Mapping Host-Related Correlates of Influenza Vaccine-Induced Immune Response: An Umbrella Review of the Available Systematic Reviews and Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective
2.2. General Methodology
2.3. Search Strategy
2.4. Eligibility Criteria and Inclusion Process
2.5. Data Extraction
2.6. Quality Appraisal
2.7. Data Analysis and Synthesis
2.8. Cumulative Evidence Synthesis (CES)
- Convincing (class I): pooled number of cases (N) > 1000, p < 0.000001, I2 < 50%, 95% prediction interval excluded the null, no small-study effect/publication bias;
- Highly suggestive (class II): N > 1000, p < 0.000001, largest study with a statistically significant effect (p < 0.05) and class I criteria not met;
- Suggestive (class III): N > 1000, p < 0.001 and class I–II criteria not met;
- Weak (class IV): p < 0.05 and class I–III criteria not met;
- Non-significant: p for the observed ES > 0.05.
3. Results
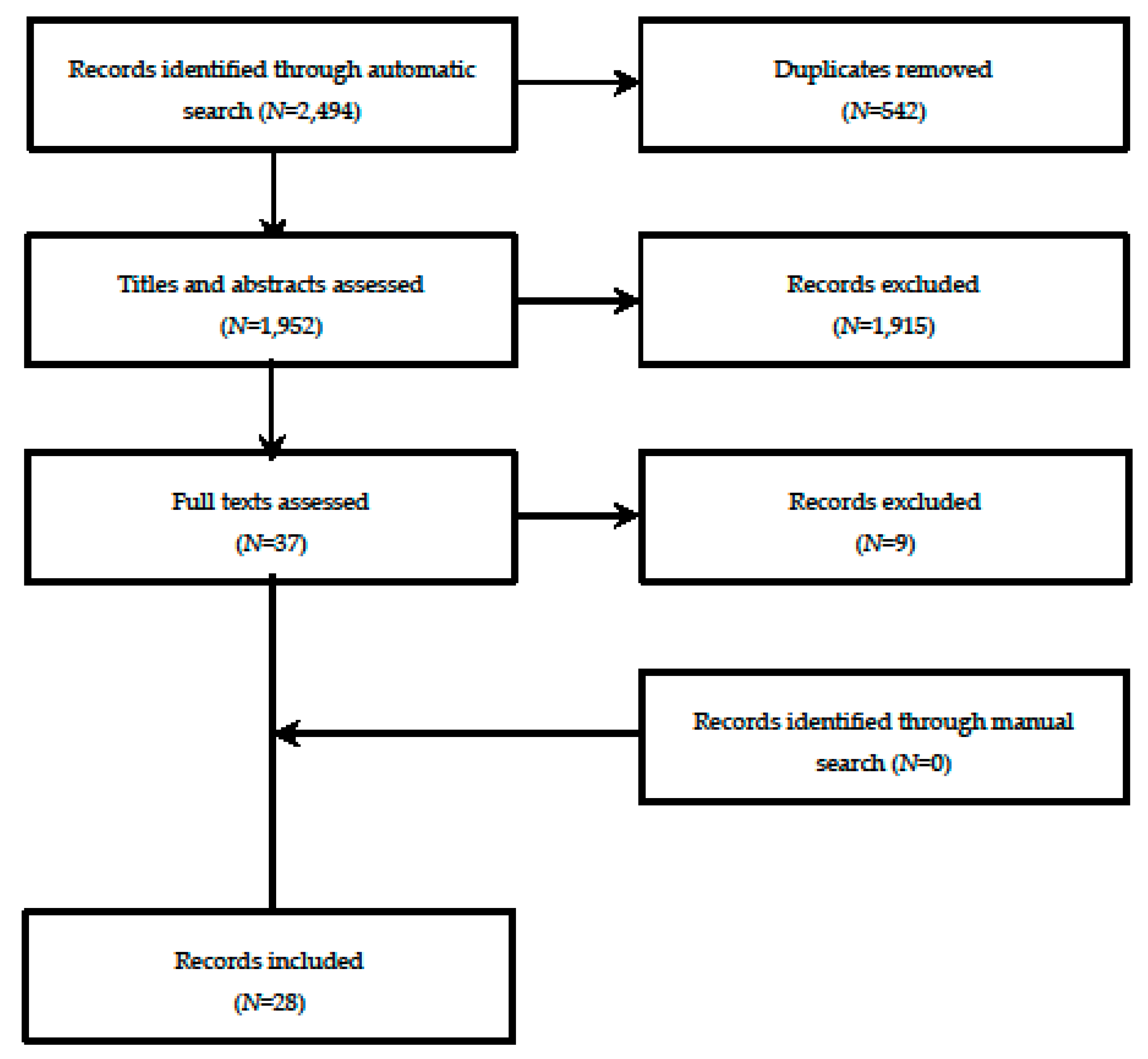
3.1. Selection Process and Main Characteristics of the Systematic Reviews and/or Meta-Analyses Included
3.2. Use of Probiotics, Prebiotics or Symbiotics
3.3. BCG (Bacillus Calmette–Guérin) Vaccination
3.4. Genetic Polymorphisms
3.5. Intravenous Drug Use
3.6. Vitamin D Supplementation/Deficiency
3.7. Immunosuppressive Conditions
3.8. Diabetes Mellitus
3.9. Physical Exercise
3.10. Latent Cytomegalovirus Infection
3.11. Psychological Stress
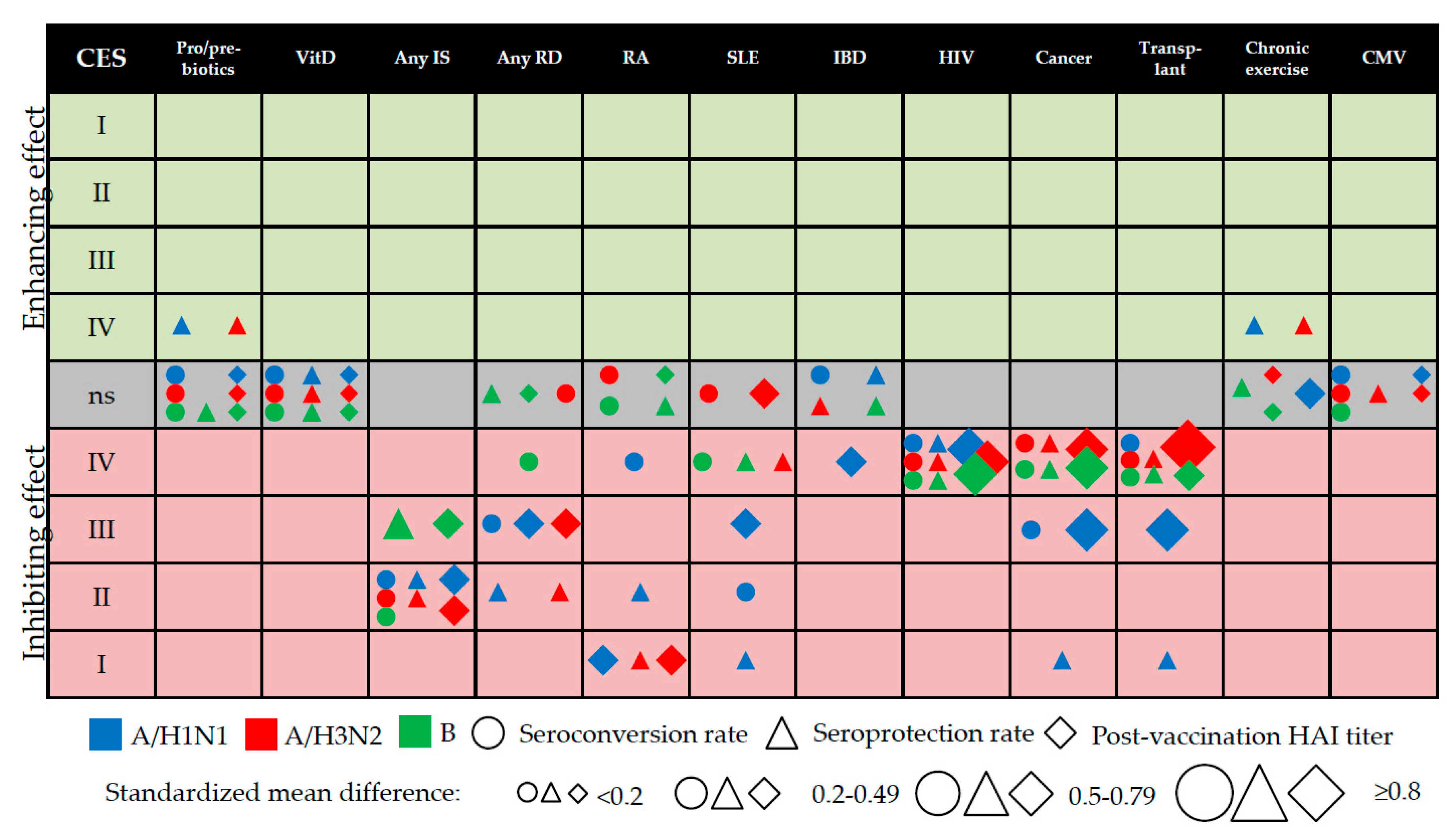
3.12. Evidence Mapping
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Vaccines against influenza WHO position paper – November 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476. [Google Scholar]
- Cassini, A.; Colzani, E.; Pini, A.; Mangen, M.J.; Plass, D.; McDonald, S.A.; Maringhini, G.; van Lier, A.; Haagsma, J.A.; Havelaar, A.H.; et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018, 23, 17-00454. [Google Scholar] [CrossRef] [PubMed]
- de Lusignan, S.; Correa, A.; Ellis, J.; Pebody, R. Influenza vaccination: in the UK and across Europe. Br. J. Gen. Pract. 2016, 66, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Influenza Vaccines — United States, 2019–20 Influenza Season. Available online: https://www.cdc.gov/flu/professionals/vaccines.htm (accessed on 25 October 2019).
- European Centre for Disease Prevention and Control (ECDC). Types of seasonal influenza vaccine. Available online: https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/types-of-seasonal-influenza-vaccine (accessed on 25 October 2019).
- Sambala, E.Z.; Ngcobo, N.; Machingaidze, S.; Wiyeh, A.B.; Mahasha, P.W.; Jaca, A.; Cooper, S.; Wiysonge, C.S. A global review of seasonal influenza vaccine introduction: analysis of the WHO/UNICEF Joint Reporting Form. Expert Rev. Vaccines 2019, 18, 859–865. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Seasonal vaccination policies and coverage in the European Region. Available online: http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/seasonal-vaccination-policies-and-coverage-in-the-european-region (accessed on 25 October 2019).
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2018-19 influenza season. MMWR Recomm. Rep. 2018, 67, 1–20. [Google Scholar] [CrossRef]
- Dewe, W.; Benoit, A.; Legrand, C. Assessing vaccine efficacy in influenza clinical trials: challenges and difficulties. Expert Rev. Clin. Pharmacol. 2013, 6, 403–411. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Correlates of vaccine-induced protection: methods and implications. Available online: https://apps.who.int/iris/bitstream/handle/10665/84288/WHO_IVB_13.01_eng.pdf;jsessionid=2F01853E2174C74A96304EC67F352EA5?sequence=1 (accessed on 25 October 2019).
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Montomoli, E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev. Vaccines. 2016, 15, 967–976. [Google Scholar] [CrossRef]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond). 1972, 70, 767–777. [Google Scholar] [CrossRef]
- de Jong, J.C.; Palache, A.M.; Beyer, W.E.; Rimmelzwaan, G.F.; Boon, A.C.; Osterhaus, A.D. Haemagglutination-inhibiting antibody to influenza virus. Dev. Biol. (Basel) 2003, 115, 63–73. [Google Scholar]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a Bayesian random-effects model. BMC Med. Res. Methodol. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085, Erratum in: Pediatr. Infect. Dis. J. 2012, 31, 537. [Google Scholar] [CrossRef] [PubMed]
- Delem, A.; Jovanovic, D. Correlation between rate of infection and preexisting titer of serum antibody as determined by single radial hemolysis during and epidemic of influenza A/Victoria/3/75. J. Infect. Dis. 1978, 137, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Torelli, A.; Montomoli, E. The use of cell-mediated immunity for the evaluation of influenza vaccines: an upcoming necessity. Hum. Vaccin. Immunother. 2019, 15, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; McLean, H.Q. Influenza vaccine effectiveness: defining the H3N2 problem. Clin. Infect. Dis. 2019, 17, 1817–1823. [Google Scholar] [CrossRef]
- Mugitani, A.; Ito, K.; Irie, S.; Eto, T.; Ishibashi, M.; Ohfuji, S.; Fukushima, W.; Maeda, A.; Hirota, Y.; Fukuoka Pediatricians Group for Vaccine Efficacy. Immunogenicity of the trivalent inactivated influenza vaccine in young children less than 4 years of age, with a focus on age and baseline antibodies. Clin. Vaccine Immunol. 2014, 21, 1253–1260. [Google Scholar] [CrossRef]
- Haq, K.; McElhaney, J.E. Immunosenescence: Influenza vaccination and the elderly. Curr. Opin. Immunol. 2014, 29, 38–42. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Galvani, A.P.; Bush, R.M. Ecological and immunological determinants of influenza evolution. Nature 2003, 422, 428–433. [Google Scholar] [CrossRef]
- Ng, T.W.Y.; Cowling, B.J.; Gao, H.Z.; Thompson, M.G. Comparative immunogenicity of enhanced seasonal influenza vaccines in older adults: A systematic review and meta-analysis. J. Infect. Dis. 2019, 219, 1525–1535. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Castrucci, M.R. Factors affecting immune responses to the influenza vaccine. Hum. Vaccin. Immunother. 2018, 14, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based. Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The role of google scholar in evidence reviews and tts applicability to grey literature searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA); Committee for Medicinal Products for Human Use (CHMP). Guideline on Influenza Vaccines: Non-Clinical and Clinical Module. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-vaccines-non-clinical-clinical-module_en.pdf (accessed on 25 October 2019).
- US Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-data-needed-support-licensure-seasonal-inactivated-influenza-vaccines (accessed on 25 October 2019).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef] [PubMed]
- The United States Advisory Committee on Immunization Practices (ACIP). Handbook for Developing Evidence-Based Recommendations. Version 1.2. Available online: http://www.cdc.gov/vaccines/acip/recs/GRADE/downloads/handbook.pdf (accessed on 25 October 2019).
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available online: https://handbook-5-1.cochrane.org/ (accessed on 25 October 2019).
- Ioannidis, J.P.; Trikalinos, T.A. An exploratory test for an excess of significant findings. Clin. Trials 2007, 4, 245–253. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Available online: http://www.R-project.org/ (accessed on 25 October 2019).
- R package “meta”. Available online: https://cran.r-project.org/web/packages/meta/meta.pdf (accessed on 25 October 2019).
- R Package “metafor”. Available online: https://cran.r-project.org/web/packages/metafor/metafor.pdf (accessed on 25 October 2019).
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid. Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef]
- Wang, D.D.; Shams-White, M.; Bright, O.J.; Parrott, J.S.; Chung, M. Creating a literature database of low-calorie sweeteners and health studies: evidence mapping. BMC Med. Res. Methodol. 2016, 16, 1. [Google Scholar] [CrossRef]
- Baral, S.; Sherman, S.G.; Millson, P.; Beyrer, C. Vaccine immunogenicity in injecting drug users: a systematic review. Lancet Infect. Dis. 2007, 7, 667–674. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Zachariae, R.; Bovbjerg, D.H. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav. Immun. 2009, 23, 427–433. [Google Scholar] [CrossRef]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; Zanuzdana, A.; Agboado, G.; Orton, E.; Béchard-Evans, L.; Morgan, G.; Stevenson, C.; et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis from a public health policy perspective. PLoS One 2011, 6, e29249. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Ollington, K.; Kaneshiro, M.; Frenck, R.; Melmed, G.Y. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine 2012, 30, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group; Nguyen-Van-Tam, J.S. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J. Infect. Dis. 2012, 206, 1250–1259. [Google Scholar] [CrossRef]
- Eckerle, I.; Rosenberger, K.D.; Zwahlen, M.; Junghanss, T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS ONE 2013, 8, e56974. [Google Scholar] [CrossRef] [PubMed]
- Goossen, G.M.; Kremer, L.C.; van de Wetering, M.D. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev. 2013, 8, CD006484. [Google Scholar] [CrossRef]
- Hua, C.; Barnetche, T.; Combe, B.; Morel, J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. (Hoboken) 2014, 66, 1016–1026. [Google Scholar] [CrossRef]
- McMahan, Z.H.; Bingham, C.O., 3rd. Effects of biological and non-biological immunomodulatory therapies on the immunogenicity of vaccines in patients with rheumatic diseases. Arthritis Res. Ther. 2014, 16, 506. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Fiatarone Singh, M.A.; Edwards, K.M. The effects of exercise on vaccination responses: a review of chronic and acute exercise interventions in humans. Brain Behav. Immun. 2014, 39, 33–41. [Google Scholar] [CrossRef]
- Posteraro, B.; Pastorino, R.; Di Giannantonio, P.; Ianuale, C.; Amore, R.; Ricciardi, W.; Boccia, S. The link between genetic variation and variability in vaccine responses: systematic review and meta-analyses. Vaccine 2014, 32, 1661–1669. [Google Scholar] [CrossRef]
- Shehata, M.A.; Karim, N.A. Influenza vaccination in cancer patients undergoing systemic therapy. Clin. Med. Insights Oncol. 2014, 8, 57–64. [Google Scholar] [CrossRef]
- Karbasi-Afshar, R.; Izadi, M.; Fazel, M.; Khedmat, H. Response of transplant recipients to influenza vaccination based on type of immunosuppression: A meta-analysis. Saudi J. Kidney Dis. Transpl. 2015, 26, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.L.; Nguyen, E.T.; Bechtold, M.L. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: A meta-analysis. Dig. Dis. Sci. 2015, 60, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, H.; Wan, L.; Lu, X.; Tam, W.W. Is systemic lupus erythematosus associated with a declined immunogenicity and poor safety of influenza vaccination?: A systematic review and meta-analysis. Medicine (Baltimore) 2016, 95, e3637. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tang, H.; Xu, X.; Liang, Y.; Xiong, Y.; Ni, J. Immunogenicity and safety of influenza vaccination in systemic lupus erythematosus patients compared with healthy controls: A meta-analysis. PLoS ONE 2016, 11, e0147856. [Google Scholar] [CrossRef] [PubMed]
- Pugès, M.; Biscay, P.; Barnetche, T.; Truchetet, M.É.; Richez, C.; Seneschal, J.; Gensous, N.; Lazaro, E.; Duffau, P. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: a systematic literature review and meta-analysis. Rheumatology (Oxford) 2016, 55, 1664–1672. [Google Scholar]
- Huang, Y.; Wang, H.; Tam, W.W.S. Is rheumatoid arthritis associated with reduced immunogenicity of the influenza vaccination? A systematic review and meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1901–1908. [Google Scholar] [CrossRef]
- Lei, W.T.; Shih, P.C.; Liu, S.J.; Lin, C.Y.; Yeh, T.L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1175. [Google Scholar] [CrossRef]
- Sousa, S.; Duarte, A.C.; Cordeiro, I.; Ferreira, J.; Gonçalves, M.J.; Meirinhos, T.; Rocha, T.M.; Romão, V.C.; Santos, M.J. Efficacy and safety of vaccination in pediatric patients with systemic inflammatory rheumatic diseases: A systematic review of the literature. Acta Reumatol. Port. 2017, 42, 8–16. [Google Scholar]
- Vollaard, A.; Schreuder, I.; Slok-Raijmakers, L.; Opstelten, W.; Rimmelzwaan, G.; Gelderblom, H. Influenza vaccination in adult patients with solid tumours treated with chemotherapy. Eur. J. Cancer 2017, 76, 134–143. [Google Scholar] [CrossRef]
- Dos Santos, G.; Tahrat, H.; Bekkat-Berkani, R. Immunogenicity, safety, and effectiveness of seasonal influenza vaccination in patients with diabetes mellitus: A systematic review. Hum. Vaccin. Immunother. 2018, 14, 1853–1866. [Google Scholar] [CrossRef]
- Lee, M.D.; Lin, C.H.; Lei, W.T.; Chang, H.Y.; Lee, H.C.; Yeung, C.Y.; Chiu, N.C.; Chi, H.; Liu, J.M.; Hsu, R.J.; et al. Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients 2018, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Subesinghe, S.; Bechman, K.; Rutherford, A.I.; Goldblatt, D.; Galloway, J.B. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J. Rheumatol. 2018, 45, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.L.; Shih, P.C.; Liu, S.J.; Lin, C.H.; Liu, J.M.; Lei, W.T.; Lin, C.Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: a systematic review and meta-analysis of randomized controlled trials. Drug Des. Devel. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The influence of probiotics on vaccine responses – A systematic review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The influence of BCG on vaccine responses – a systematic review. Expert Rev. Vaccines 2018, 17, 547–554. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, S.P.H.; Warmink, K.; Borghans, J.A.M.; Knol, M.J.; van Baarle, D. Effect of latent cytomegalovirus infection on the antibody response to influenza vaccination: a systematic review and meta-analysis. Med. Microbiol. Immunol. 2019, 208, 305–321. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons Ltd.: Chichester, West Sussex, UK, 2009; pp. 45–50. [Google Scholar]
- Selmi, C.; Generali, E.; Massarotti, M.; Bianchi, G.; Sciré, C.A. New treatments for inflammatory rheumatic disease. Immunol. Res. 2014, 60, 277–288. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Seasonal Influenza Vaccination in Europe. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/influenza-vaccination-2007%E2%80%932008-to-2014%E2%80%932015.pdf (accessed on 25 October 2019).
- Iorio, A.M.; Francisci, D.; Camilloni, B.; Stagni, G.; De Martino, M.; Toneatto, D.; Bugarini, R.; Neri, M.; Podda, A. Antibody responses and HIV-1 viral load in HIV-1-seropositive subjects immunised with either the MF59-adjuvanted influenza vaccine or a conventional non-adjuvanted subunit vaccine during highly active antiretroviral therapy. Vaccine 2003, 21, 3629–3637. [Google Scholar] [CrossRef]
- Baldo, V.; Baldovin, T.; Floreani, A.; Carraro, A.M.; Trivello, R.; Family Medicine Group of Pianiga. MF59-adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18-60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine 2007, 25, 3955–3961. [Google Scholar] [CrossRef]
- McKittrick, N.; Frank, I.; Jacobson, J.M.; White, C.J.; Kim, D.; Kappes, R.; DiGiorgio, C.; Kenney, T.; Boyer, J.; Tebas, P. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann. Intern. Med. 2013, 158, 19–26. [Google Scholar] [CrossRef] [PubMed]
- GiaQuinta, S.; Michaels, M.G.; McCullers, J.A.; Wang, L.; Fonnesbeck, C.; O’Shea, A.; Green, M.; Halasa, N.B. Randomized, double-blind comparison of standard-dose vs. high-dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatr. Transplant. 2015, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Xu, X.; Liang, Y.; Xiong, Y.; Chen, R.; Ni, J. Effect of a booster dose of influenza vaccine in patients with hemodialysis, peritoneal dialysis and renal transplant recipients: A systematic literature review and meta-analysis. Hum. Vaccin. Immunother. 2016, 12, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Manini, I.; Trombetta, C.M.; Lazzeri, G.; Pozzi, T.; Rossi, S.; Montomoli, E. Egg-independent influenza vaccines and vaccine candidates. Vaccines (Basel) 2017, 5, 18. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Marchi, S.; Manini, I.; Lazzeri, G.; Montomoli, E. Challenges in the development of egg-independent vaccines for influenza. Expert Rev. Vaccines 2019, 18, 737–750. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Seasonal Influenza Vaccination Strategies. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/vaccination-strategies (accessed on 25 October 2019).
- Centers for Disease Control and Prevention (CDC). 2019-20 Summary of Recommendations. Available online: https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm (accessed on 25 October 2019).
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Centers of Disease Control and Prevention (CDC). Immunogenicity, Efficacy, and Effectiveness of Influenza Vaccines. Available online: https://www.cdc.gov/flu/professionals/acip/immunogenicity.htm (accessed on 25 October 2019).
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized vaccinology: A review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Poland, G.A.; Kennedy, R.B.; Ovsyannikova, I.G. Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery? PLoS Pathog. 2011, 7, e1002344. [Google Scholar] [CrossRef]
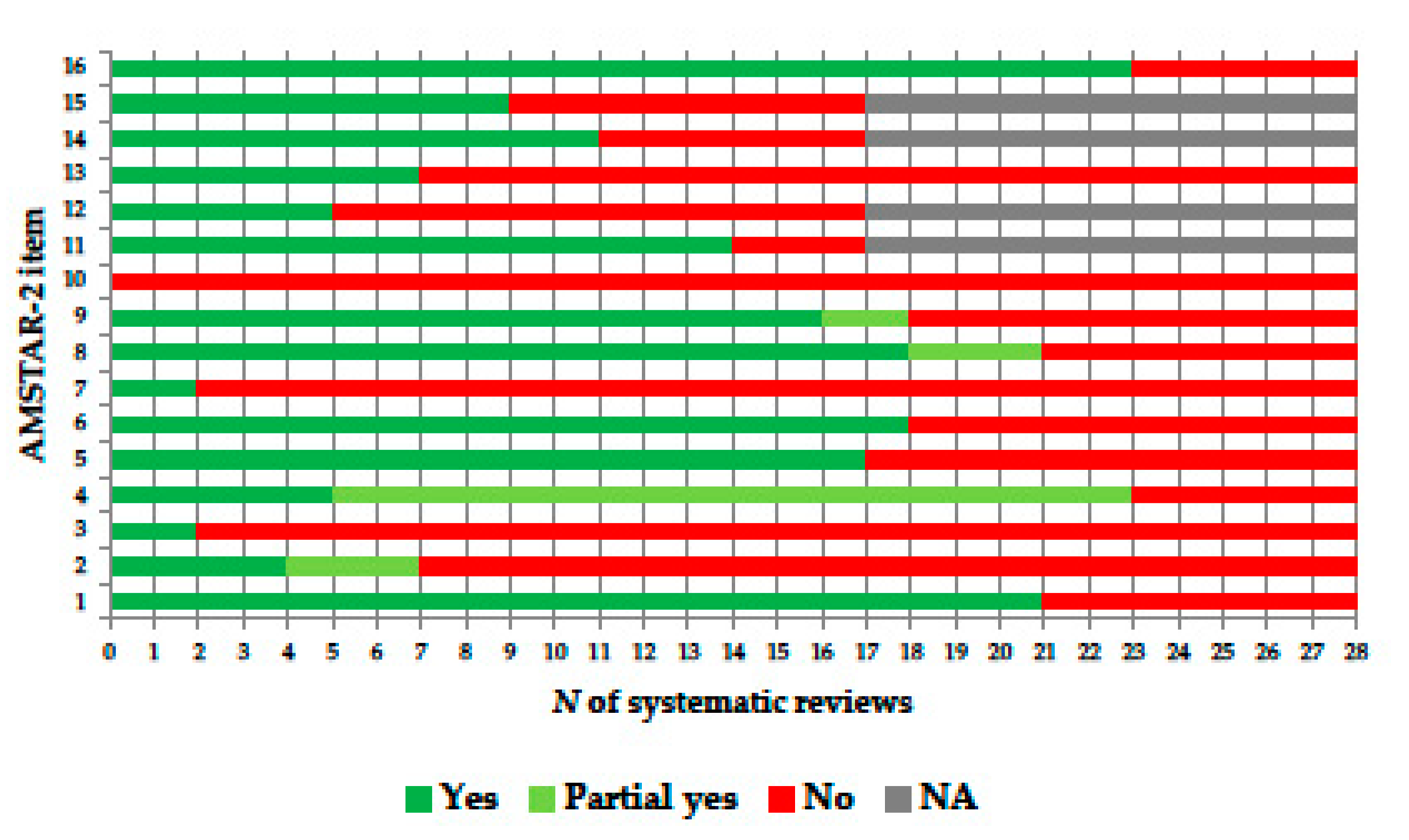
| Study [Ref] | Year | Factor(s) Assessed | Type of Study | Population † | k† | Meta-Analysis Performed | AMSTAR-2 ‡ |
|---|---|---|---|---|---|---|---|
| Baral [40] | 2007 | Intravenous drug use | Obs | Adults | 2 | No | 2/2/8/4 |
| Pedersen [41] | 2009 | Psychological stress | Obs | All | 13 | Yes | 7/2/7/0 |
| Beck [42] | 2011 | Immunosuppression of any etiology | RCT, obs | All | 209 | Yes | 12/0/4/0 |
| Agarwal [43] | 2012 | Immunosuppressive drugs | RCT, obs | All | 11 | No | 3/1/8/4 |
| Beck [44] | 2012 | Immunosuppression by etiology | RCT, obs | All | 209 | Yes | 12/0/4/0 |
| Eckerle [45] | 2013 | Solid organ transplants | RCT, obs | All | 36 | Yes | 6/1/9/0 |
| Goossen [46] | 2013 | Chemotherapy in cancer patients | RCT, CCT | Children | 9 | No | 10/0/2/4 |
| Hua [47] | 2014 | Antirheumatic drugs in rheumatoid arthritis patients | Obs | Adults | 7 | Yes | 5/2/9/0 |
| McMahan [48] | 2014 | Biological and non-biological drugs in rheumatic disease patients | Unclear | All | 18 | No | 1/0/11/4 |
| Pascoe [49] | 2014 | Acute and chronic physical exercise | RCT, obs | All | 15 | No | 5/1/6/4 |
| Posteraro [50] | 2014 | Genetic variations | Obs | Adults | 1 | Yes | 7/1/8/0 |
| Shehata [51] | 2014 | Cancer patients on systemic treatment | Unclear | All | 16 | No | 2/0/10/4 |
| Karbasi-Afshar [52] | 2015 | Transplant recipients | Obs | Unclear | 15 | Yes | 1/0/15/0 |
| Nguyen [53] | 2015 | Immunosuppressive drugs in inflammatory bowel disease patients | Obs | All | 2 | Yes | 2/3/11/0 |
| Huang [54] | 2016 | Systemic lupus erythematosus | Obs | All | 15 | Yes | 11/1/4/0 |
| Liao [55] | 2016 | Systemic lupus erythematosus | Obs | All | 18 | Yes | 8/2/6/0 |
| Pugès [56] | 2016 | Systemic lupus erythematosus | Obs | Adults | 17 | Yes | 10/1/5/0 |
| Huang [57] | 2017 | Rheumatoid arthritis | Obs | Adults | 13 | Yes | 8/1/7/0 |
| Lei [58] | 2017 | Probiotics, prebiotics and symbiotics | RCT | Adults | 20 | Yes | 9/1/6/0 |
| Sousa [59] | 2017 | Rheumatic diseases | RCT, obs | Children | 11 | No | 3/0/9/4 |
| Vollaard [60] | 2017 | Solid tumor patients on chemotherapy | Obs | Adults | 20 | No | 1/0/15/0 |
| Dos Santos [61] | 2018 | Diabetes mellitus | RCT, obs | All | 15 | No | 6/0/6/4 |
| Lee [62] | 2018 | Vitamin D deficiency/supplementation | RCT | All | 9 | Yes | 6/2/8/0 |
| Subesinghe [63] | 2018 | Antirheumatic drugs in rheumatoid arthritis patients | Obs | Adults | 7 | Yes | 9/1/6/0 |
| Yeh [64] | 2018 | Probiotics, prebiotics and symbiotics | RCT | Adults | 20 | Yes | 8/2/6/0 |
| Zimmermann [65] | 2018 | Probiotics | RCT | Adults | 12 | No | 3/1/8/4 |
| Zimmermann [66] | 2018 | BCG vaccination | RCT, CCT | Adults | 3 | No | 3/1/8/4 |
| van den Berg [67] | 2019 | Latent CMV infection | RCT, obs | All | 15 | Yes | 12/0/4/0 |
| Parameter | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Seroconversion rate | |||
| k | 10 | 8 | 9 |
| N | 277/274 | 235/229 | 266/250 |
| OR RE (95% CI) | 1.55 (0.86, 2.90) | 1.34 (0.72, 2.50) | 1.14 (0.75, 1.74) |
| p RE | 0.14 | 0.35 | 0.54 |
| 95% PI | 0.38, 6.50 | 0.36, 5.00 | 0.75, 1.74 |
| OR FE (95% CI) | 1.42 (0.96, 2.09) | 1.12 (0.75, 1.66) | 1.14 (0.75, 1.74) |
| p FE | 0.075 | 0.58 | 0.54 |
| I2, % | 49.6 | 49.0 | 0 |
| τ2 | 0.42 | 0.35 | 0 |
| SSE, p | 0.49 | NA | NA |
| LS | No | No | No |
| CES | ns | ns | ns |
| Seroprotection rate | |||
| k | 13 | 11 | 11 |
| N | 845/855 | 805/812 | 800/810 |
| OR RE (95% CI) | 1.68 (1.02, 2.75) | 1.93 (1.08, 3.44) | 0.94 (0.73, 1.23) |
| p RE | 0.040 | 0.026 | 0.66 |
| 95% PI | 0.47, 5.98 | 0.64, 3.13 | 0.73, 1.23 |
| OR FE (95% CI) | 1.25 (0.98, 1.59) | 1.94 (1.20, 3.13) | 0.94 (0.73, 1.23) |
| p FE | 0.067 | 0.006 | 0.66 |
| I2, % | 56.2 | 24.9 | 0 |
| τ2 | 0.36 | 0.23 | 0 |
| SSE, p | 0.18 | 0.58 | 0.38 |
| LS | No | No | No |
| CES | IV | IV | ns |
| Post-vaccination HAI titer | |||
| k | 11 | 10 | 10 |
| N | 399/398 | 380/374 | 380/374 |
| g RE (95% CI) | 0.05 (−0.09, 0.19) | 0.05 (−0.10, 0.19) | 0.00 (−0.15, 0.14) |
| p RE | 0.49 | 0.53 | 0.96 |
| 95% PI | −0.09, 0.19 | −0.10, 0.19 | −0.15, 0.14 |
| g FE (95% CI) | 0.05 (−0.09, 0.19) | 0.05 (−0.10, 0.19) | 0.00 (−0.15, 0.14) |
| p FE | 0.49 | 0.53 | 0.96 |
| I2, % | 0 | 0 | 0 |
| τ2 | 0 | 0 | 0 |
| SSE, p | 0.84 | 0.78 | 0.009 |
| LS | No | No | No |
| CES | ns | ns | ns |
| Parameter | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Seroconversion rate | |||
| k | 3 | 3 | 3 |
| N | 176/276 | 176/276 | 176/276 |
| OR RE (95% CI) | 0.79 (0.52, 1.20) | 1.02 (0.69, 1.51) | 0.93 (0.57, 1.52) |
| p RE | 0.27 | 0.94 | 0.77 |
| 95% PI | 0.52, 1.20 | 0.69, 1.51 | 0.57, 1.52 |
| OR FE (95% CI) | 0.79 (0.52, 1.20) | 1.02 (0.69, 1.51) | 0.93 (0.57, 1.52) |
| p FE | 0.27 | 0.94 | 0.77 |
| I2, % | 0 | 0 | 0 |
| τ2 | 0 | 0 | 0 |
| SSE, p | NA | NA | NA |
| LS | No | No | No |
| CES | ns | ns | ns |
| Seroprotection rate | |||
| k | 3 | 3 | 3 |
| N | 176/276 | 176/276 | 176/276 |
| OR RE (95% CI) | 0.85 (0.51, 1.41) | 0.98 (0.60, 1.58) | 0.75 (0.44, 1.28) |
| p RE | 0.53 | 0.92 | 0.29 |
| 95% PI | 0.51, 1.41 | 0.60, 1.58 | 0.44, 1.28 |
| OR FE (95% CI) | 0.85 (0.51, 1.41) | 0.98 (0.60, 1.58) | 0.75 (0.44, 1.28) |
| p FE | 0.53 | 0.92 | 0.29 |
| I2, % | 0 | 0 | 0 |
| τ2 | 0 | 0 | 0 |
| SSE, p | NA | NA | NA |
| LS | No | No | No |
| CES | ns | ns | ns |
| Post-vaccination HAI titer | |||
| k | 3 | 3 | 3 |
| N | 154/153 | 154/153 | 154/153 |
| g RE (95% CI) | 0.07 (−0.17, 0.30) | −0.05 (−0.27, 0.17) | 0.02 (−0.21, 0.24) |
| p RE | 0.58 | 0.66 | 0.89 |
| 95% PI | −0.18, 0.31 | −0.27, 0.17 | −0.21, 0.24 |
| g FE (95% CI) | 0.06 (−0.16, 0.29) | −0.05 (−0.27, 0.17) | 0.02 (−0.21, 0.24) |
| p FE | 0.57 | 0.66 | 0.89 |
| I2, % | 4.2 | 0 | 0 |
| τ2 | 0 | 0 | 0 |
| SSE, p | NA | NA | NA |
| LS | No | No | No |
| CES | ns | ns | ns |
| Parameter | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Seroconversion rate | |||
| k | 116 | 94 | 85 |
| N | 8673/4638 | 4193/3023 | 3888/2944 |
| OR RE (95% CI) | 0.50 (0.42, 0.59) | 0.51 (0.41, 0.63) | 0.53 (0.44, 0.64) |
| p RE | 2∙10−15 | 2∙10−10 | 4∙10−11 |
| 95% PI | 0.13, 1.99 | 0.11, 2.37 | 0.16, 1.75 |
| OR FE (95% CI) | 0.53 (0.49, 0.59) | 0.56 (0.50, 0.62) | 0.54 (0.48, 0.61) |
| p FE | <1∙10−14 | <1∙10−14 | <1∙10−14 |
| I2, % | 65.7 | 65.8 | 53.9 |
| τ2 | 0.49 | 0.60 | 0.36 |
| SSE, p | 0.30 | 0.30 | 0.75 |
| LS | Yes | Yes | Yes |
| CES | II | II | II |
| Seroprotection rate | |||
| k | 102 | 76 | 75 |
| N | 8452/4272 | 3780/2605 | 3759/2505 |
| OR RE (95% CI) | 0.42 (0.35, 0.51) | 0.35 (0.27, 0.45) | 0.53 (0.41, 0.69) |
| p RE | <1∙10−15 | 1∙10−15 | 3∙10−6 |
| 95% PI | 0.12, 1.54 | 0.08, 1.46 | 0.10, 2.76 |
| OR FE (95% CI) | 0.44 (0.39, 0.49) | 0.38 (0.32, 0.45) | 0.51 (0.44, 0.60) |
| p FE | <1∙10−15 | <1∙10−15 | <1∙10−15 |
| I2, % | 54.5 | 45.7 | 59.9 |
| τ2 | 0.43 | 0.52 | 0.69 |
| SSE, p | >0.99 | 0.78 | 0.12 |
| LS | Yes | Yes | Yes |
| CES | II | II | III |
| Post-vaccination HAI titer | |||
| k | 99 | 77 | 69 |
| N | 7909/4438 | 3889/2922 | 3720/2751 |
| g RE (95% CI) | −0.36 (−0.45, −0.28) | −0.44 (−0.55, −0.34) | −0.34 (−0.43, −0.24) |
| p RE | <1∙10−15 | 2∙10−15 | 2∙10−12 |
| 95% PI | −1.05, 0.32 | −1.25, 0.36 | −0.94, 0.26 |
| g FE (95% CI) | −0.33 (−0.37, −0.29) | −0.43 (−0.49, −0.38) | −0.32 (−0.38, −0.27) |
| p FE | <1∙10−15 | <1∙10−15 | <1∙10−15 |
| I2, % | 73.8 | 75.0 | 63.6 |
| τ2 | 0.12 | 0.17 | 0.09 |
| SSE, p | 0.17 | 0.64 | 0.42 |
| LS | Yes | Yes | No |
| CES | II | II | III |
| Virus | Parameter | Any RD | RA | SLE | IBD | HIV | Cancer | Transplantation | |
|---|---|---|---|---|---|---|---|---|---|
| A/H1N1 | SC | OR | 0.57 | 0.64 | 0.35 | 0.54 | 0.51 | 0.49 | 0.49 |
| CES | III | IV | II | ns | IV | III | IV | ||
| SP | OR | 0.47 | 0.39 | 0.38 | 0.80 | 0.35 | 0.28 | 0.28 | |
| CES | II | II | I | ns | IV | I | I | ||
| HAI titer | g | −0.21 | −0.37 | −0.42 | −0.30 | −0.51 | −0.61 | −0.61 | |
| CES | III | I | III | IV | IV | III | III | ||
| A/H3N2 | SC | OR | 0.78 | 0.97 | 0.55 | NA | 0.46 | 0.44 | 0.35 |
| CES | ns | ns | ns | NA | IV | IV | IV | ||
| SP | OR | 0.40 | 0.37 | 0.39 | 0.74 | 0.21 | 0.37 | 0.26 | |
| CES | II | IV | IV | ns | IV | IV | IV | ||
| HAI titer | g | −0.26 | −0.26 | −0.23 | NA | −0.77 | −0.54 | −0.89 | |
| CES | III | IV | ns | NA | IV | IV | IV | ||
| B | SC | OR | 0.75 | 0.72 | 0.57 | NA | 0.37 | 0.41 | 0.54 |
| CES | IV | ns | IV | NA | IV | IV | IV | ||
| SP | OR | 0.76 | 0.84 | 0.60 | 1.12 | 0.33 | 0.46 | 0.40 | |
| CES | ns | ns | IV | ns | IV | IV | IV | ||
| HAI titer | g | −0.05 | −0.11 | NA | NA | −0.54 | −0.54 | −0.49 | |
| CES | ns | ns | NA | NA | IV | IV | IV | ||
| Parameter | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Seroprotection rate | |||
| k | 3 | 3 | 3 |
| N | 115/106 | 115/106 | 115/106 |
| OR RE (95% CI) | 2.70 (1.45, 5.02) | 1.95 (1.11, 3.43) | 1.31 (0.74, 2.30) |
| p RE | 0.0017 | 0.020 | 0.36 |
| 95% PI | 1.45, 5.02 | 1.11, 3.43 | 0.74, 2.30 |
| OR FE (95% CI) | 2.70 (1.45, 5.02) | 1.95 (1.11, 3.43) | 1.31 (0.74, 2.30) |
| p FE | 0.0017 | 0.020 | 0.36 |
| I2, % | 0 | 0 | 0 |
| τ2 | 0 | 0 | 0 |
| SSE, p | NA | NA | NA |
| LS | Yes | Yes | No |
| CES | IV | IV | ns |
| Post-vaccination HAI titer | |||
| k | 2 | 2 | 2 |
| N | 88/83 | 88/83 | 88/83 |
| g RE (95% CI) | 0.28 (−0.39, 0.96) | 0.19 (−0.12, 0.49) | −0.07 (−0.37, 0.23) |
| p RE | 0.41 | 0.23 | 0.65 |
| 95% PI | −0.75, 1.32 | −0.12, 0.49 | −0.37, 0.23 |
| g FE (95% CI) | 0.13 (−0.18, 0.43) | 0.19 (−0.12, 0.49) | −0.07 (−0.37, 0.23) |
| p FE | 0.42 | 0.23 | 0.65 |
| I2, % | 63.4 | 0 | 0 |
| τ2 | 0.16 | 0 | 0 |
| SSE, p | NA | NA | NA |
| LS | No | No | No |
| CES | ns | ns | ns |
| Parameter | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Seroconversion rate | |||
| k | 6 | 7 | 9 |
| N | 192/83 | 670/203 | 99/43 |
| OR RE (95% CI) | 0.46 (0.14, 1.56) | 1.05 (0.74, 1.49) | 0.64 (0.27, 1.56) |
| p RE | 0.21 | 0.79 | 0.33 |
| 95% PI | 0.03, 6.65 | 0.74, 1.49 | 0.27, 1.56 |
| OR FE (95% CI) | 0.58 (0.29, 1.16) | 1.05 (0.74, 1.49) | 0.64 (0.27, 1.56) |
| p FE | 0.12 | 0.79 | 0.33 |
| I2, % | 65.7 | 0 | 0 |
| τ2 | 1.47 | 0 | 0 |
| SSE, p | NA | NA | NA |
| LS | Yes | No | No |
| CES | ns | ns | ns |
| Seroprotection rate | |||
| k | NA | 5 | NA |
| N | NA | 616/191 | NA |
| OR RE (95% CI) | NA | 1.08 (0.76, 1.54) | NA |
| p RE | NA | 0.42 | NA |
| 95% PI | NA | 0.76, 1.54 | NA |
| OR FE (95% CI) | NA | 1.08 (0.76, 1.54) | NA |
| p FE | NA | 0.42 | NA |
| I2, % | NA | 0 | NA |
| τ2 | NA | 0 | NA |
| SSE, p | NA | NA | NA |
| LS | NA | No | NA |
| CES | NA | ns | NA |
| Post-vaccination HAI titer | |||
| k | 7 | 7 | NA |
| N | 371/221 | 716/260 | NA |
| g RE (95% CI) | −0.25 (−0.58, 0.08) | −0.06 (−0.22, 0.11) | NA |
| p RE | 0.14 | 0.50 | NA |
| 95% PI | −0.99, 0.50 | −0.31, 0.20 | NA |
| g FE (95% CI) | −0.13 (−0.31, 0.04) | −0.06 (−0.20, 0.09) | NA |
| p FE | 0.13 | 0.45 | NA |
| I2, % | 65.0 | 20.3 | NA |
| τ2 | 0.12 | 0.01 | NA |
| SSE, p | NA | NA | NA |
| LS | No | No | NA |
| CES | ns | ns | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domnich, A.; Manini, I.; Calabrò, G.E.; de Waure, C.; Montomoli, E. Mapping Host-Related Correlates of Influenza Vaccine-Induced Immune Response: An Umbrella Review of the Available Systematic Reviews and Meta-Analyses. Vaccines 2019, 7, 215. https://doi.org/10.3390/vaccines7040215
Domnich A, Manini I, Calabrò GE, de Waure C, Montomoli E. Mapping Host-Related Correlates of Influenza Vaccine-Induced Immune Response: An Umbrella Review of the Available Systematic Reviews and Meta-Analyses. Vaccines. 2019; 7(4):215. https://doi.org/10.3390/vaccines7040215
Chicago/Turabian StyleDomnich, Alexander, Ilaria Manini, Giovanna Elisa Calabrò, Chiara de Waure, and Emanuele Montomoli. 2019. "Mapping Host-Related Correlates of Influenza Vaccine-Induced Immune Response: An Umbrella Review of the Available Systematic Reviews and Meta-Analyses" Vaccines 7, no. 4: 215. https://doi.org/10.3390/vaccines7040215
APA StyleDomnich, A., Manini, I., Calabrò, G. E., de Waure, C., & Montomoli, E. (2019). Mapping Host-Related Correlates of Influenza Vaccine-Induced Immune Response: An Umbrella Review of the Available Systematic Reviews and Meta-Analyses. Vaccines, 7(4), 215. https://doi.org/10.3390/vaccines7040215







