The Potential of Influenza HA-Specific Immunity in Mitigating Lethality of Postinfluenza Pneumococcal Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Cells
2.3. Infectious Agents
2.4. Production and Purification of Influenza HA-Gag VLPs
2.5. Characterization of HA-Gag VLPs
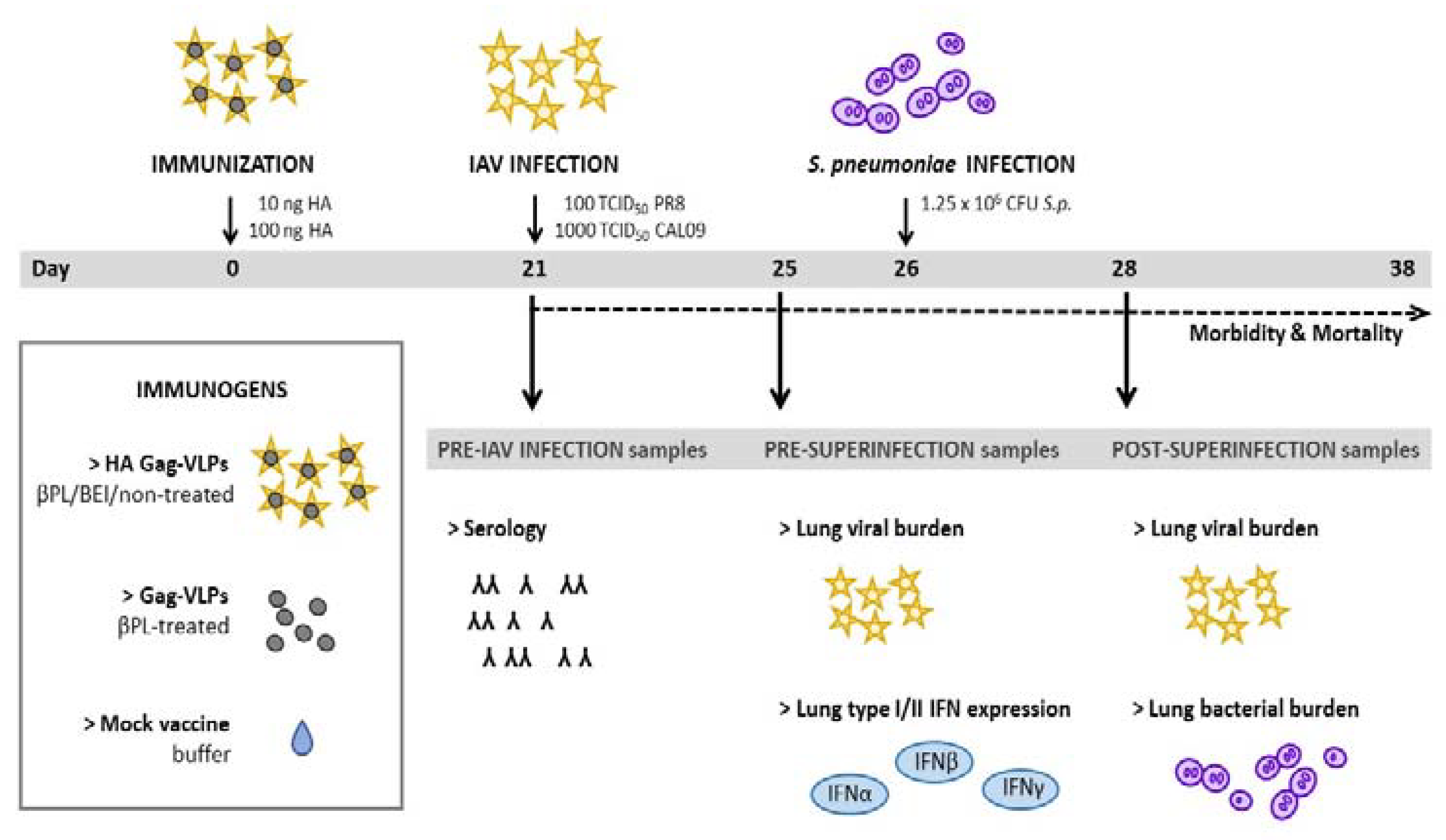
2.6. Immunization and Infection Protocol
2.7. Serology
2.8. Analysis of Lung Pathogen Burden
2.9. Analysis of type I/II Interferon Expression
2.10. Statistical Analyses
3. Results
3.1. Study Design and Murine Superinfection Model
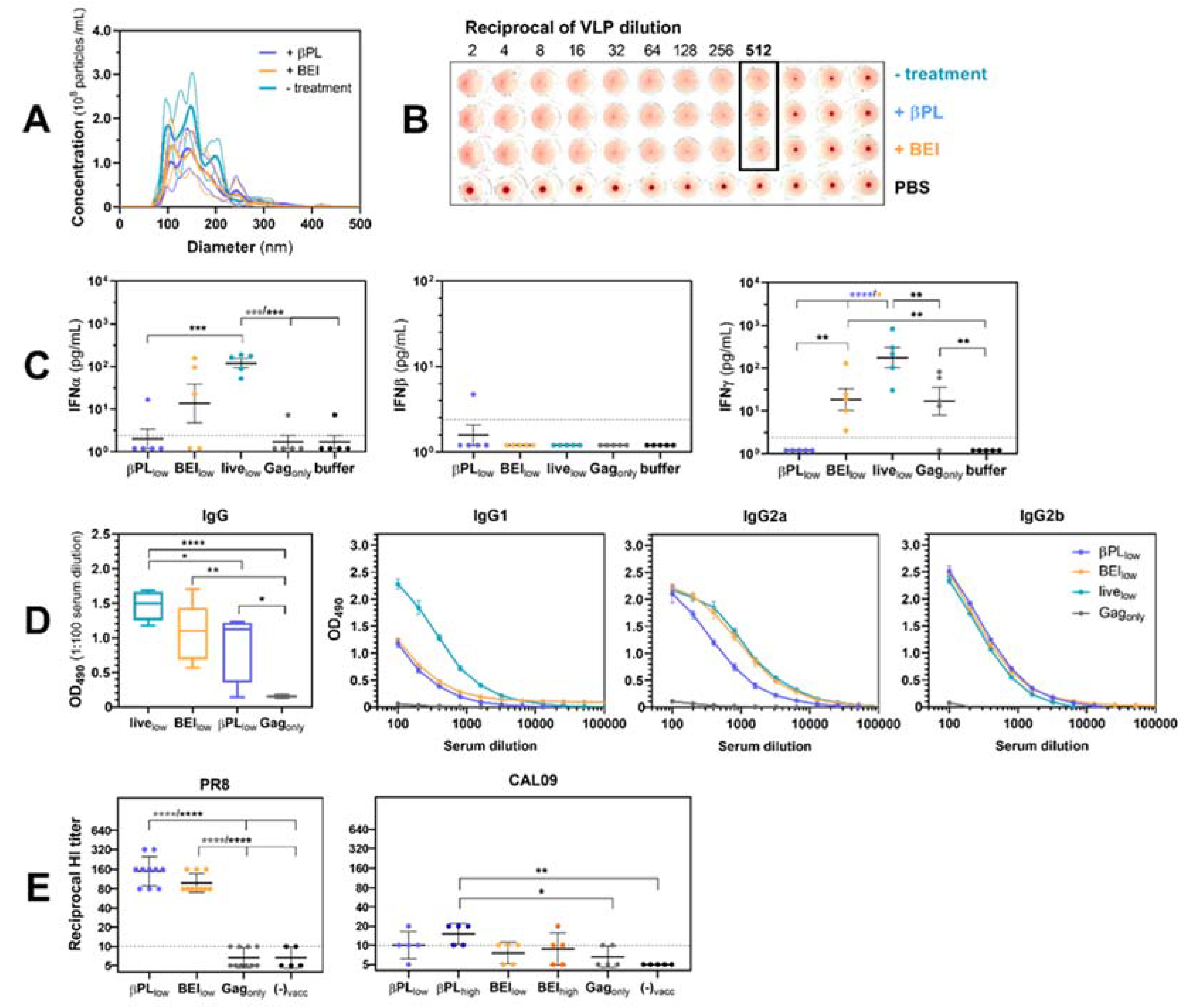
3.2. Effect of Baculovirus Inactivation on Influenza VLP Immunogenicity
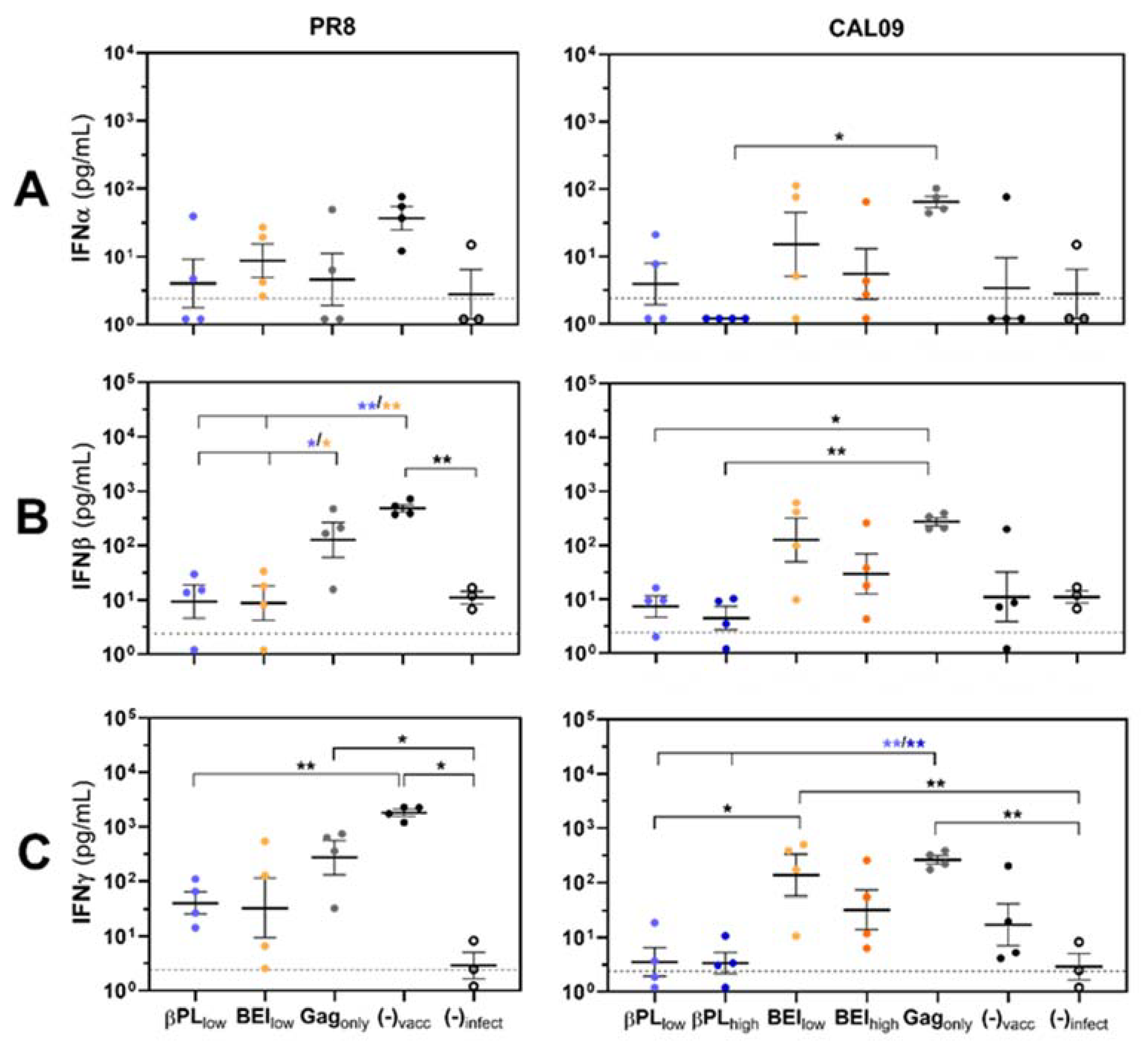
3.3. Effective Anti-HA Immunity Suppresses Influenza-Mediated IFN Type I/II Responses in the Lungs of Infected Mice
3.4. Neutralizing and Non-Neutralizing Anti-HA Immunity Restrain Viral Replication and Viral Rebound After Secondary Pneumococcus Infection
3.5. Neutralizing Anti-Influenza HA Immunity Renders Mice Capable of Controlling a Secondary Pneumococcal Infection
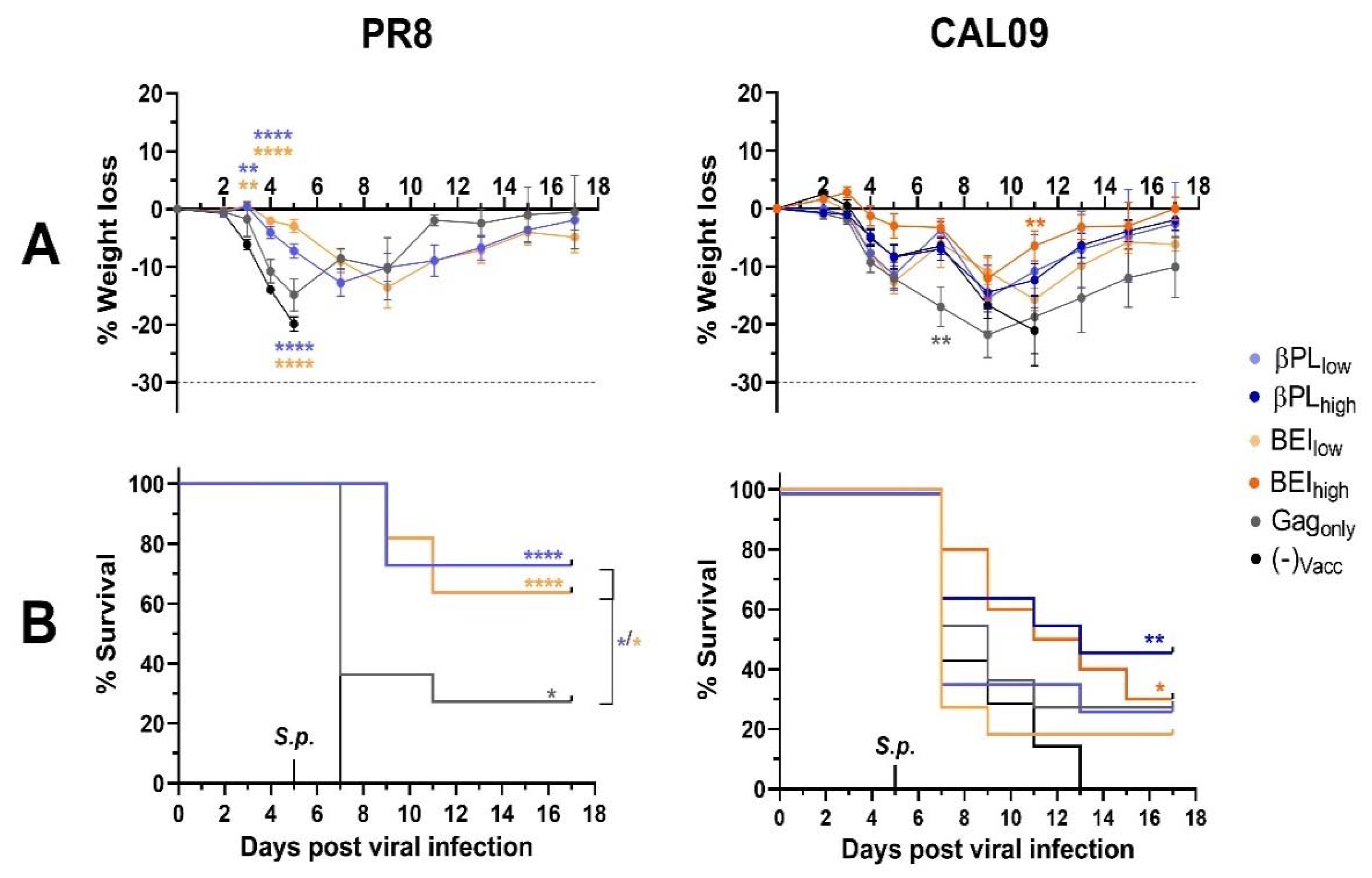
3.6. Influenza-Specific and Non-Specific Immunity Provide a Survival Advantage After Secondary Pneumococcal Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Donkor, E.S. Understanding the pneumococcus: transmission and evolution. Front. Cell. Infect. Microbiol. 2013, 3, 7. [Google Scholar] [CrossRef]
- Domenech de Cellès, M.; Arduin, H.; Lévy-Bruhl, D.; Georges, S.; Souty, C.; Guillemot, D.; Watier, L.; Opatowski, L. Unraveling the seasonal epidemiology of pneumococcus. Proc. Natl. Acad. Sci. USA 2019, 116, 1802–1807. [Google Scholar] [CrossRef]
- Sharma-Chawla, N.; Sender, V.; Kershaw, O.; Gruber, A.D.; Volckmar, J.; Henriques-Normark, B.; Stegemann-Koniszewski, S.; Bruder, D. Influenza A Virus Infection Predisposes Hosts to Secondary Infection with Different Streptococcus pneumoniae Serotypes with Similar Outcome but Serotype-Specific Manifestation. Infect. Immun. 2016, 84, 3445–3457. [Google Scholar] [CrossRef]
- Li, W.; Moltedo, B.; Moran, T.M. Type I Interferon Induction during Influenza Virus Infection Increases Susceptibility to Secondary Streptococcus pneumoniae Infection by Negative Regulation of T Cells. J. Virol. 2012, 86, 12304–12312. [Google Scholar] [CrossRef]
- Navarini, A.A.; Recher, M.; Lang, K.S.; Georgiev, P.; Meury, S.; Bergthaler, A.; Flatz, L.; Bille, J.; Landmann, R.; Odermatt, B.; et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc. Natl. Acad. Sci. USA 2006, 103, 15535–15539. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D.W. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Shepardson, K.M.; Larson, K.; Morton, R.V.; Prigge, J.R.; Schmidt, E.E.; Huber, V.C.; Rynda-Apple, A. Differential Type I Interferon Signaling Is a Master Regulator of Susceptibility to Postinfluenza Bacterial Superinfection. MBio 2016. [Google Scholar] [CrossRef]
- Kudva, A.; Scheller, E.V.; Robinson, K.M.; Crowe, C.R.; Choi, S.M.; Slight, S.R.; Khader, S.A.; Dubin, P.J.; Enelow, R.I.; Kolls, J.K.; et al. Influenza A Inhibits Th17-Mediated Host Defense against Bacterial Pneumonia in Mice. J. Immunol. 2011, 186, 1666–1674. [Google Scholar] [CrossRef]
- Smith, A.M.; Adler, F.R.; Ribeiro, R.M.; Gutenkunst, R.N.; McAuley, J.L.; McCullers, J.A.; Perelson, A.S. Kinetics of Coinfection with Influenza A Virus and Streptococcus pneumoniae. PLoS Pathog. 2013, 9, e1003238. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Thomas, P.G.; McCullers, J.A. Depletion of Alveolar Macrophages during Influenza Infection Facilitates Bacterial Superinfections. J. Immunol. 2013, 191, 1250–1259. [Google Scholar] [CrossRef]
- McNamee, L.A.; Harmsen, A.G. Both Influenza-Induced Neutrophil Dysfunction and Neutrophil-Independent Mechanisms Contribute to Increased Susceptibility to a Secondary Streptococcus pneumoniae Infection. Infect. Immuny 2006, 74, 6707–6721. [Google Scholar] [CrossRef]
- Small, C.-L.; Shaler, C.R.; McCormick, S.; Jeyanathan, M.; Damjanovic, D.; Brown, E.G.; Arck, P.; Jordana, M.; Kaushic, C.; Ashkar, A.A.; et al. Influenza Infection Leads to Increased Susceptibility to Subsequent Bacterial Superinfection by Impairing NK Cell Responses in the Lung. J. Immunol. 2010, 184, 2048–2056. [Google Scholar] [CrossRef]
- Loughran, S.T.; Power, P.A.; Maguire, P.T.; McQuaid, S.L.; Buchanan, P.J.; Jonsdottir, I.; Newman, R.W.; Harvey, R.; Johnson, P.A. Influenza infection directly alters innate IL-23 and IL-12p70 and subsequent IL-17A and IFN-γ responses to pneumococcus in vitro in human monocytes. PLOS ONE 2018, 13, e0203521. [Google Scholar] [CrossRef]
- Mina, M.J.; Klugman, K.P.; McCullers, J.A. Live Attenuated Influenza Vaccine, But Not Pneumococcal Conjugate Vaccine, Protects Against Increased Density and Duration of Pneumococcal Carriage After Influenza Infection in Pneumococcal Colonized Mice. J. Infect. Dis. 2013, 208, 1281–1285. [Google Scholar] [CrossRef]
- Song, J.Y.; Lee, J.S.; Wie, S.-H.; Kim, H.Y.; Lee, J.; Seo, Y.B.; Jeong, H.W.; Kim, S.W.; Lee, S.H.; Park, K.-H.; et al. Prospective Cohort Study on the Effectiveness of Influenza and Pneumococcal Vaccines in Preventing Pneumonia Development and Hospitalization. Clin. Vaccine Immunol. 2015, 22, 229–234. [Google Scholar] [CrossRef]
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J. Pediatr. Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]
- Heikkinen, T.; Block, S.L.; Toback, S.L.; Wu, X.; Ambrose, C.S. Effectiveness of Intranasal Live Attenuated Influenza Vaccine Against All-cause Acute Otitis Media in Children. Pediatr. Infect. Dis. J. 2013, 32, 669–674. [Google Scholar] [CrossRef]
- Choi, A.; Christopoulou, I.; Saelens, X.; García-Sastre, A.; Schotsaert, M. TIV Vaccination Modulates Host Responses to Influenza Virus Infection that Correlate with Protection against Bacterial Superinfection. Vaccines 2019, 7, 113. [Google Scholar] [CrossRef]
- Huber, V.C.; Peltola, V.; Iverson, A.R.; McCullers, J.A. Contribution of Vaccine-Induced Immunity toward either the HA or the NA Component of Influenza Viruses Limits Secondary Bacterial Complications. J. Virol. 2010, 84, 4105–4108. [Google Scholar] [CrossRef]
- Klausberger, M.; Leneva, I.A.; Egorov, A.; Strobl, F.; Ghorbanpour, S.M.; Falynskova, I.N.; Poddubikov, A.V.; Makhmudova, N.R.; Krokhin, A.; Svitich, O.A.; et al. Off-target effects of an insect cell-expressed influenza HA-pseudotyped Gag-VLP preparation in limiting postinfluenza Staphylococcus aureus infections. Vaccine 2019, in press. [Google Scholar] [CrossRef]
- Flannery, B.; Zimmerman, R.K.; Gubareva, L.V.; Garten, R.J.; Chung, J.R.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Ohmit, S.E.; et al. Enhanced Genetic Characterization of Influenza A(H3N2) Viruses and Vaccine Effectiveness by Genetic Group, 2014–2015. J. Infect. Dis. 2016, 214, 1010–1019. [Google Scholar] [CrossRef]
- Jacobsen, H.; Rajendran, M.; Choi, A.; Sjursen, H.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F.; Nachbagauer, R. Influenza Virus Hemagglutinin Stalk-Specific Antibodies in Human Serum are a Surrogate Marker for In Vivo Protection in a Serum Transfer Mouse Challenge Model. mBio 2017, 8. [Google Scholar] [CrossRef]
- Krammer, F.; Margine, I.; Hai, R.; Flood, A.; Hirsh, A.; Tsvetnitsky, V.; Chen, D.; Palese, P. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J. Virol. 2014, 88, 2340–2343. [Google Scholar] [CrossRef]
- Klausberger, M.; Tscheliessnig, R.; Neff, S.; Nachbagauer, R.; Wohlbold, T.J.; Wilde, M.; Palmberger, D.; Krammer, F.; Jungbauer, A.; Grabherr, R. Globular Head-Displayed Conserved Influenza H1 Hemagglutinin Stalk Epitopes Confer Protection against Heterologous H1N1 Virus. PLoS ONE 2016, 11, e0153579. [Google Scholar] [CrossRef]
- Shrestha, A.; Bao, K.; Chen, Y.-R.; Chen, W.; Wang, P.; Fei, Z.; Blissard, G.W. Global Analysis of Baculovirus Autographa californica Multiple Nucleopolyhedrovirus Gene Expression in the Midgut of the Lepidopteran Host Trichoplusia ni. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Per Cent Endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Reiter, K.; Aguilar, P.P.; Wetter, V.; Steppert, P.; Tover, A.; Jungbauer, A. Separation of virus-like particles and extracellular vesicles by flow-through and heparin affinity chromatography. J. Chromatogr. A 2019, 1588, 77–84. [Google Scholar] [CrossRef]
- Krammer, F.; Margine, I.; Tan, G.S.; Pica, N.; Krause, J.C.; Palese, P. A Carboxy-Terminal Trimerization Domain Stabilizes Conformational Epitopes on the Stalk Domain of Soluble Recombinant Hemagglutinin Substrates. PLoS ONE 2012, 7, e43603. [Google Scholar] [CrossRef]
- Klausberger, M.; Wilde, M.; Palmberger, D.; Hai, R.; Albrecht, R.A.; Margine, I.; Hirsh, A.; García-Sastre, A.; Grabherr, R.; Krammer, F. One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine 2014, 32, 355–362. [Google Scholar] [CrossRef]
- Bright, R.A.; Carter, D.M.; Daniluk, S.; Toapanta, F.R.; Ahmad, A.; Gavrilov, V.; Massare, M.; Pushko, P.; Mytle, N.; Rowe, T.; et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007, 25, 3871–3878. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Khadke, S.; Korsholm, K.S.; Perrie, Y.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. The administration route is decisive for the ability of the vaccine adjuvant CAF09 to induce antigen-specific CD8 + T-cell responses: The immunological consequences of the biodistribution profile. J. Control. Release 2016, 239, 107–117. [Google Scholar] [CrossRef]
- Shahangian, A.; Chow, E.K.; Tian, X.; Kang, J.R.; Ghaffari, A.; Liu, S.Y.; Belperio, J.A.; Cheng, G.; Deng, J.C. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Investig. 2009, 119, 1910–1920. [Google Scholar] [CrossRef]
- McCullers, J.A.; Rehg, J.E. Lethal Synergism between Influenza Virus and Streptococcus pneumoniae: Characterization of a Mouse Model and the Role of Platelet-Activating Factor Receptor. J. Infect. Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef]
- Pushko, P.; Tretyakova, I.; Hidajat, R.; Sun, X.; Belser, J.A.; Tumpey, T.M. Multi-clade H5N1 virus-like particles: Immunogenicity and protection against H5N1 virus and effects of beta-propiolactone. Vaccine 2018, 36, 4346–4353. [Google Scholar] [CrossRef]
- Rueda, P.; Fominaya, J.; Langeveld, J.P.; Bruschke, C.; Vela, C.; Casal, J.I. Effect of different baculovirus inactivation procedures on the integrity and immunogenicity of porcine parvovirus-like particles. Vaccine 2000, 19, 726–734. [Google Scholar] [CrossRef]
- She, Y.-M.; Cheng, K.; Farnsworth, A.; Li, X.; Cyr, T.D. Surface modifications of influenza proteins upon virus inactivation by β-propiolactone. PROTEOMICS 2013, 13, 3537–3547. [Google Scholar] [CrossRef]
- Herrera-Rodriguez, J.; Signorazzi, A.; Holtrop, M.; de Vries-Idema, J.; Huckriede, A. Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production. Vaccine 2019, 37, 1630–1637. [Google Scholar] [CrossRef]
- Blackburn, N.K.; Besselaar, T.G. A study of the effect of chemical inactivants on the epitopes of Rift Valley fever virus glycoproteins using monoclonal antibodies. J. Virol. Methods 1991, 33, 367–374. [Google Scholar] [CrossRef]
- Hervas-Stubbs, S.; Rueda, P.; Lopez, L.; Leclerc, C. Insect Baculoviruses Strongly Potentiate Adaptive Immune Responses by Inducing Type I IFN. J. Immunol. 2007, 178, 2361–2369. [Google Scholar] [CrossRef]
- Suzuki, T.; Chang, M.O.; Kitajima, M.; Takaku, H. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell. Immunol. 2010, 262, 35–43. [Google Scholar] [CrossRef]
- Heinimäki, S.; Tamminen, K.; Malm, M.; Vesikari, T.; Blazevic, V. Live baculovirus acts as a strong B and T cell adjuvant for monomeric and oligomeric protein antigens. Virology 2017, 511, 114–122. [Google Scholar] [CrossRef]
- Margine, I.; Martinez-Gil, L.; Chou, Y.-Y.; Krammer, F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS ONE 2012, 7, e51559. [Google Scholar] [CrossRef]
- Klonoski, J.M.; Watson, T.; Bickett, T.E.; Svendsen, J.M.; Gau, T.J.; Britt, A.; Nelson, J.T.; Schlenker, E.H.; Chaussee, M.S.; Rynda-Apple, A.; et al. Contributions of Influenza Virus Hemagglutinin and Host Immune Responses Toward the Severity of Influenza Virus: Streptococcus pyogenes Superinfections. Viral Immunol. 2018, 31, 457–469. [Google Scholar] [CrossRef]
- Lee, B.; Robinson, K.M.; McHugh, K.J.; Scheller, E.V.; Mandalapu, S.; Chen, C.; Di, Y.P.; Clay, M.E.; Enelow, R.I.; Dubin, P.J.; et al. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L158–L167. [Google Scholar] [CrossRef]
- McCullers, J.A.; Bartmess, K.C. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 2003, 187, 1000–1009. [Google Scholar] [CrossRef]
- Sareneva, T.; Matikainen, S.; Kurimoto, M.; Julkunen, I. Influenza A virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J. Immunol. 1998, 160, 6032–6038. [Google Scholar]
- Price, G.E.; Gaszewska-Mastarlarz, A.; Moskophidis, D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 2000, 74, 3996–4003. [Google Scholar] [CrossRef]
- Abe, T.; Hemmi, H.; Miyamoto, H.; Moriishi, K.; Tamura, S.; Takaku, H.; Akira, S.; Matsuura, Y. Involvement of the Toll-Like Receptor 9 Signaling Pathway in the Induction of Innate Immunity by Baculovirus. J. Virol. 2005, 79, 2847–2858. [Google Scholar] [CrossRef]
- Moriyama, T.; Suzuki, T.; Chang, M.O.; Kitajima, M.; Takaku, H. Baculovirus directly activates murine NK cells via TLR9. Cancer Gene. Ther. 2017, 24, 175–179. [Google Scholar] [CrossRef]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Norton, E.B.; Clements, J.D.; Voss, T.G.; Cardenas-Freytag, L. Prophylactic Administration of Bacterially Derived Immunomodulators Improves the Outcome of Influenza Virus Infection in a Murine Model. J. Virol. 2010, 84, 2983–2995. [Google Scholar] [CrossRef]
- Uittenbogaard, J.P.; Zomer, B.; Hoogerhout, P.; Metz, B. Reactions of β-Propiolactone with Nucleobase Analogues, Nucleosides, and Peptides: Implications for The Inactivation of Viruses. J. Biol. Chem. 2011, 286, 36198–36214. [Google Scholar] [CrossRef]
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges. Expert Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Tan, G.S.; Palese, P.; Ravetch, J.V. Broadly neutralizing hemagglutinin stalk–specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med. 2014, 20, 143–151. [Google Scholar] [CrossRef]
- Petersen, H.; Mostafa, A.; Tantawy, M.A.; Iqbal, A.A.; Hoffmann, D.; Tallam, A.; Selvakumar, B.; Pessler, F.; Beer, M.; Rautenschlein, S.; et al. NS Segment of a 1918 Influenza A Virus-Descendent Enhances Replication of H1N1pdm09 and Virus-Induced Cellular Immune Response in Mammalian and Avian Systems. Front. Microbiol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Reading, P.C.; Tate, M.D.; Pickett, D.L.; Brooks, A.G. Glycosylation as a Target for Recognition of Influenza Viruses by the Innate Immune System. In Current Topics in Innate Immunity; Lambris, J.D., Ed.; Springer: New York, NY, USA, 2007; Volume 598, pp. 279–292. ISBN 978-0-387-71765-4. [Google Scholar]
- Sun, X.; Jayaraman, A.; Maniprasad, P.; Raman, R.; Houser, K.V.; Pappas, C.; Zeng, H.; Sasisekharan, R.; Katz, J.M.; Tumpey, T.M. N-Linked Glycosylation of the Hemagglutinin Protein Influences Virulence and Antigenicity of the 1918 Pandemic and Seasonal H1N1 Influenza A Viruses. J. Virol. 2013, 87, 8756–8766. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klausberger, M.; Leneva, I.A.; Falynskova, I.N.; Vasiliev, K.; Poddubikov, A.V.; Lindner, C.; Kartaschova, N.P.; Svitich, O.A.; Stukova, M.; Grabherr, R.; et al. The Potential of Influenza HA-Specific Immunity in Mitigating Lethality of Postinfluenza Pneumococcal Infections. Vaccines 2019, 7, 187. https://doi.org/10.3390/vaccines7040187
Klausberger M, Leneva IA, Falynskova IN, Vasiliev K, Poddubikov AV, Lindner C, Kartaschova NP, Svitich OA, Stukova M, Grabherr R, et al. The Potential of Influenza HA-Specific Immunity in Mitigating Lethality of Postinfluenza Pneumococcal Infections. Vaccines. 2019; 7(4):187. https://doi.org/10.3390/vaccines7040187
Chicago/Turabian StyleKlausberger, Miriam, Irina A. Leneva, Irina N. Falynskova, Kirill Vasiliev, Alexander V. Poddubikov, Claudia Lindner, Nadezhda P. Kartaschova, Oxana A. Svitich, Marina Stukova, Reingard Grabherr, and et al. 2019. "The Potential of Influenza HA-Specific Immunity in Mitigating Lethality of Postinfluenza Pneumococcal Infections" Vaccines 7, no. 4: 187. https://doi.org/10.3390/vaccines7040187
APA StyleKlausberger, M., Leneva, I. A., Falynskova, I. N., Vasiliev, K., Poddubikov, A. V., Lindner, C., Kartaschova, N. P., Svitich, O. A., Stukova, M., Grabherr, R., & Egorov, A. (2019). The Potential of Influenza HA-Specific Immunity in Mitigating Lethality of Postinfluenza Pneumococcal Infections. Vaccines, 7(4), 187. https://doi.org/10.3390/vaccines7040187




