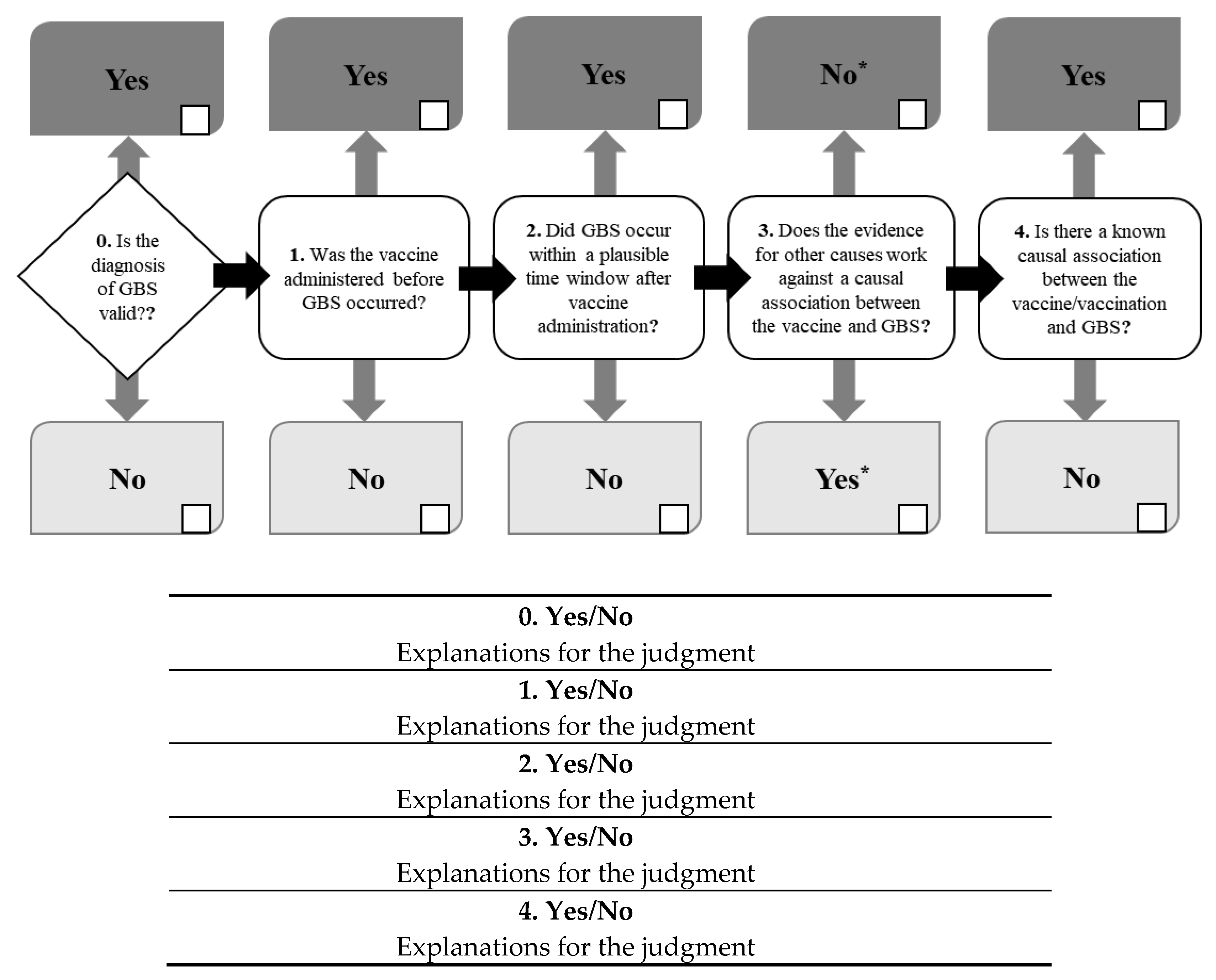
3.4. Algorithm and Classification
3.5. Application
4. Discussion
4.1. First Guidelines for Causality Assessment between Guillain–Barré Syndrome and the Vaccine Following Vaccination
4.2. Securing the Objectivity of the Clinical Diagnosis of Guillain–Barré Syndrome through Multi-Step Verification
4.3. Recommended Application Method of Guidelines Aligned with Policy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Looker, C.; Kelly, B. No-fault compensation following adverse events attributed to vaccination: a review of international programmes. Bull. World Health Organ. 2011, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Council for International Organizations of Medical Sciences (CIOMS). Definition and Application of Terms for Vaccine Pharmacovigilance; Report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance: Geneva, Switzerland, 2012. [Google Scholar]
- Edwards, I.R.; Jeffrey, K.A. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 1255–1259. [Google Scholar] [CrossRef]
- Nebeker, J.R.; Barach, P.; Samore, M.H. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann. Intern. Med. 2004, 140, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Kramer, M.S.; Leventhal, J.M.; Hutchinson, T.A.; Feinstein, A.R. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA 1979, 242, 623–632. [Google Scholar] [CrossRef]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Louet, H.L. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef]
- World Health Organization. Causality Assessment of an adverse event following immunization (AEFI). In User Manual for the Revised WHO Classification Second Edition; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barre syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Guillain-Barré syndrome and Flu Vaccine. Available online: https://www.cdc.gov/flu/prevent/guillainbarre.htm (accessed on 3 December 2019).
- Sejvar, J.J.; Kohl, K.S.; Gidudu, J.; Amato, A.; Bakshi, N.; Baxter, R.; Burwen, D.R.; Cornblath, D.R.; Cleerbout, J.; Edwards, K.M.; et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011, 29, 599–612. [Google Scholar] [CrossRef] [Green Version]
- Leonhard, S.E.; Mandarakas, M.R.; Gondim, F.A.A.; Bateman, K.; Ferreira, M.L.B.; Cornblath, D.R.; van Doorn, P.A.; Dourado, M.E.; Hughes, R.A.C.; Islam, B.; et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat. Rev. Neurol. 2019, 15, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Longo, M.R. Guillain-Barré syndrome. Autoimmun. Rev. 2017, 16, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Yang, H.W.; Liao, T.L.; Wu, W.J.; Yang, S.E.; Chih, Y.C.; Chuang, J.H. Safety of pandemic (H1N1) 2009 monovalent vaccines in Taiwan: A self-controlled case series study. PLoS ONE 2013, 8, e58827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestel, J.; Volkers, P.; Mentzer, D.; Lehmann, H.C.; Hartung, H.P.; Keller-Stanislawski, B.; for the GBS study group. Risk of Guillain–Barré syndrome following pandemic influenza A (H1N1) 2009 vaccination in Germany. Pharmacoepidemiol. Drug Saf. 2014, 23, 1192–1204. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, B.C.; Rothbarth, P.H.; Van der Meché, F.G.A.; Herbrink, P.; Schmitz, P.I.M.; De Klerk, M.A.; Van Doorn, P.A. The spectrum of antecedent infections in Guillain-Barré syndrome: A case-control study. Neurology 1998, 51, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, P.A.; Ruts, L.; Jacobs, B.C. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008, 7, 939–950. [Google Scholar] [CrossRef]
- Hughes, R.A.C.; Hadden, R.D.M.; Gregson, N.A.; Smith, K.J. Pathogenesis of Guillain–Barré syndrome. J. Neuroimmunol. 1999, 100, 74–97. [Google Scholar] [CrossRef]
- Koga, M.; Yuki, N.; Hirata, K. Antecedent symptoms in Guillain–Barré syndrome: an important indicator for clinical and serological subgroups. Acta Neurol. Scand. 2001, 103, 278–287. [Google Scholar] [CrossRef]
- van Koningsveld, R.; Rico, R.; Gerstenbluth, I.; Schmitz, P.I.M.; Ang, C.W.; Merkies, I.S.J.; Jacobs, B.C.; Halabi, Y.; Endtz, H.P.; van der Meché, F.G.A.; et al. Gastroenteritis-associated Guillain–Barre syndrome on the Caribbean island Curacao. Neurology 2001, 56, 1467–1472. [Google Scholar] [CrossRef]
- Lynch, R.M.; Mantus, G.; Encinales, L.; Pacheco, N.; Li, G.; Porras, A.; Mendoza, A.R.; Peng, J.; Rengifo-Pardo, M.; Cruz, M.M.; et al. Augmented Zika and dengue neutralizing antibodies are associated with Guillain-Barre syndrome. J. Infect. Dis. 2019, 219, 26–30. [Google Scholar] [CrossRef]
- Gangula, R.S.; Stanley, W.; Vandanapu, A.; Prabhu, M. Guillain-Barre syndrome with falciparum malaria and scrub typhus mixed infection-an unusual combination. J. Clin. Diagn. Res. 2017, 11, OD10–OD11. [Google Scholar] [CrossRef] [PubMed]
- Wijesundere, A. Guillain-Barré syndrome in Plasmodium falciparum malaria. Postgrad. Med. J. 1992, 68, 376. [Google Scholar] [CrossRef] [PubMed]
- Ju, I.N.; Lee, J.W.; Cho, S.Y.; Ryu, S.J.; Kim, Y.J.; Kim, S.I.; Kang, M.W. Two cases of scrub typhus presenting with Guillain-Barré syndrome with respiratory failure. Korean J. Intern. Med. 2011, 26, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, N.; Khealani, B.A.; Shariff, A.H.; Wasay, M. Guillain–Barré syndrome (demyelinating) six weeks after bariatric surgery: A case report and literature review. Obes. Res. Clin. Pract. 2015, 9, 416–419. [Google Scholar] [CrossRef]
- Qi, B.; Chunyang, M. Retrospective Analysis and Comment on the Time Interval Between Surgery and Onset of Guillain-Barré Syndrome. World Neurosurg. 2018, 109, 499. [Google Scholar] [CrossRef]
- Ebrahim, S.Z.; Rahmani, F.; Rezaei, N. Autoimmunity and cytokines in Guillain-Barré syndrome revisited: review of pathomechanisms with an eye on therapeutic options. Eur. Cytokine Netw. 2019, 30, 1–14. [Google Scholar]
- Israeli, E.; Agmon-Levin, N.; Blank, M.; Chapman, J.; Shoenfeld, Y. Guillain-Barré syndrome--a classical autoimmune disease triggered by infection or vaccination. Clin. Rev. Allergy Immunol. 2012, 42, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter species and Guillain-Barre syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Leonard, E.E.; Tompkins, L.S.; Falkow, S.; Nachamkin, I. Comparison of Campylobacter jejuni isolates implicated in Guillain-Barre syndrome and strains that cause enteritis by a DNA microarray. Infect. Immun. 2004, 72, 1199–1203. [Google Scholar] [CrossRef] [Green Version]
- Hadden, R.D.M.; Karch, H.; Hartung, H.P.; Zielasek, J.; Weissbrich, B.; Schubert, J.; Weishaupt, A.; Cornblath, D.R.; Swan, A.V.; Hughes, R.A.C.; et al. Preceding infections, immune factors, and outcome in Guillain–Barré syndrome. Neurology 2001, 56, 758–765. [Google Scholar] [CrossRef]
- Orlikowski, D.; Quijano-Roy, S.; Sivadon-Tardy, V.; Raphael, J.C.; Gaillard, J.L. Campylobacter jejuni and cytomegalovirus (CMV) infections in patients with the Guillain-Barre syndrome. Arch. Pediatr. 2006, 13, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.H.; Van der Meché, F.G.A.; Meulstee, J.; Rothbarth, P.; Jacobs, B.C.; Schmitz, P.I.M.; Van Doorn, P.A. Cytomegalovirus infection and Guillain-Barré syndrome: The clinical, electrophysiologic, and prognostic features. Neurology 1996, 47, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Steininger, C.; Seiser, A.; Gueler, N.; Puchhammer-Stöckl, E.; Aberle, S.W.; Stanek, G.; Popow-Kraupp, T. Primary cytomegalovirus infection in patients with Guillain-Barre syndrome. J. Neuroimmunol. 2007, 183, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kaida, K.; Ariga, T.; Yu, R.K. Antiganglioside antibodies and their pathophysiological effects on Guillain-Barré syndrome and related disorders—A review. Glycobiology 2009, 19, 676–692. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, S. Guillain-Barré syndrome: Epidemiology, pathophysiology and management. Drugs 2004, 64, 597–610. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Rojas, M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramirez-Santana, C.; Monsalve, D.M.; Gershwin, M.E.; Anaya, J.M. Guillain-Barre syndrome, transverse myelitis and infectious diseases. Cell. Mol. Immunol. 2018, 15, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Tam, C.C.; O’Brien, S.J.; Petersen, I.; Islam, A.; Hayward, A.; Rodrigues, L.C. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS ONE 2007, 2, e344. [Google Scholar] [CrossRef] [Green Version]
- Grose, C.; Feorino, P. Epstein-Barr virus and Guillain-Barré syndrome. Lancet 1972, 300, 1285–1287. [Google Scholar] [CrossRef]
- Islam, B.; Islam, Z.; GeurtsvanKessel, C.H.; Jahan, I.; Endtz, H.P.; Mohammad, Q.D.; Jacobs, B.C. Guillain-Barré syndrome following varicella-zoster virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Meyer Sauteur, P.M.; Huizinga, R.; Tio-Gillen, A.P.; Roodbol, J.; Hoogenboezem, T.; Jacobs, E.; van Rijn, M.; van der Eijk, A.A.; Vink, C.; de Wit, M.C.; et al. Mycoplasma pneumoniae triggering the Guillain-Barré syndrome: A case-control study. Ann. Neurol. 2016, 80, 566–580. [Google Scholar] [CrossRef]
- Narita, M. Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection. Pediatr. Neurol. 2009, 41, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nafissi, S.; Vahabi, Z.; Sadeghi Ghahar, M.; Amirzargar, A.A.; Naderi, S. The role of cytomegalovirus, Haemophilus influenzae and Epstein Barr virus in Guillain Barre syndrome. Acta Med. Iran. 2013, 51, 372–376. [Google Scholar] [PubMed]
- Yamana, M.; Kuwahara, M.; Fukumoto, Y.; Yoshikawa, K.; Takada, K.; Kusunoki, S. Guillain-Barré syndrome and related diseases after influenza virus infection. Neurol. Neuroimmunol. Neuroinflammation 2019, 6, e575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaderi, S.; Gunnes, N.; Bakken, I.J.; Magnus, P.; Trogstad, L.; Håberg, S.E. Risk of Guillain-Barré syndrome after exposure to pandemic influenza A(H1N1)pdm09 vaccination or infection: A Norwegian population-based cohort study. Eur. J. Epidemiol. 2016, 31, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.B.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef]
- Michel, D.L. Drug-Induced Liver Injury. Mayo Clin. Proc. 2014, 89, 95–106. [Google Scholar] [CrossRef]
- Yu, Y.C.; Mao, Y.M.; Chen, C.W.; Chen, J.J.; Chen, J.; Cong, W.M.; Ding, Y.; Duan, Z.P.; Fu, Q.C.; Guo, X.Y.; et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol. Int. 2017, 11, 221–241. [Google Scholar] [CrossRef]
- Kaplowitz, N. Drug-induced liver injury. Clin. Infect. Dis. 2004, 38, S44–S48. [Google Scholar] [CrossRef]
- Fritsch, P.O.; Sidoroff, A. Drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Am. J. Clin. Dermatol. 2000, 1, 349–360. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control & Prevention–Vaccine Compensation Program. Available online: http://www.cdc.go.kr/menu.es?mid=a30301000000 (accessed on 10 October 2018).
- Cha, S.H. The history of vaccination and current vaccination policies in Korea. Clin. Exp. Vaccine Res. 2012, 1, 3–8. [Google Scholar] [CrossRef] [Green Version]

| 1. Diagnostic code | |||||||
| ICD-10th code | |||||||
| 2. Brighton Collaboration criteria * | |||||||
| No. | Brighton Collaboration criteria | Y | N | NA/UJ | |||
| 2.1. | Bilateral AND flaccid weakness of the limbs | ||||||
| 2.2 | Decreased or absent deep tendon reflexes in weak limbs | ||||||
| 2.3. | Monophasic illness pattern AND interval between onset and nadir of weakness between 12 h and 28 days AND subsequent clinical plateau | ||||||
| 2.4. | Absence of an identified alternative diagnosis for weakness | ||||||
| 2.5. | Cytoalbuminologic dissociation (i.e., elevation of CSF protein level above normal values AND CSF total white cell count <50 cells/μL) | ||||||
| 2.6. | Electrophysiologic findings consistent with GBS | ||||||
| |||||||
| Brighton Collaboration diagnostic level result | |||||||
| 3. Questions to help diagnosis | |||||||
| No. | Examinations to diagnose Guillain–Barré syndrome | Y | N | NA | Note | ||
| 3.1. | Was a test for nerve conduction performed? Write the date of the test and specifics in the note column. | ||||||
| 3.2. | Was a test for cerebrospinal fluid performed? Write the date of the test and specifics in the note column. | ||||||
| Questions to help diagnose Guillain–Barré syndrome | Y | N | NA | UJ | Note | ||
| 3.3. | Was the paralysis ascending? | ||||||
| 3.4. | Did it take less than 4 weeks for symptoms to stop worsening? | ||||||
| Questions that might indicate diseases other than Guillain–Barré syndrome | |||||||
| 3.5. | Were the symptoms accompanied by cognitive deterioration? | ||||||
| 3.6. | Did the initial symptoms include fever? | ||||||
| 3.7. | Did the initial symptoms include bowel or bladder dysfunction? | ||||||
| 3.8. | Was there any other cause of muscular weakness or paralysis? | ||||||
| 1. Order of the incidence | |||||||
| ⚬ Was the vaccine administered before Guillain–Barré syndrome occurred? | Yes/No | ||||||
| 2. Temporal proximity (time interval) | |||||||
| ⚬Did Guillain–Barré syndrome occur within 6 weeks following vaccination? | Yes/No | ||||||
| ⚬Write the time interval between vaccination and the occurrence of Guillain–Barré syndrome (e.g., 4 weeks following vaccination). | |||||||
| 3. Evidence for other causes | |||||||
| |||||||
| Y | N | UK | NA | Note | |||
| History * | 3.1 Has the patient ever experienced Guillain–Barré syndrome or other neuromuscular disorders regardless of vaccination? Please write the name of the disease in the note column. | ||||||
| 3.2. Has the patient ever been vaccinated with the same vaccine in the past? If so, please write the vaccination date. | |||||||
| 3.3. Has the patient ever experienced Guillain–Barré syndrome or any other neuromuscular disease after any type of vaccination? | |||||||
| Patient’s condition before appearance of GBS * | ⚬ Has the patient ever experienced any items listed below before the manifestation of Guillain–Barré syndrome? If so, please write the date of the infection. | ||||||
| 3.4. Upper respiratory infections | |||||||
| 3.5. GI troubles | |||||||
| 3.6. Any other symptoms (Zika, Dengue, etc.) | |||||||
| 3.7. Malaria | |||||||
| 3.8. Tsutsugamushi | |||||||
| 3.9. Surgery | |||||||
| Patient examination | ⚬ Was a test for any pathogens listed below performed after the manifestation of Guillain–Barré syndrome? If so, write the date of the test, test result, and specific notes. | ||||||
| 3.10. Campylobacter jejuni | |||||||
| 3.11. Cytomegalo virus (CMV) | |||||||
| 3.12. Epstein-Barr virus (EBV) | |||||||
| 3.13. Herpes Simplex virus (HSV) | |||||||
| 3.14. Varicella-zoster virus (VZV) | |||||||
| 3.15. Mycoplasma pneumonia | |||||||
| 3.16. Hemophilus influenza | |||||||
| 3.17. Influenza virus | |||||||
| 3.18. Other pathogens | |||||||
| Immunization anxiety | 3.19. Was the Guillain–Barré syndrome a stress response to vaccination? (e.g., acute stress response, vasovagal syncope, hyperventilation, anxiety, etc.) | ||||||
| Vaccine quality | 3.20. Could the vaccine given to this patient have a quality defect or is substandard or counterfeit? | ||||||
| Immunization errors (please write type of error, if any) | 3.21. Did anything unusual occur during vaccination preparation? (e.g., incorrect mixing, use of expired vaccine, abnormal physical condition, etc.) | ||||||
| 3.22. Did anything unusual occur during the vaccination procedure? (e.g., Inoculation timing/dose/site/route, needle size error, etc.) | |||||||
| |||||||
| 4. Published evidence (literature, WHO GACVS, IOM etc.) regarding a causal association between the vaccine and GBS | |||||||
| Influenza vaccine | |||||||
| Meningococcal vaccine | |||||||
| Hepatitis A vaccine | |||||||
| Hepatitis B vaccine | |||||||
| MMR vaccine | |||||||
| HPV vaccine | |||||||
| DTP vaccine | |||||||
| HiB vaccine | |||||||
| Polio vaccine | |||||||
| Varicella vaccine | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kang, H.-Y.; Cho, S.; Park, S.; Kim, A.-Y.; Jung, S.-Y.; Seong, B.L.; Lee, Y.-M. Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain–Barré Syndrome. Vaccines 2020, 8, 101. https://doi.org/10.3390/vaccines8010101
Lee H, Kang H-Y, Cho S, Park S, Kim A-Y, Jung S-Y, Seong BL, Lee Y-M. Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain–Barré Syndrome. Vaccines. 2020; 8(1):101. https://doi.org/10.3390/vaccines8010101
Chicago/Turabian StyleLee, Hankil, Hye-Young Kang, Sunghwa Cho, Seonyoung Park, Ah-Young Kim, Sun-Young Jung, Baik Lin Seong, and Young-Mock Lee. 2020. "Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain–Barré Syndrome" Vaccines 8, no. 1: 101. https://doi.org/10.3390/vaccines8010101
APA StyleLee, H., Kang, H.-Y., Cho, S., Park, S., Kim, A.-Y., Jung, S.-Y., Seong, B. L., & Lee, Y.-M. (2020). Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain–Barré Syndrome. Vaccines, 8(1), 101. https://doi.org/10.3390/vaccines8010101





